Assessment of Retinal Microcirculation in Primary Open-Angle Glaucoma Using Adaptive Optics and OCT Angiography: Correlation with Structural and Functional Damage
Abstract
1. Introduction
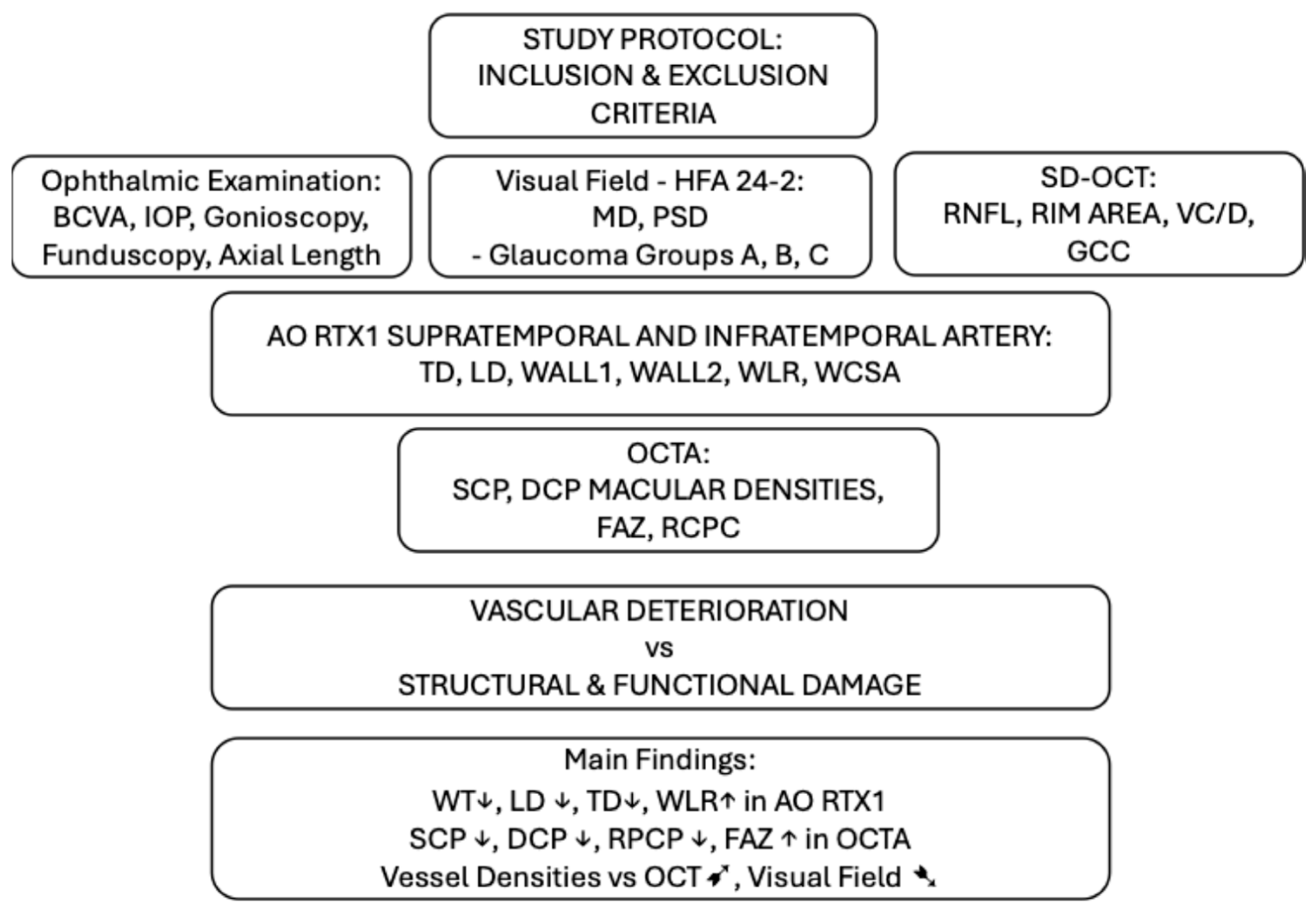
2. Materials and Methods
2.1. Inclusion Criteria
- Glaucoma A–pre-perimetric glaucoma: no visual field scotoma in perimetry, MD greater than 6 dB (16 eyes);
- Glaucoma B–early perimetric glaucoma, MD less than −6 dB (24 eyes);
- Glaucoma C–moderate perimetric glaucoma, MD less than −12 dB (17 eyes).
2.2. Exclusion Criteria
- refractive errors > 6 D or cylindrical lens ≥ 2.5 D,
- axial eye length ≥ 26 mm,
- distance best-corrected visual acuity (BCVA) ≤ 0.4 on Snellen charts,
- media opacities resulting in low image quality,
- diabetes mellitus,
- history of trauma,
- any other ocular diseases that may influence retinal vessel morphology.
2.3. Study Protocol
2.4. Statistical Analyses
3. Results
3.1. Glaucoma vs. Healthy Eyes
3.2. Comparison of AO Rtx1 Arteriolar Parameters Between Glaucoma and Healthy Eyes
3.3. Comparison of AO Rtx1 Arteriolar Parameters Between Glaucoma Groups
3.4. Comparison of OCTA Parameters Between Glaucoma and Healthy Eyes
3.5. Comparison of OCTA Parameters Between Glaucoma Groups
3.6. Correlations of the OCTA Results with Other Analysed Parameters
Assessment of the correlation of the RPCP
4. Discussion
4.1. AO rtx1™
4.2. OCTA: SCP, DCP, RPCP, FAZ
4.3. SCP Correlations with OCT, VF, AO rtx1™
4.4. RPCP Correlations with OCT, VF, AO rtx1™
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Martucci, A.; Nucci, C.; Pinazo-Duran, M.D. Editorial: New perspectives in glaucoma pathophysiology, diagnosis, and treatment. Front. Med. 2023, 10, 1200427. [Google Scholar] [CrossRef]
- Shalaby, W.S.; Ahmed, O.M.; Waisbourd, M.; Katz, L.J. A review of potential novel glaucoma therapeutic options independent of intraocular pressure. Surv. Ophthalmol. 2022, 67, 1062–1080. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J. The vascular concept of glaucoma. Surv. Ophthalmol. 1994, 38 (Suppl. S3), S3–S6. [Google Scholar] [CrossRef]
- Szewczuk, A.; Wawrzyniak, Z.M.; Szaflik, J.P.; Zaleska-Żmijewska, A. Is Primary Open-Angle Glaucoma a Vascular Disease? Assessment of the Relationship between Retinal Arteriolar Morphology and Glaucoma Severity Using Adaptive Optics. J. Clin. Med. 2024, 13, 478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flammer, J.; Orgül, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.-P.; Stefánsson, E. The impact of ocular blood flow in glaucoma. Prog. Retin Eye Res. 2002, 21, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Deokule, S.; Vizzeri, G.; Boehm, A.; Bowd, C.; Weinreb, R.N. Association of visual field severity and parapapillary retinal blood flow in open-angle glaucoma. J. Glaucoma 2010, 19, 293–298. [Google Scholar] [CrossRef]
- Kuang, T.M.; Zhang, C.; Zangwill, L.M.; Weinreb, R.N.; Medeiros, F.A. Estimating lead time gained by optical coherence tomography in detecting glaucoma before the development of visual field defects. Ophthalmology 2015, 122, 2002–2009. [Google Scholar] [CrossRef]
- Chang, R.T.; Singh, K. Glaucoma suspect: Diagnosis suspect: Diagnosis and management. J. Ophthalmol. 2016, 5, 32–37. [Google Scholar] [CrossRef]
- Rizzoni, D.; Porteri, E.; Duse, S.; De Ciuceis, C.; Rosei, C.A.; La Boria, E.; Semeraro, F.; Costagliola, C.; Sebastiani, A.; Danzi, P.; et al. Relationship between media-to-lumen ratio of subcutaneous small arteries and wall-to-lumen ratio of retinal arterioles evaluated noninvasively by scanning laser Doppler flowmetry. J. Hypertens. 2012, 30, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.J.; Harazny, J.M.; Kistner, I.; Friedrich, S.; Wojtkiewicz, J.; Schmieder, R.E. Retinal capillary rarefaction in patients with untreated mild-moderate hypertension. BMC Cardiovasc. Disord. 2017, 17, 300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Ciuceis, C.; Agabiti Rosei, C.; Caletti, S.; Trapletti, V.; Coschignano, M.A.; Tiberio, G.A.M.; Duse, S.; Docchio, F.; Pasinetti, S.; Zambonardi, F.; et al. Comparison between invasive and noninvasive techniques of evaluation of microvascular structural alterations. J. Hypertens. 2018, 36, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.K.; Ong, Y.T.; Cheung, C.Y.; Wong, T.Y. Retinal vascular calibre measurements: Clinical significance, current knowledge and future perspectives. Ophthalmologica 2013, 229, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Akyol, E.; Hagag, A.M.; Sivaprasad, S.; Lotery, A.J. Adaptive optics: Principles and applications in ophthalmology. Eye 2021, 35, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Szewczuk, A.; Zaleska-Żmijewska, A.; Dziedziak, J.; Szaflik, J.P. Clinical Application of Adaptive Optics Imaging in Diagnosis, Management, and Monitoring of Ophthalmological Diseases: A Narrative Review. Med. Sci. Monit. 2023, 29, e941926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nitta, E.; Hirooka, K.; Shimazaki, T.; Sato, S.; Ukegawa, K.; Nakano, Y.; Tsujikawa, A. Retinal oxygen saturation before and after glaucoma surgery. Acta Ophthalmol. 2017, 95, e350–e353. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.L.; Pradhan, Z.S.; Suh, M.H.; Moghimi, S.; Mansouri, K.; Weinreb, R.N. Optical coherence tomography angiography in glaucoma. J. Glaucoma 2020, 29, 312. [Google Scholar] [CrossRef]
- Van Melkebeke, L.; Barbosa-Breda, J.; Huygens, M.; Stalmans, I. Optical coherence tomography angiography in glaucoma: A review. Ophthalmic Res. 2018, 60, 139–151. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, L.; Jia, Y.; Takusagawa, H.L.; Pechauer, A.D.; Edmunds, B.; Lombardi, L.; Davis, E.; Morrison, J.C.; Huang, D. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol. 2015, 133, 1045–1052. [Google Scholar] [CrossRef]
- Chen, C.L.; Zhang, A.; Bojikian, K.D.; Wen, J.C.; Zhang, Q.; Xin, C.; Mudumbai, R.C.; Johnstone, M.A.; Chen, P.P.; Wang, R.K. Peripapillary Retinal Nerve Fiber Layer Vascular Microcirculation in Glaucoma Using Optical Coherence Tomography-Based Microangiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Iida, Y.; Nakanishi, H.; Terada, N.; Morooka, S.; Yamada, H.; Hasegawa, T.; Yokota, S.; Yoshikawa, M.; Yoshimura, N. Microvascular Density in Glaucomatous Eyes With Hemifield Visual Field Defects: An Optical Coherence Tomography Angiography Study. Am. J. Ophthalmol. 2016, 168, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, A.; Zangwill, L.M.; Diniz-Filho, A.; Suh, M.H.; Yousefi, S.; Saunders, L.J.; Belghith, A.; Manalastas, P.I.; Medeiros, F.A.; Weinreb, R.N. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology 2016, 123, 2498–2508. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, C.; Ko, T.; Kong, X.; Yu, X.; Min, W.; Shi, G.; Sun, X. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: An optical coherence tomography angiography study. Arch. Clin. Exp. Ophthalmol. 2015, 253, 1557–1564. [Google Scholar] [CrossRef]
- Available online: https://optopol.com/ (accessed on 30 May 2025).
- Hamard, P.; Hamard, H.; Dufaux, J.; Quesnot, S. Optic nerve head blood flow using a laser Doppler velocimeter and haemorheology in primary open-angle glaucoma and normal pressure glaucoma. Br. J. Ophthalmol. 1994, 78, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Michelson, G.; Langhans, M.J.; Groh, M.J. Perfusion of the juxtapapillary retina and the neuroretinal rim area in primary open angle glaucoma. J. Glaucoma 1996, 5, 91–98. [Google Scholar] [CrossRef]
- Yarmohammadi, A.; Zangwill, L.M.; Diniz-Filho, A.; Suh, M.H.; Manalastas, P.I.; Fatehee, N.; Yousefi, S.; Belghith, A.; Saunders, L.J.; Medeiros, F.A.; et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Ophthalmol. Vis. Sci. 2016, 57, OCT451–OCT459. [Google Scholar] [CrossRef]
- Jia, Y.; Wei, E.; Wang, X.; Zhang, X.; Morrison, J.C.; Parikh, M.; Lombardi, L.H.; Gattey, D.M.; Armour, R.L.; Edmunds, B.; et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014, 121, 1322–1332. [Google Scholar] [CrossRef]
- Jonas, J.B.; Nguyen, X.N.; Naumann, G.O. Parapapillary retinal vessel diameter in normal and glaucoma eyes. I. Morphometric data. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1599–1603. [Google Scholar]
- Wang, S.; Xu, L.; Wang, Y.; Wang, Y.; Jonas, J.B. Retinal vessel diameter in normal and glaucomatous eyes: The Beijing eye study. Clin. Exp. Ophthalmol. 2007, 35, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Nguyen, X.N.; Gusek, G.C.; Naumann, G.O. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Investig. Ophthalmol. Vis. Sci. 1989, 30, 908–918. [Google Scholar]
- Rader, J.; Feuer, W.J.; Anderson, D.R. Peripapillary vasoconstriction in the glaucomas and the anterior ischemic optic neuropathies. Arch. Ophthalmol. 1994, 117, 72–80. [Google Scholar] [CrossRef]
- Zhang, Q.; Jan, C.; Guo, C.Y.; Wang, F.H.; Liang, Y.B.; Cao, K.; Zhang, Z.; Yang, D.Y.; Thomas, R.; Wang, N.L.; et al. Association of intraocular pressure-related factors and retinal vessel diameter with optic disc rim area in subjects with and without primary open angle glaucoma. Clin. Exp. Ophthalmol. 2018, 46, 389–399. [Google Scholar] [CrossRef]
- Hugo, J.; Chavane, F.; Beylerian, M.; Callet, M.; Denis, D.; Matonti, F. Morphologic Analysis of Peripapillary Retinal Arteriole Using Adaptive Optics in Primary Open-angle Glaucoma. Eur. J. Gastroenterol. Hepatol. 2020, 29, 271–275. [Google Scholar] [CrossRef]
- Meixner, E.; Michelson, G. Measurement of retinal wall-to-lumen ratio by adaptive optics retinal camera: A clinical research. Graefe’s Arch. Clin. Exp. Ophthalmol 2015, 253, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Kanno, J.; Weinreb, R.N.; Yoshikawa, Y.; Mine, I.; Ishii, H.; Ibuki, H.; Shinoda, K. OCT angiography measured changes in the foveal avascular zone area after glaucoma surgery. Br. J. Ophthalmol. 2022, 106, 80–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, F.Y.; Ng, D.S.; Lam, A.; Luk, F.; Wong, R.; Chan, C.; Mohamed, S.; Fong, A.; Lok, J.; Tso, T.; et al. Determinants of quantitative optical coherence tomography angiography metrics in patients with diabetes. Sci. Rep. 2017, 7, 2575. [Google Scholar] [CrossRef]
- Choi, J.; Kwon, J.; Shin, J.W.; Lee, J.; Lee, S.; Kook, M.S. Quantitative optical coherence tomography angiography of macular vascular structure and foveal avascular zone in glaucoma. PLoS ONE 2017, 12, e0184948. [Google Scholar] [CrossRef]
- Kwon, J.; Choi, J.; Shin, J.W.; Lee, J.; Kook, M.S. Glaucoma diagnostic capabilities of foveal avascular zone parameters using optical coherence tomography angiography according to visual field defect location. J. Glaucoma 2017, 26, 1120–1129. [Google Scholar] [CrossRef]
- Kwon, J.; Choi, J.; Shin, J.W.; Lee, J.; Kook, M.S. An Optical Coherence Tomography Angiography Study of the Relationship Between Foveal Avascular Zone Size and Retinal Vessel Density. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.L.; Pradhan, Z.S.; Weinreb, R.N.; Reddy, H.B.; Riyazuddin, M.; Dasari, S.; Palakurthy, M.; Puttaiah, N.K.; Rao, D.A.S.; Webers, C.A.B. Regional comparisons of optical coherence tomography angiography vessel density in primary open angle glaucoma. Am. J. Ophthalmol. 2016, 171, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Zangwill, L.M.; Akagi, T.; Saunders, L.J.; Yarmohammadi, A.; Manalastas, P.I.C.; Penteado, R.C.; Weinreb, R.N. Progressive macula vessel density loss in primary open-angle glaucoma: A longitudinal study. Am. J. Ophthalmol. 2017, 182, 107–117. [Google Scholar] [CrossRef]
- Yarmohammadi, A.; Zangwill, L.M.; Manalastas, P.I.C.; Fuller, N.J.; Diniz-Filho, A.; Saunders, L.J.; Suh, M.H.; Hasenstab, K.; Weinreb, R.N. Peripapillary and Macular Vessel Density in Patients with Primary Open-Angle Glaucoma and Unilateral Visual Field Loss. Ophthalmology 2018, 125, 578–587. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yarmohammadi, A.; Zangwill, L.M.; Diniz-Filho, A.; Saunders, L.J.; Suh, M.H.; Wu, Z.; Manalastas, P.I.C.; Akagi, T.; Medeiros, F.A.; Weinreb, R.N. Peripapillary and Macular Vessel Density in Patients with Glaucoma and Single-Hemifield Visual Field Defect. Ophthalmology 2017, 124, 709–719. [Google Scholar] [CrossRef]
- Yospon, T.; Rojananuangnit, K. Optical Coherence Tomography Angiography (OCTA) Differences in Vessel Perfusion Density and Flux Index of the Optic Nerve and Peri-Papillary Area in Healthy, Glaucoma Suspect and Glaucomatous Eyes. Clin. Ophthalmol. 2023, 17, 3011–3021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tonti, E.; Tonti, S.; Mancini, F.; Bonini, C.; Spadea, L.; D’Esposito, F.; Gagliano, C.; Musa, M.; Zeppieri, M. Artificial Intelligence and Advanced Technology in Glaucoma: A Review. J. Pers. Med. 2024, 14, 1062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Tang, L.; Xia, M.; Cao, G. The application of artificial intelligence in glaucoma diagnosis and prediction. Front. Cell. Dev. Biol. 2023, 11, 1173094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, Y.J.; Sun, S.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Diagnostic ability of macular microvasculature with swept-source OCT angiography for highly myopic glaucoma using deep learning. Sci. Rep. 2023, 13, 5209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.H.; Lin, S.; Moghimi, S. Application of artificial intelligence in glaucoma care: An updated review. Taiwan J. Ophthalmol. 2024, 14, 340–351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soltanian-Zadeh, S.; Kurokawa, K.; Liu, Z.; Zhang, F.; Saeedi, O.; Hammer, D.X.; Miller, D.T.; Farsiu, S. Weakly supervised individual ganglion cell segmentation from adaptive optics OCT images for glaucomatous damage assessment. Optica 2021, 8, 642–651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lan, C.H.; Chiu, T.H.; Yen, W.T.; Lu, D.W. Artificial Intelligence in Glaucoma: Advances in Diagnosis, Progression Forecasting, and Surgical Outcome Prediction. Int. J. Mol. Sci. 2025, 26, 4473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Technology | Evaluated Parameters |
|---|---|
| Scanning Laser Doppler Flowmetry (SLDF) (Heidelberg Retina Flowmeter; Heidelberg Engineering, Heidelberg, Germany) | Volumetric total capillary blood flow and flow velocity in the retina, wall-to-lumen ratio (WLR), wall cross-sectional area (WCSA), wall thickness (WT), lumen diameter (LD), total diameter (TD) [11,12,13] |
| Retinal Vessel Analyzer (RVA) (Dynamic Vessel Analyzer, Imedos Systems UG, Jena, Germany) | Dynamic vessel diameter response to flickering light, arterial and venous calibres [14] |
| Adaptive Optics Fundus Camera (AO-FC) (Rtx1, Imagine Eyes, Orsay, France) | Wall-to-lumen ratio (WLR), wall cross-sectional area (WCSA), wall thickness (WT), lumen diameter (LD), and total diameter (TD) [15,16] |
| Retinal Oximeter (RO) (Oxymap T1, Oxymap ehf., Reykjavik, Iceland) | Oxygen saturation of haemoglobin in retinal arterioles and venules [17] |
| Laser Doppler Velocimeter (LDV) (CLBF 100, Canon Inc., Tokyo, Japan) | Peak blood flow velocity in major retinal vessels [14] |
| Optical Coherence Tomography Angiography (OCTA) (Revo NX 130, Optopol Technology Sp. z o.o., Zawiercie, Poland); (AngioVue Imaging System, Optovue Inc., Fremont, CA, USA); Cirrus HD-OCT with AngioPlex, Carl Zeiss Meditec AG, Jena, Germany); (DRI OCT Triton, Topcon Corp., Tokyo, Japan). | Vessel density (VD), perfusion density, foveal avascular zone (FAZ), radial peripapillary capillaries (RPC), macular capillary plexus density [18,19,20,21,22] |
| Parameters | Control Group | Glaucoma Group | p-Value * |
|---|---|---|---|
| age (m ± SD) (years) | 49.5 ± 4.4 | 51.7 ± 6.4 | 0.067 |
| HA | 8.3% | 9.8% | 0.896 |
| CHD | 4.2% | 14.8% | 0.346 |
| BMI (m ± SD) (kg/m2) | 23.7 ± 2.1 | 24.4 ± 1.8 | 0.069 |
| SBP (m ± SD) (mm Hg) | 123.4 ± 11.2 | 127.5 ± 10.1 | 0.117 |
| DBP (m ± SD) (mm Hg) | 80.5 ± 9.2 | 77.7 ± 7.1 | 0.069 |
| BCVA (m ± SD) | 0.998 ± 0.014 | 0.895 ± 0.142 | <0.001 |
| IOP (m ± SD) (mm Hg) | 15.1 ± 2.5 | 13.2 ± 2.7 | <0.001 † |
| AL (m ± SD) (mm) | 23.8 ± 0.8 | 24.0 ± 1.2 | 0.683 |
| OCT c/d v (m ± SD) | 0.501 ± 0.153 | 0.811 ± 0.118 | <0.001 |
| OCT RNFL (m ± SD) (µm) | 101.3 ± 7.4 | 78.8 ± 13.9 | <0.001 |
| OCT GCC (m ± SD) (µm) | 97.7 ± 4.8 | 80.74 ± 11.8 | <0.001 |
| OCT Parameters | Group Glaucoma A | Group Glaucoma B | Group Glaucoma C | p-Value * |
|---|---|---|---|---|
| Total number of eyes | 16 | 24 | 17 | 0.702 ‡ |
| RIM AREA (m ± SD) | 0.854 ± 0.304 | 0.824 ± 0.324 | 0.492 † ± 0.146 (p < 0.001) | <0.001 |
| Vertical Cup-to-disc ratio (m ± SD) | 0.556 ± 0.051 | 0.596 ± 0.021 | 0.863 † ± 0.806 (p < 0.001) | <0.001 |
| RNFL average (m ± SD) (µm) | 88.6 ± 11.4 | 82.1 ± 10.7 | 65.3 † ± 10.7 (p < 0.001) | <0.001 |
| RNFL superior (m ± SD) (µm) | 88.7 ± 13.7 | 84.1 ± 14.0 | 68.3 † ± 13.5 (p = 0.004) | <0.001 |
| RNFL inferior (m ± SD) (µm) | 89.1 ± 11.2 | 80.1 ± 10.1 | 62.1 † ± 9.7 (p < 0.001) | <0.001 |
| GCC (m ± SD) (µm) | 90.0 ± 8.8 | 83.0 ± 9.4 | 68.8 † ± 8.2 (p < 0.001) | <0.001 |
| Visual Field Parameters | Group Glaucoma A | Group Glaucoma B | Group Glaucoma C | p-Value * |
|---|---|---|---|---|
| Total number of eyes | 16 | 24 | 17 | 0.702 ‡ |
| MD (m ± SD) (dB) | −0.17 ± 0.91 | −1.89 ± 1.49 | −13.55 ± 7.66 (p † < 0.001; p †† = 0.014) | <0.001 |
| PSD (m ± SD) (dB) | 1.56 ± 0.25 | 3.55 ± 2.04 | 10.30± 3.75 (p † < 0.001) | <0.001 |
| AO rtx1™ Parameters | Control Group | Glaucoma Group | p-Value * |
|---|---|---|---|
| TDm S (m ± SD) (µm) | 124.1 ± 12.4 | 111.0 ± 10.4 | <0.001 † |
| LDm S (m ± SD) (µm) | 100.6 ± 11.1 | 85.5 ± 10.4 | <0.001 † |
| 1 WTm S (m ± SD) (µm) | 12.2 ± 1.3 | 12.8 ± 1.4 | 0.030 |
| 2 WTm S (m ± SD) (µm) | 11.6 ± 1.2 | 12.6 ± 1.2 | <0.001 † |
| WLRm S (m ± SD) | 0.238 ± 0.002 | 0.301 ± 0.04 | <0.001 |
| WCSAm S (m ± SD) (µm2) | 4197.3 ± 657.9 | 3909.5 ± 531.6 | 0.019 † |
| TDm I (m ± SD) (µm) | 124.4 ± 12.0 | 114.4 ± 11.0 | <0.001 † |
| LDm I (m ± SD) (µm) | 100.4 ± 10.9 | 87.7 ± 10.8 | <0.001 † |
| 1 WTm I (m ± SD) (µm) | 12.1 ± 1.3 | 13.4 ± 1.8 | <0.001 |
| 2 WTm I (m ± SD) (µm) | 11.9 ± 1.1 | 13.3 ± 1.74 | <0.001 |
| WLRm I (m ± SD) | 0.241 ± 0.021 | 0.308 ± 0.047 | <0.001 |
| WCSAm I (m ± SD) (µm2) | 4245.6 ± 690.9 | 4257.5 ± 812.1 | 0.937 |
| AO rtx1™ Parameters | Group Glaucoma A | Group Glaucoma B | Group Glaucoma C | p-Value * |
|---|---|---|---|---|
| Total number of eyes | 16 | 24 | 17 | 0.702 † |
| TDm S (m ± SD) (µm) | 113.1 ± 11.8 | 111.6 ± 10.2 | 109.4 ± 8.7 | 0.384 |
| LDm S (m ± SD) (µm) | 87.8 ± 11.3 | 85.8 ± 10.2 | 84.0 ± 9.5 | 0.416 |
| 1 WTm S (m ± SD) (µm) | 12.9 ± 1.7 | 12.9 ± 1.3 | 12.5 ± 1.1 | 0.225 |
| 2 WTm S (m ± SD) (µm) | 12.5 ± 1.4 | 12.7 ± 1.2 | 12.5 ± 1.2 | 0.867 |
| WLRm S (m ± SD) | 0.293 ± 0.043 | 0.304 ± 0.048 | 0.301 ± 0.041 | 0.744 |
| WCSAm S (m ± SD) (µm2) | 3982.5 ± 657.3 | 3971.2 ± 500.3 | 3808.0 ± 394.2 | 0.539 |
| TDm I (m ± SD) (µm) | 113.2 ± 10.6 | 116.1 ± 12.2 | 112.7 ± 14.0 | 0.595 |
| LDm I (m ± SD) (µm) | 87.7 ± 9.5 | 89.3 ± 11.3 | 85.8 ± 12.2 | 0.558 |
| 1 WTm I (m ± SD) (µm) | 13.0 ± 1.6 | 13.4 ± 2.2 | 13.6 ± 1.5 | 0.506 |
| 2 WTm I (m ± SD) (µm) | 13.0 ± 1.8 | 13.5 ± 1.8 | 13.2 ± 1.6 | 0.503 |
| WLRm I (m ± SD) | 0.302 ± 0.042 | 0.306 ± 0.057 | 0.316 ± 0.040 | 0.656 |
| WCSAm I (m ± SD) (µm2) | 4114.3 ± 746.1 | 4349.1 ± 838.6 | 4218.9 ± 910.7 | 0.584 |
| OCTA Parameters | Control Group | Glaucoma Group | p-Value * |
|---|---|---|---|
| SCP FAZ (m ± SD) (mm2) | 0.295 ± 0.125 | 0.546 ± 0.299 | <0.001 |
| SCP perimeter (m ± SD) (mm) | 2.006 ± 0.497 | 3.444 ± 1.494 | <0.001 |
| SCP circularity (m ± SD) | 0.700 ± 0.082 | 0.555 ± 0.159 | <0.001 |
| DCP FAZ (m ± SD) (mm2) | 0.430 ± 0.172 | 0.651 ± 0.303 | <0.001 |
| DCP perimeter (m ± SD) (mm) | 2.394 ± 0.471 | 3.342 ± 0.896 | <0.001 |
| DCP circularity (m ± SD) | 0.716 ± 0.072 | 0.591 ± 0.122 | <0.001 |
| SCP Total (m ± SD) | 38.61 ± 1.31 | 36.78 ± 1.85 | <0.001 |
| SCP Super (m ± SD) | 38.76 ± 1.17 | 36.91 ± 1.84 | <0.001 |
| SCP Infer (m ± SD) | 38.40 ± 2.06 | 36.63 ± 2.63 | <0.001 |
| SCP Etdrs (m ± SD) | 38.46 ± 1.41 | 36.39 ± 3.60 | <0.001 |
| DCP Total (m ± SD) | 41.06 ± 1.21 | 40.17 ± 2.54 | 0.024 |
| DCP Super (m ± SD) | 41.07 ± 1.21 | 40.23 ± 2.60 | 0.073 |
| DCP Infer (m ± SD) | 41.05 ± 1.62 | 40.10 ± 2.75 | 0.025 |
| DCP Etdrs (m ± SD) | 41.33 ± 1.38 | 40.67 ± 2.51 | 0.090 |
| RPCP Total (m ± SD) | 39.27 ± 1.48 | 35.42 ± 4.97 | <0.001 |
| RPCP Super (m ± SD) | 39.44 ± 1.17 | 35.59 ± 5.01 | <0.001 |
| RPCP Infer (m ± SD) | 39.03 ± 2.24 | 35.17 ± 5.14 | <0.001 |
| OCTA Parameters | Group Glaucoma A | Group Glaucoma B | Group Glaucoma C | p-Value * |
|---|---|---|---|---|
| Total number of eyes | 16 | 24 | 17 | 0.702 ‡ |
| SCP FAZ (m ± SD) (mm2) | 0.564 ± 0.356 | 0.449 ± 0.190 | 0.566 ± 0.289 | 0.711 |
| DCP FAZ (m ± SD) (mm2) | 0.569 ± 0.219 | 0.588 ± 0.308 | 0.762 ± 0.344 | 0.904 |
| SCP Total (m ± SD) | 37.9 ± 1.2 | 36.8 ± 2.2 | 36.6 ± 0.9 p †† = 0.011 | 0.014 |
| SCP Super (m ± SD) | 37.7 ± 1.4 | 36.9 ± 2.1 | 36.7 ± 1.4 | 0.171 |
| SCP Infer (m ± SD) | 38.2 ± 1.2 | 36.6 ± 3.1 | 36.5 ± 1.0 p †† = 0.004 | 0.004 |
| DCP Total (m ± SD) | 40.7 ± 3.2 | 39.9 ± 3.0 | 40.5 ± 1.0 | 0.063 |
| DCP Super (m ± SD) | 40.6 ± 3.3 | 40.0 ± 3.1 | 40.3 ± 1.3 | 0.126 |
| DCP Infer (m ± SD) | 40.7 ± 3.2 | 39.8 ± 3.0 | 40.7 ± 1.1 | 0.157 |
| RPCP Total (m ± SD) | 37.7 ± 0.8 | 34.3 ± 6.6 | 34.7 ± 4.4 p †† = 0.003 | 0.033 |
| RPCP Super (m ± SD) | 37.8 ± 1.2 | 34.2 ± 6.7 | 34.6 ± 4.6 p †† = 0.013 | 0.017 |
| RPCP Infer (m ± SD) | 37.5 ± 1.5 | 34.2 ± 6.8 | 34.8 ± 4.5 p †† = 0.035 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaleska-Żmijewska, A.; Szewczuk, A.; Wawrzyniak, Z.M.; Żmijewska, M.; Szaflik, J.P. Assessment of Retinal Microcirculation in Primary Open-Angle Glaucoma Using Adaptive Optics and OCT Angiography: Correlation with Structural and Functional Damage. J. Clin. Med. 2025, 14, 4978. https://doi.org/10.3390/jcm14144978
Zaleska-Żmijewska A, Szewczuk A, Wawrzyniak ZM, Żmijewska M, Szaflik JP. Assessment of Retinal Microcirculation in Primary Open-Angle Glaucoma Using Adaptive Optics and OCT Angiography: Correlation with Structural and Functional Damage. Journal of Clinical Medicine. 2025; 14(14):4978. https://doi.org/10.3390/jcm14144978
Chicago/Turabian StyleZaleska-Żmijewska, Anna, Alina Szewczuk, Zbigniew M. Wawrzyniak, Maria Żmijewska, and Jacek P. Szaflik. 2025. "Assessment of Retinal Microcirculation in Primary Open-Angle Glaucoma Using Adaptive Optics and OCT Angiography: Correlation with Structural and Functional Damage" Journal of Clinical Medicine 14, no. 14: 4978. https://doi.org/10.3390/jcm14144978
APA StyleZaleska-Żmijewska, A., Szewczuk, A., Wawrzyniak, Z. M., Żmijewska, M., & Szaflik, J. P. (2025). Assessment of Retinal Microcirculation in Primary Open-Angle Glaucoma Using Adaptive Optics and OCT Angiography: Correlation with Structural and Functional Damage. Journal of Clinical Medicine, 14(14), 4978. https://doi.org/10.3390/jcm14144978








