The Angiography Pattern of Buerger’s Disease: Challenges and Recommendations
Abstract
1. Introduction
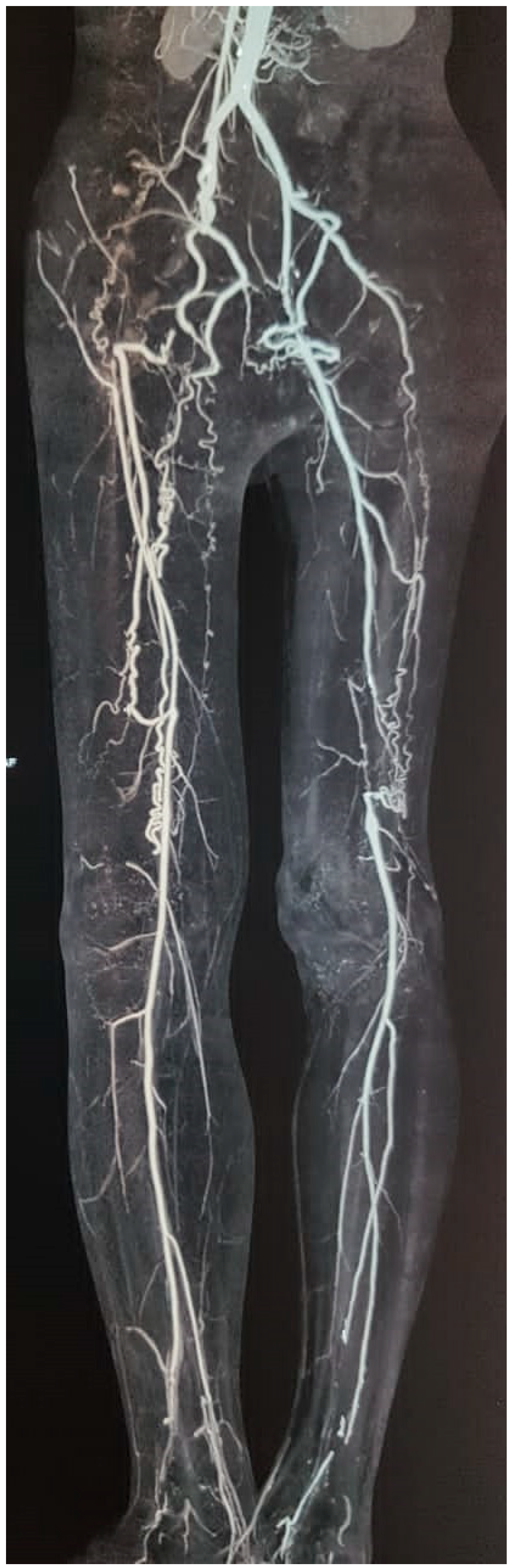
1.1. Angiography Pattern of Lower Limbs in BD Patients
- A.
- Normal aortoiliac and femoropopliteal arteries
- B.
- Segmental occlusions
- C.
- Collaterals
- D.
- Vasospasm
- E.
- Early venous opacification
1.2. Phlebography of Lower Limbs in BD Patients:
1.3. Angiography Pattern of Upper Limbs of BD Patients:
1.4. BD Diagnosis According to Angiography in Atypical Cases:
1.5. Angiography Pattern of Visceral Vessels in the Patients with Previous BD Diagnosis:
2. Discussion
3. Conclusions
Funding
Conflicts of Interest
References
- Fazeli, B.; Poredos, P.; Patel, M.; Klein-Weigel, P.; Catalano, M.; Stephen, E.; AL Salman, M.M.; Altarazi, L.; Bashar, A.H.; Chua, B.; et al. Milestones in thromboangiitis obliterans: A position paper of the VAS-European independent foundation in angiology/vascular medicine. Int. Angiol. 2021, 40, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, B.; Ligi, D.; Keramat, S.; Maniscalco, R.; Sharebiani, H.; Mannello, F. Recent Updates and Advances in Winiwarter-Buerger Disease (Thromboangiitis Obliterans): Biomolecular Mechanisms, Diagnostics and Clinical Consequences. Diagnostics 2021, 11, 1736. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, B.; Poredos, P.; Kozak, M.; Pecsvarady, Z.; Catalano, M.; AL Salman, M.M.; Altarazi, L.; Ali, A.A.; Bashar, A.H.; Bozkurt, K.; et al. Diagnostic criteria for Buerger’s disease: International Consensus of VAS—European Independent Foundation in Angiology/Vascular Medicine. Int. Angiol. 2023, 42, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Wessler, S. Buerger’s disease revisited. Surg. Clin. N. Am. 1969, 49, 703–713. [Google Scholar] [CrossRef]
- Wessler, S.; Ming, S.C.; Gurewich, V.; Freiman, D.G. A critical evaluation of thromboangiitis obliterans. The case against Buerger’s disease. N. Engl. J. Med. 1960, 262, 1149–1160. [Google Scholar] [CrossRef]
- Schatz, I.J.; Fine, G.; Eyler, W.R. Thromboangiitis obliterans. Br. Heart J. 1966, 28, 84–91. [Google Scholar] [CrossRef]
- Iwai, T.; Kume, H.; Koizumi, S.; Sakurazawa, K.; Honma, K.; Ogasawara, H.; Takemura, T.; Kishino, M.; Kagayama, T. Buerger Disease: Pathological Changes in Elderly Patients. Ann. Vasc. Dis. 2022, 15, 29–36. [Google Scholar] [CrossRef]
- Olin, J.W. Thromboangiitis obliterans (Buerger’s disease). N. Engl. J. Med. 2000, 343, 864–869. [Google Scholar] [CrossRef]
- Mills, J.L., Sr. Buerger’s disease in the 21st century: Diagnosis, clinical features, and therapy. Semin. Vasc. Surg. 2003, 16, 179–189. [Google Scholar] [CrossRef]
- Lazarides, M.K.; Georgiadis, G.S.; Papas, T.T.; Nikolopoulos, E.S. Diagnostic criteria and treatment of Buerger’s disease: A review. Int. J. Low. Extrem. Wounds 2006, 5, 89–95. [Google Scholar] [CrossRef]
- Suzuki, S.; Mine, H.; Umehara, I.; Yoshida, T.; Okada, Y. Buerger’s disease (thromboangiitis obliterans): An analysis of the arteriograms of 119 cases. Clin. Radiol. 1982, 33, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Hagen, B.; Lohse, S. Clinical and radiologic aspects of Buerger’s disease. Cardiovasc. Interv. Radiol. 1984, 7, 283–293. [Google Scholar] [CrossRef]
- Goodman, R.M.; Elian, B.; Mozes, M.; Deutsch, V. Buerger’s disease in Israel. Am. J. Med. 1965, 39, 601–615. [Google Scholar] [CrossRef]
- Gilkes, R.; Dow, J. Aortic involvement in Buerger’s disease. Br. J. Radiol. 1973, 46, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, A.; Munk, J.; Schramek, A.; Ben Arieh, J. The angiographic appearance of thromboangiitis obliterans (Buerger’s disease) in the abdominal visceral vessels. Br. J. Radiol. 1973, 46, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Shionoya, S.; Ban, I.; Nakata, Y.; Matsubara, J.; Hirai, M.; Kawai, S. Involvement of the iliac artery in Buerger’s disease (pathogenesis and arterial reconstruction). J. Cardiovasc. Surg. 1978, 19, 69–76. [Google Scholar]
- Inada, K.; Kattsumura, T. The entity of Buerger’s disease. Angiology 1972, 23, 668–687. [Google Scholar] [CrossRef]
- Eadie, D.G.; Mann, C.V.; Smith, P.G. Buerger’s disease. A clinical and pathological re-examination. Br. J. Surg. 1968, 55, 452–456. [Google Scholar] [CrossRef]
- Szilagyi, D.E.; Derusso, F.J.; Elliott, J.P., Jr. Thromboangiitis Obliterans. Clinico-Angiographic Correlations. Arch. Surg. 1964, 88, 824–835. [Google Scholar] [CrossRef]
- McKusick, V.A.; Harris, W.S.; Ottesen, O.E.; Goodman, R.M.; Shelley, W.M.; Bloodwell, R.D. Buerger’s Disease: A Distinct Clinical and Pathologic Entity. JAMA 1962, 181, 5–12. [Google Scholar] [CrossRef]
- Welling, R.E. Buerger’s disease revisited. Angiology 1982, 33, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Lörelius, L.E.; Erikson, U.; Aberg, H. Arterial segmental vasoconstriction. Frequency and pathogenetic factors. Acta Radiol. Diagn. 1977, 18, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Theis, F.V. Thromboangiitis obliterans: A 30-year study. J. Am. Geriatr. Soc. 1958, 6, 106–117. [Google Scholar] [CrossRef]
- Martorell, F.; Valls-Serra, J.; Martorell, A. Tromboangeitis obliterante; revision de 103 casos. J. Int. Chir. 1951, 11, 44–56. [Google Scholar]
- Klein-Weigel, P.; Volz, T.S.; Zange, L.; Richter, J. Buerger’s disease: Providing integrated care. J. Multidiscip. Healthc. 2016, 9, 511–518. [Google Scholar] [CrossRef]
- Debakey, M.E.; Crawford, E.S.; Garrett, H.E.; Cooley, D.A.; Morris, G.C., Jr.; Abbott, J.P. Occlusive disease of the lower extremities in patients 16 to 37 years of age. Ann. Surg. 1964, 159, 873–890. [Google Scholar] [CrossRef]
- Fujii, Y.; Soga, J.; Nakamura, S.; Hidaka, T.; Hata, T.; Idei, N.; Fujimura, N.; Nishioka, K.; Chayama, K.; Kihara, Y.; et al. Classification of corkscrew collaterals in thromboangiitis obliterans (Buerger’s disease): Relationship between corkscrew type and prevalence of ischemic ulcers. Circ. J. 2010, 74, 1684–1688. [Google Scholar] [CrossRef]
- Suzuki, S.; Yamada, I.; Himeno, Y. Angiographic findings in Buerger disease. Int. J. Cardiol. 1996, 54 (Suppl. S1), 89–95. [Google Scholar] [CrossRef]
- Brown, H.; Sellwood, R.A.; Harrison, C.V.; Martin, P. Thromboangiitis obliterans. Br. J. Surg. 1969, 56, 59–63. [Google Scholar] [CrossRef]
- Yoshimuta, T.; Akutsu, K.; Okajima, T.; Tamori, Y.; Kubota, Y.; Takeshita, S. Corkscrew collaterals in Buerger’s disease. Can. J. Cardiol. 2009, 25, 365. [Google Scholar] [CrossRef]
- Fujii, Y.; Ueda, T.; Uchimura, Y.; Teragawa, H. Corkscrew collaterals in atherosclerosis obliterans. Clin. Case Rep. 2017, 5, 1948–1949. [Google Scholar] [CrossRef]
- Fujii, Y.; Teragawa, H.; Kihara, Y.; Higashi, Y. Corkscrew collaterals in Raynaud’s syndrome. BMJ Case Rep. 2016, 2016, bcr2016215841. [Google Scholar] [CrossRef] [PubMed]
- Marvi, U.; Chung, L. Digital ischemic loss in systemic sclerosis. Int. J. Rheumatol. 2010, 2010, 130717. [Google Scholar] [CrossRef] [PubMed]
- Baş, A.; Dikici, A.S.; Gülşen, F.; Samancı, C.; Mihmanlı, İ.; Beşirli, K.; Numan, F.; Kantarci, F. Corkscrew Collateral Vessels in Buerger Disease: Vasa Vasorum or Vasa Nervorum. J. Vasc. Interv. Radiol. 2016, 27, 735–739. [Google Scholar] [CrossRef]
- Farzadnia, M.; Ravari, H.; Masoudian, M.; Valizadeh, N.; Fazeli, B. Unexpected Inflammation in the Sympathetic Ganglia in Thromboangiitis Obliterans. Int. J. Angiol. 2017, 26, 212–217. [Google Scholar] [CrossRef]
- Lambeth, J.T.; Yong, N.K. Arteriographic findings in thromboangiitis obliterans with emphasis on femoropopliteal involvement. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1970, 109, 553–562. [Google Scholar] [CrossRef]
- Köhler, R. Regular alternating changes in arterial width in lower limb angiograms. Acta Radiol. Diagn. 1965, 3, 529–542. [Google Scholar] [CrossRef]
- Chopra, B.S.; Zakariah, T.; Sodhi, J.S.; Khanna, S.K.; Wahi, P.L. Thromboangitis obliterans: A clinical study with special emphasis on venous involvement. Angiology 1976, 27, 126–132. [Google Scholar] [CrossRef]
- Hirai, M.; Shionoya, S. Arterial obstruction of the upper limb in Buerger’s disease: Its incidence and primary lesion. Br. J. Surg. 1979, 66, 124–128. [Google Scholar] [CrossRef]
- Dimmick, S.J.; Goh, A.C.; Cauzza, E.; Steinbach, L.S.; Baumgartner, I.; Stauffer, E.; Voegelin, E.; Anderson, S.E. Imaging appearances of Buerger’s disease complications in the upper and lower limbs. Clin. Radiol. 2012, 67, 1207–1211. [Google Scholar] [CrossRef]
- Ejiyooye, T.F.; Ajibowo, A.O.; Dirisanala, S.; Olagbende, B.; Ezenagu, U.E.; Khan, A. A Rare Case of Thromboangiitis Obliterans of Bilateral Upper Extremities in an Adult Male. Cureus 2022, 14, e24975. [Google Scholar] [CrossRef]
- Murali, U.; Ahmad, M.A.A.; Najihah, F. Thromboangitis Obliterans involving Bilateral Upper limb Extremities—A Rare Case Report from Malaysia. J. Clin. Diagn. Res. 2017, 11, PD06–PD08. [Google Scholar] [CrossRef]
- Koh, Y.B. Buerger’s Disease; Any Current Advances? Ann. Vasc. Dis. 2008, 1, 63–65. [Google Scholar] [CrossRef]
- Fujii, Y.; Ohmura, Y.; Takeuchi, R.; Morimoto, S.; Uchida, H.; Hayashi, K.; Sano, S. Buerger’s disease in a middle-aged woman with diabetes mellitus. A case report. Angiology 1996, 47, 97–102. [Google Scholar]
- Mandel, L.R.; Vidmar, D.A. Buerger’s disease in a middle-aged woman. A unique presentation. Postgrad. Med. 1988, 83, 265–268, 270-2. [Google Scholar] [CrossRef] [PubMed]
- Hus, I.; Sokolowska, B.; Walter-Croneck, A.; Chrapko, M.; Nowaczynska, A.; Dmoszynska, A. Assessment of plasma prothrombotic factors in patients with Buerger’s disease. Blood Coagul. Fibrinolysis 2013, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ramin, M.; Salimi, J.; Meysamie, A. An Iranian scoring system for diagnosing Buerger’s disease. Acta Med. Iran. 2014, 52, 60–65. [Google Scholar] [PubMed]
- Igari, K.; Kudo, T.; Toyofuku, T.; Inoue, Y. Endothelial dysfunction in patients with Buerger disease. Vasc. Health Risk Manag. 2017, 13, 317–323. [Google Scholar] [CrossRef]
- Zannini, G.; Cotrufo, M. Epidemiological, angiographic and clinical aspects of Buerger’s disease. J. Cardiovasc. Surg. 1973, 14, 17–20. [Google Scholar]
- Tamura, A.; Aso, N.; Kadota, J. Corkscrew appearance in the right coronary artery in a patient with Buerger’s disease. Heart 2006, 92, 944. [Google Scholar] [CrossRef]
- Hong, T.E.; Faxon, D.P. Coronary artery disease in patients with Buerger’s disease. Rev. Cardiovasc. Med. 2005, 6, 222–226. [Google Scholar] [PubMed]
- Fakour, F.; Fazeli, B. Visceral bed involvement in thromboangiitis obliterans: A systematic review. Vasc. Health Risk Manag. 2019, 15, 317–353. [Google Scholar] [CrossRef] [PubMed]
- Michail, P.O.; Filis, K.A.; Delladetsima, J.K.; Koronarchis, D.N.; Bastounis, E.A. Thromboangiitis obliterans (Buerger’s disease) in visceral vessels confirmed by angiographic and histological findings. Eur. J. Vasc. Endovasc. Surg. 1998, 16, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kimura, T.; Furukawa, Y.; Tadamura, E.; Kita, T. Coronary Buerger’s disease with a peripheral arterial aneurysm. Eur. Heart J. 2007, 28, 928. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, B.; Poredos, P.; Schernthaner, G.; Stephen, E.; Kozak, M.; Catalano, M.; Pecsvarady, Z.; Patel, M.; Al Salman, M.M.; Altarazi, L.; et al. An International Delphi Consensus on Diagnostic Criteria for Buerger’s Disease. Ann. Vasc. Surg. 2022, 85, 211–218. [Google Scholar] [CrossRef]
- Busch, K. Buerger’s disease (thromboangiitis obliterans): Clinical features and assessment by colour duplex ultrasound. Australas. J. Ultrasound Med. 2011, 14, 18–22. [Google Scholar] [CrossRef]
- Mitropoulos, F.; Eforakopoulos, F.; Kanakis, M.A.; Vassili, M.; Mastorakou, I.; Georgiadis, M. Diagnostic and Therapeutic Approach in a Patient with Buerger’s and Coronary Artery Disease. Case Rep. Med. 2013, 2013, 974184. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazeli, B.; Poredos, P.; Liew, A.; Stephen, E.; Bashar, A.H.M.; Kozak, M.; Catalano, M.; Al Salman, M.M.; Altarazi, L.; Antignani, P.L.; et al. The Angiography Pattern of Buerger’s Disease: Challenges and Recommendations. J. Clin. Med. 2025, 14, 4841. https://doi.org/10.3390/jcm14144841
Fazeli B, Poredos P, Liew A, Stephen E, Bashar AHM, Kozak M, Catalano M, Al Salman MM, Altarazi L, Antignani PL, et al. The Angiography Pattern of Buerger’s Disease: Challenges and Recommendations. Journal of Clinical Medicine. 2025; 14(14):4841. https://doi.org/10.3390/jcm14144841
Chicago/Turabian StyleFazeli, Bahare, Pavel Poredos, Aaron Liew, Edwin Stephen, Abul Hasan Muhammad Bashar, Matija Kozak, Mariella Catalano, Mussaad Mohammaed Al Salman, Louay Altarazi, Pier Luigi Antignani, and et al. 2025. "The Angiography Pattern of Buerger’s Disease: Challenges and Recommendations" Journal of Clinical Medicine 14, no. 14: 4841. https://doi.org/10.3390/jcm14144841
APA StyleFazeli, B., Poredos, P., Liew, A., Stephen, E., Bashar, A. H. M., Kozak, M., Catalano, M., Al Salman, M. M., Altarazi, L., Antignani, P. L., Desai, S., Dimakakos, E., Erer, D., Farkas, K., Fokou, M., Hussein, E., Ionac, M., Iwai, T., Karahan, O., ... Zor, M. H., on behalf of the VAS International Buerger Working Group. (2025). The Angiography Pattern of Buerger’s Disease: Challenges and Recommendations. Journal of Clinical Medicine, 14(14), 4841. https://doi.org/10.3390/jcm14144841










