Feasibility of Near-Infrared Spectroscopy for Monitoring Tissue Oxygenation During Uterus Transplantation and Hysterectomy
Abstract
1. Introduction
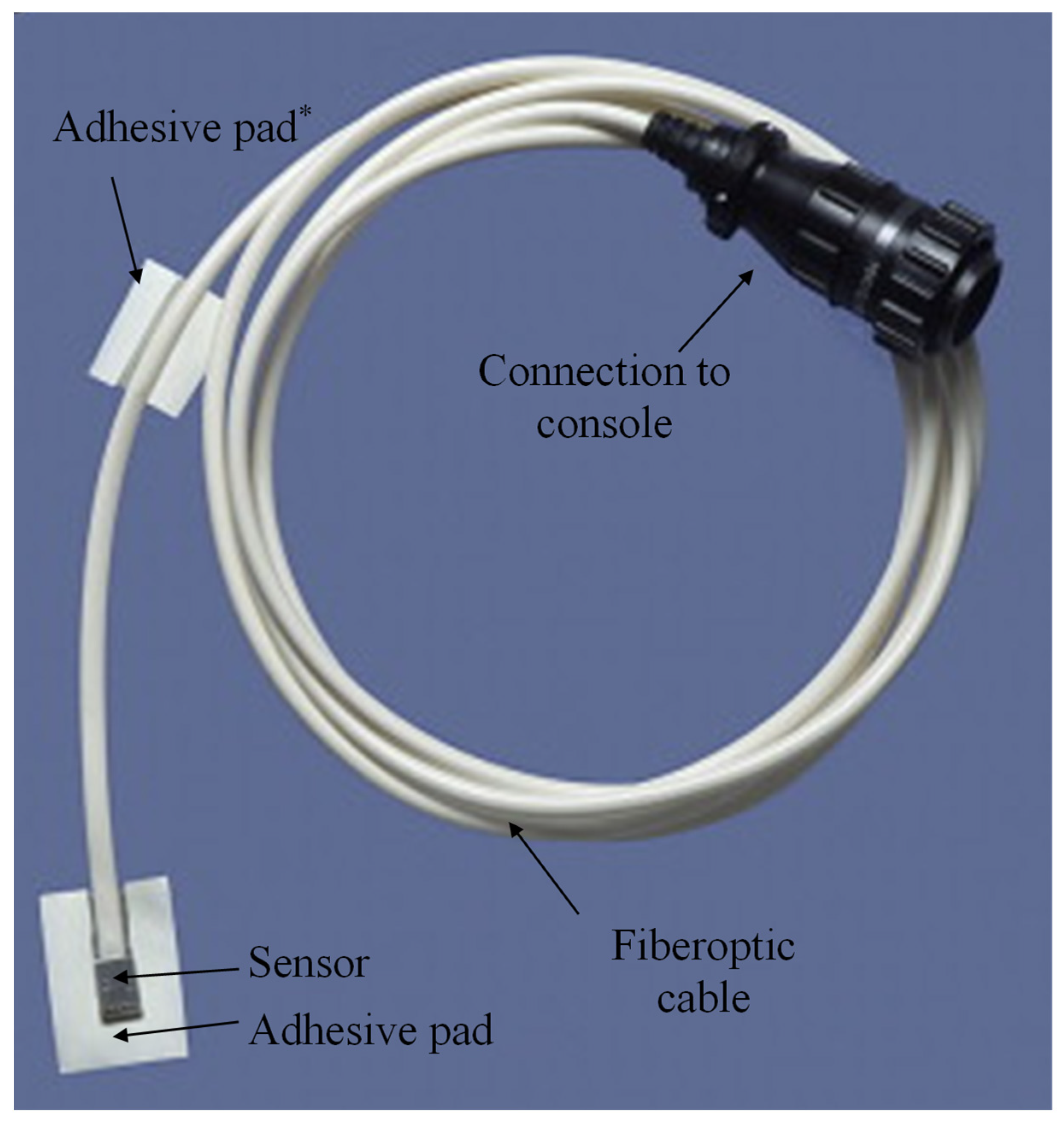
2. Materials and Methods
3. Results
3.1. Participant Demographics
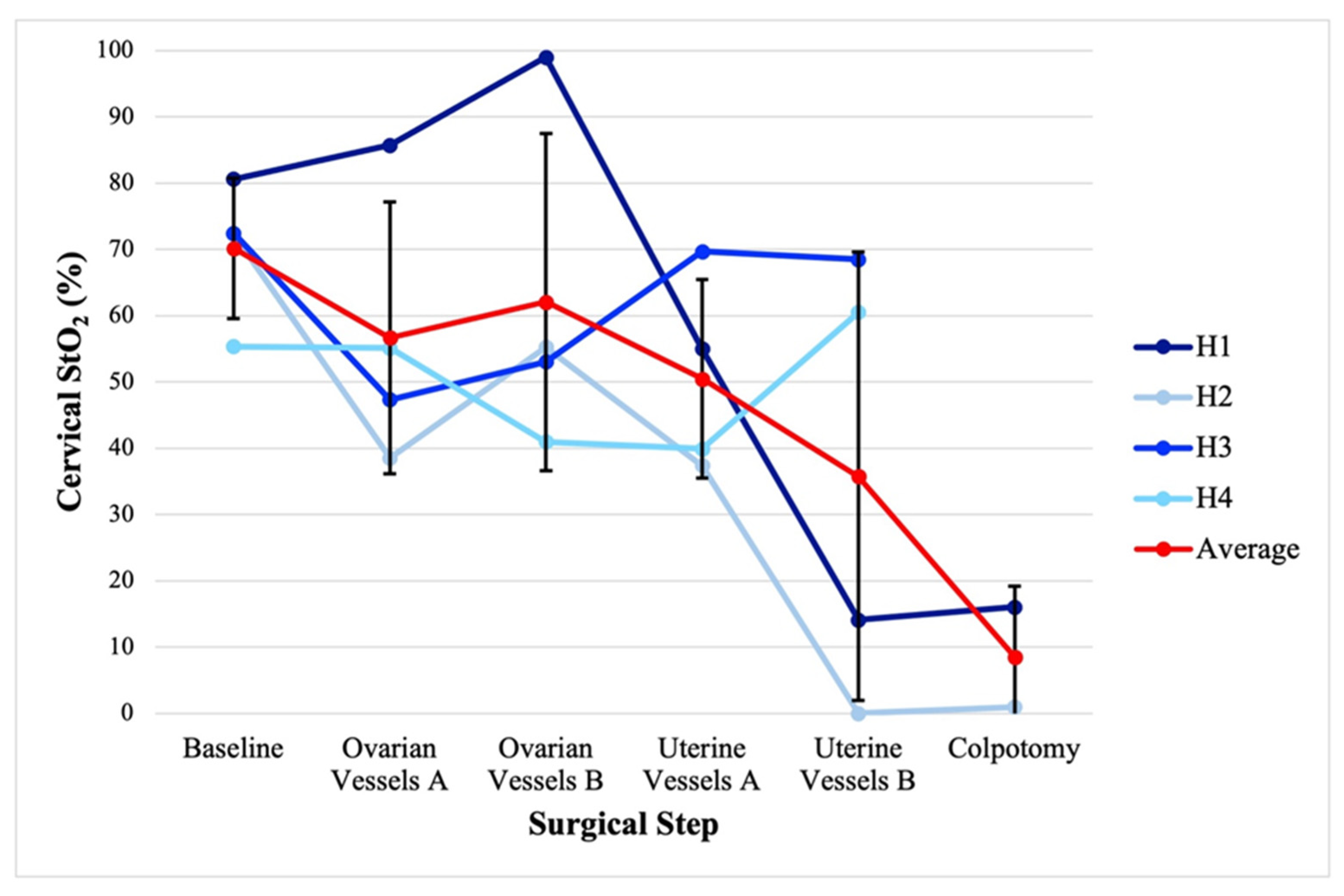
3.2. Hysterectomy and Bilateral Salpingo-Oophorectomy Intraoperative Monitoring
3.3. Uterus Transplantation Intraoperative Monitoring
3.4. Postoperative Monitoring of Uterus Allograft
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brännström, M.; Belfort, M.A.; Ayoubi, J.M. Uterus transplantation worldwide: Clinical activities and outcomes. Curr. Opin. Organ. Transplant. 2021, 26, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Kristek, J.; Johannesson, L.; Novotny, R.; Kachlik, D.; Fronek, J. Human uterine vasculature with respect to uterus transplantation: A comprehensive review. J. Obstet. Gynaecol. Res. 2020, 46, 2199–2220. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Koon, E.C.; Johannesson, L.; McKenna, G.J.; Anthony, T.; Klintmalm, G.B.; Gunby, R.T.; Warren, A.M.; Putman, J.M.; DePrisco, G.; et al. Living Donor Uterus Transplantation: A Single Center’s Observations and Lessons Learned From Early Setbacks to Technical Success. Am. J. Transplant. 2017, 17, 2901–2910. [Google Scholar] [CrossRef]
- Brännström, M.; Johannesson, L.; Dahm-Kähler, P.; Enskog, A.; Mölne, J.; Kvarnström, N.; Diaz-Garcia, C.; Hanafy, A.; Lundmark, C.; Marcickiewicz, J.; et al. First clinical uterus transplantation trial: A six-month report. Fertil. Steril. 2014, 101, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.P.; Saso, S.; Bracewell-Milnes, T.; Thum, M.Y.; Nicopoullos, J.; Diaz-Garcia, C.; Friend, P.; Ghaem-Maghami, S.; Testa, G.; Johannesson, L.; et al. Human uterine transplantation: A review of outcomes from the first 45 cases. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1310–1319. [Google Scholar] [CrossRef]
- Cook-Swartz Doppler Probe. Available online: https://www.coopersurgical.com/product/cook-swartz-doppler-probe/ (accessed on 17 May 2025).
- Kristek, J.; Johannesson, L.; Clemons, M.P.; Kautznerova, D.; Chlupac, J.; Fronek, J.; Testa, G.; dePrisco, G. Radiologic Evaluation of Uterine Vasculature of Uterus Transplant Living Donor Candidates: DUETS Classification. J. Clin. Med. 2022, 11, 4626. [Google Scholar] [CrossRef]
- Leonhardt, H.; Thilander-Klang, A.; Båth, J.; Johannesson, M.; Kvarnström, N.; Dahm-Kähler, P.; Brännström, M. Imaging evaluation of uterine arteries in potential living donors for uterus transplantation: A comparative study of MRA, CTA, and DSA. Eur. Radiol. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Perni, U.C.; Wang, E.Y.; Gregg, A.R. Antepartum Care of the Uterus Transplant Patient: The Experience of 3 Successful US Centers. Clin. Obstet. Gynecol. 2022, 65, 84–91. [Google Scholar] [CrossRef]
- McNulty, J.; Born, M.; Pozos, R.S. Near-Infrared Spectroscopy (NIRS). In Springer Handbook of Medical Technology; Kramme, R., Hoffmann, K.-P., Pozos, R.S., Eds.; Springer: Berlin/Heidelberg, Germany; pp. 423–438.
- Lin, S.J.; Nguyen, M.D.; Chen, C.; Colakoglu, S.; Curtis, M.S.; Tobias, A.M.; Lee, B.T. Tissue Oximetry Monitoring in Microsurgical Breast Reconstruction Decreases Flap Loss and Improves Rate of Flap Salvage. Plast. Reconstr. Surg. 2011, 127, 1080–1085. [Google Scholar] [CrossRef]
- Steele, M.H. Three-Year Experience Using Near Infrared Spectroscopy Tissue Oximetry Monitoring of Free Tissue Transfers. Ann. Plast. Surg. 2011, 66, 540–545. [Google Scholar] [CrossRef]
- Keller, A. A New Diagnostic Algorithm for Early Prediction of Vascular Compromise in 208 Microsurgical Flaps Using Tissue Oxygen Saturation Measurements. Ann. Plast. Surg. 2009, 62, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Keller, A. Noninvasive Tissue Oximetry for Flap Monitoring: An Initial Study. J. Reconstr. Microsurg. 2007, 23, 189–197. [Google Scholar] [CrossRef]
- Lindelauf, A.A.; Saelmans, A.G.; van Kuijk, S.M.; van der Hulst, R.R.; Schols, R.M. Near-Infrared Spectroscopy (NIRS) versus Hyperspectral Imaging (HSI) to Detect Flap Failure in Reconstructive Surgery: A Systematic Review. Life 2022, 12, 65. [Google Scholar] [CrossRef]
- T.Ox. ViOptix. Available online: https://www.vioptix.com/products/t-ox/ (accessed on 17 May 2025).
- The University of Pennsylvania Uterus Transplant for Uterine Factor Infertility Trial (UNTIL). ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03307356?locStr=Philadelphia,%20PA&country=United%20States&state=Pennsylvania&city=Philadelphia&cond=Uterus%20Transplant&rank=1 (accessed on 1 March 2025).
- Johannesson, L.; Richards, E.; Reddy, V.; Walter, J.; Olthoff, K.; Quintini, C.; Tzakis, A.; Latif, N.; Porrett, P.; O’Neill, K.; et al. The First 5 Years of Uterus Transplant in the US: A Report From the United States Uterus Transplant Consortium. JAMA Surg. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Baños, A.; Wolf, M.; Grawe, C.; Stahel, M.; Haensse, D.; Fink, D.; Hornung, R. Frequency domain near-infrared spectroscopy of the uterine cervix during cervical ripening. Lasers Surg. Med. 2007, 39, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.; Spichtig, S.; Baños, A.; Stahel, M.; Zimmermann, R.; Wolf, M. Frequency-domain near-infrared spectroscopy of the uterine cervix during regular pregnancies. Lasers Med. Sci. 2011, 26, 205–212. [Google Scholar] [CrossRef]
- Hornung, R.; Pham, T.H.; Keefe, K.A.; Berns, M.W.; Tadir, Y.; Tromberg, B.J. Quantitative near-infrared spectroscopy of cervical dysplasia in vivo. Hum. Reprod. 1999, 14, 2908–2916. [Google Scholar] [CrossRef]
- Matzinger, B.; Wolf, M.; Baños, A.; Fink, D.; Hornung, R. Optical properties, physiologic parameters and tissue composition of the human uterine cervix as a function of hormonal status. Lasers Med. Sci. 2009, 24, 561–566. [Google Scholar] [CrossRef]
- Clancy, N.T.; Saso, S.; Stoyanov, D.; Sauvage, V.; Corless, D.J.; Boyd, M.; Noakes, D.E.; Thum, M.Y.; Ghaem-Maghami, S.; Smith, J.R.; et al. Multispectral imaging of organ viability during uterine transplantation surgery in rabbits and sheep. J. Biomed. Opt. 2016, 21, 106006. [Google Scholar] [CrossRef]
- Noori, S.; Drabu, B.; McCoy, M.; Sekar, K. Non-invasive measurement of local tissue perfusion and its correlation with hemodynamic indices during the early postnatal period in term neonates. J. Perinatol. 2011, 31, 785–788. [Google Scholar] [CrossRef]
- Kagaya, Y.; Miyamoto, S. A systematic review of near-infrared spectroscopy in flap monitoring: Current basic and clinical evidence and prospects. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 246–257. [Google Scholar] [CrossRef] [PubMed]



| UTx | Patient Identifier | Age at Surgery (y) | BMI (kg/m2) | Race | Donor Allograft Status | Subsequent Pregnancy Outcomes |
| T1 | 32 | 19 | White | 29-year-old deceased donor, three prior vaginal deliveries | 1 live-born infant, cesarean hysterectomy | |
| T2 | 26 | 32 | White | 35-year-old deceased donor, two prior vaginal deliveries | 2 live-born infants: cesarean section, followed by cesarean hysterectomy | |
| T3 | 32 | 21 | White | 41-year-old living donor, one prior vaginal and one cesarean section | 2 live-born infants, cesarean section, followed by cesarean hysterectomy | |
| TAH-BSO | Patient Identifier | Age at Surgery (y) | BMI (kg/m2) | Race | Pathology | __ |
| H1 | 62 | 28 | Black | Uterine carcinosarcoma | ||
| H2 | 62 | 42 | White | Ovarian serous carcinoma | ||
| H3 | 71 | 37 | Black | Ovarian mucinous cystadenoma | ||
| H4 | 56 | 30 | Black | Endometrial endometrioid carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Applebaum, J.; Zhao, D.; Latif, N.; O’Neill, K. Feasibility of Near-Infrared Spectroscopy for Monitoring Tissue Oxygenation During Uterus Transplantation and Hysterectomy. J. Clin. Med. 2025, 14, 4832. https://doi.org/10.3390/jcm14144832
Applebaum J, Zhao D, Latif N, O’Neill K. Feasibility of Near-Infrared Spectroscopy for Monitoring Tissue Oxygenation During Uterus Transplantation and Hysterectomy. Journal of Clinical Medicine. 2025; 14(14):4832. https://doi.org/10.3390/jcm14144832
Chicago/Turabian StyleApplebaum, Jeremy, Dan Zhao, Nawar Latif, and Kathleen O’Neill. 2025. "Feasibility of Near-Infrared Spectroscopy for Monitoring Tissue Oxygenation During Uterus Transplantation and Hysterectomy" Journal of Clinical Medicine 14, no. 14: 4832. https://doi.org/10.3390/jcm14144832
APA StyleApplebaum, J., Zhao, D., Latif, N., & O’Neill, K. (2025). Feasibility of Near-Infrared Spectroscopy for Monitoring Tissue Oxygenation During Uterus Transplantation and Hysterectomy. Journal of Clinical Medicine, 14(14), 4832. https://doi.org/10.3390/jcm14144832






