A Case of Application of Computer-Aided Design and Manufacturing Technology and Extended Reality Surgical Assistance to Marginal Mandibulectomy
Abstract
:1. Introduction
2. Materials and Methods
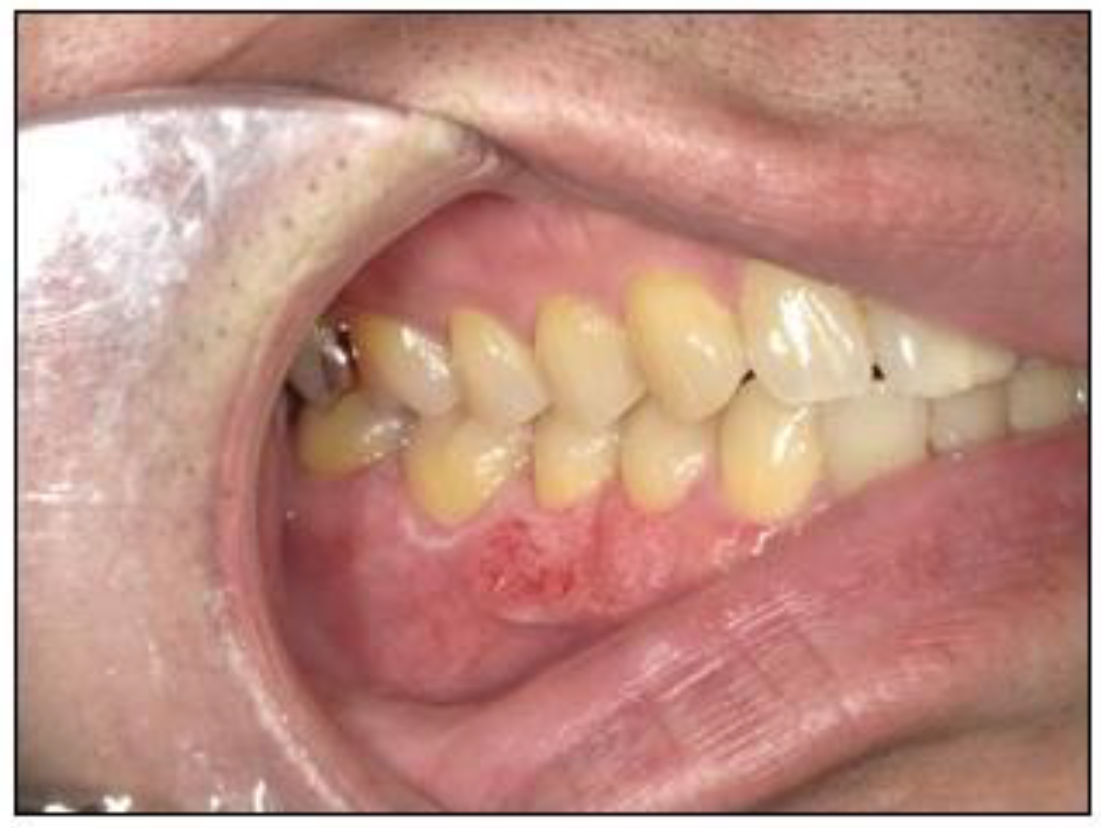
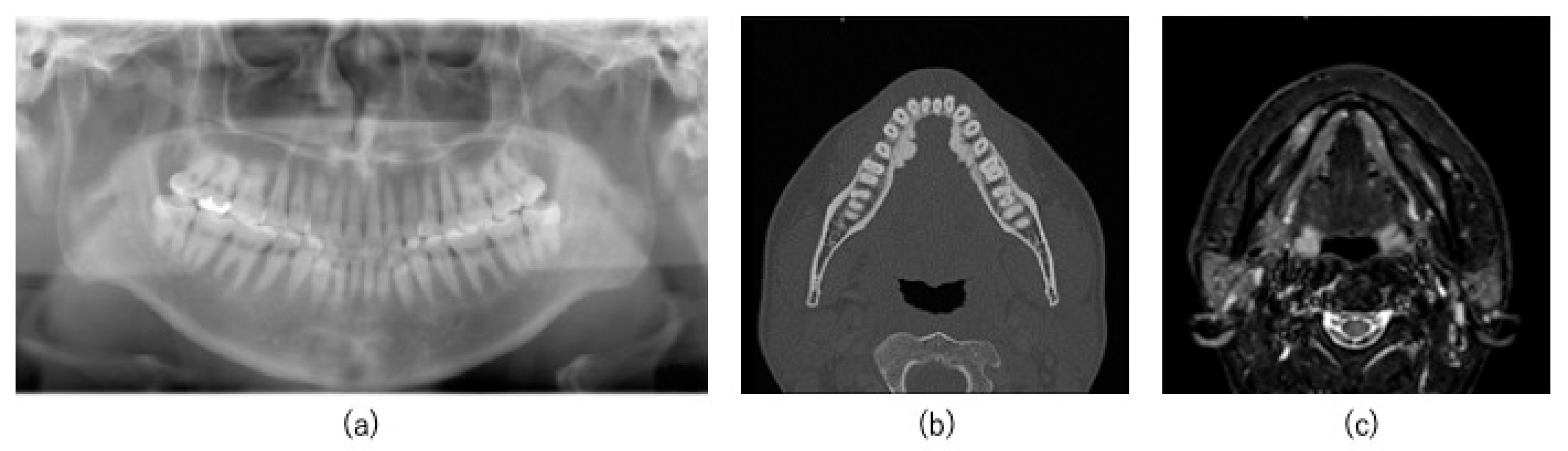
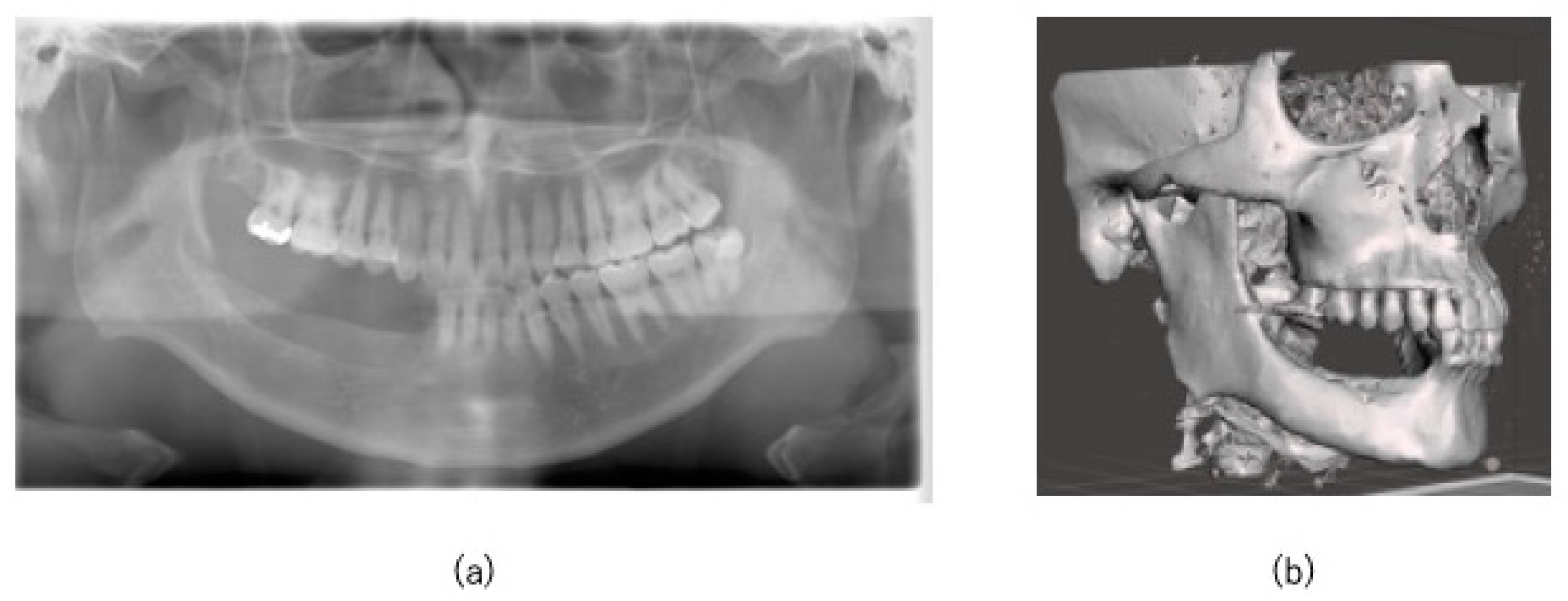
2.1. Case
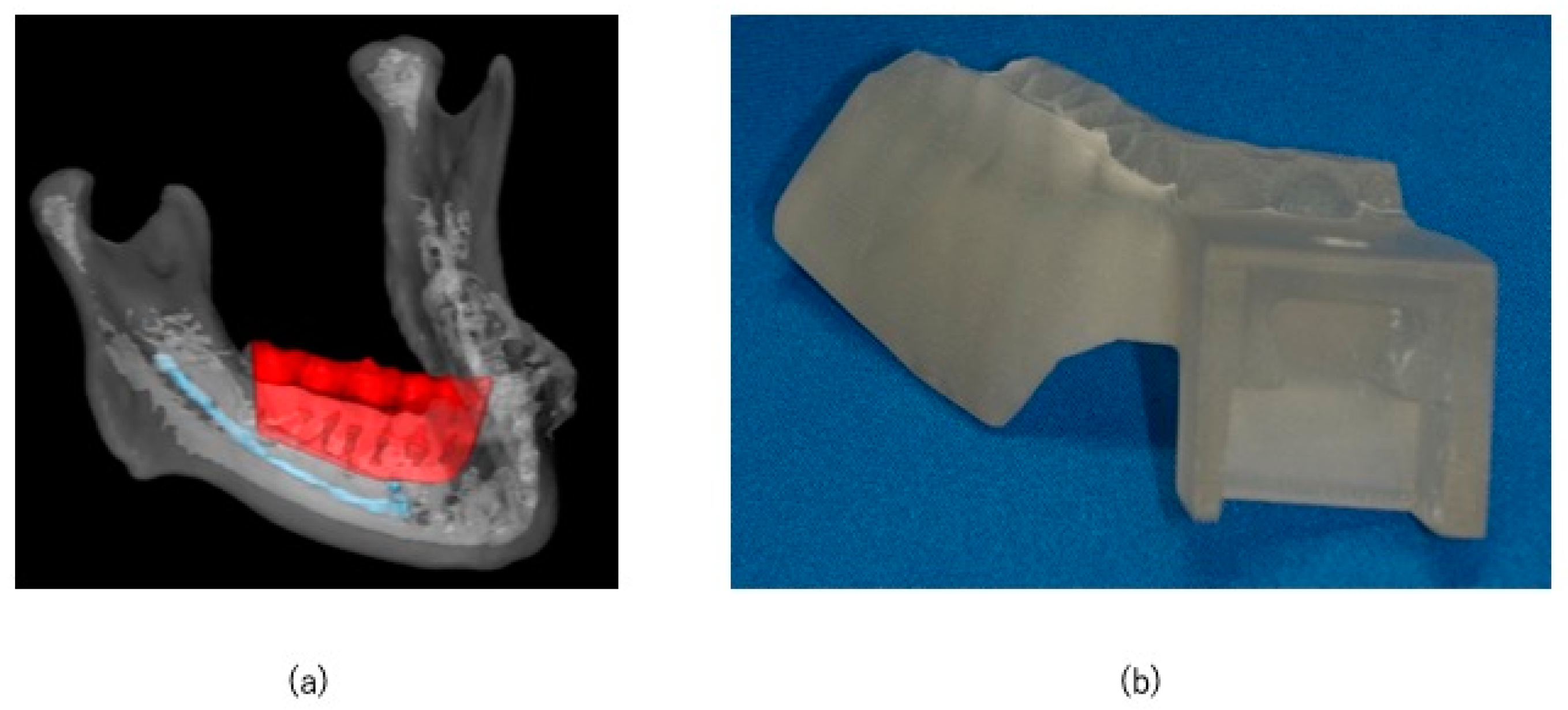
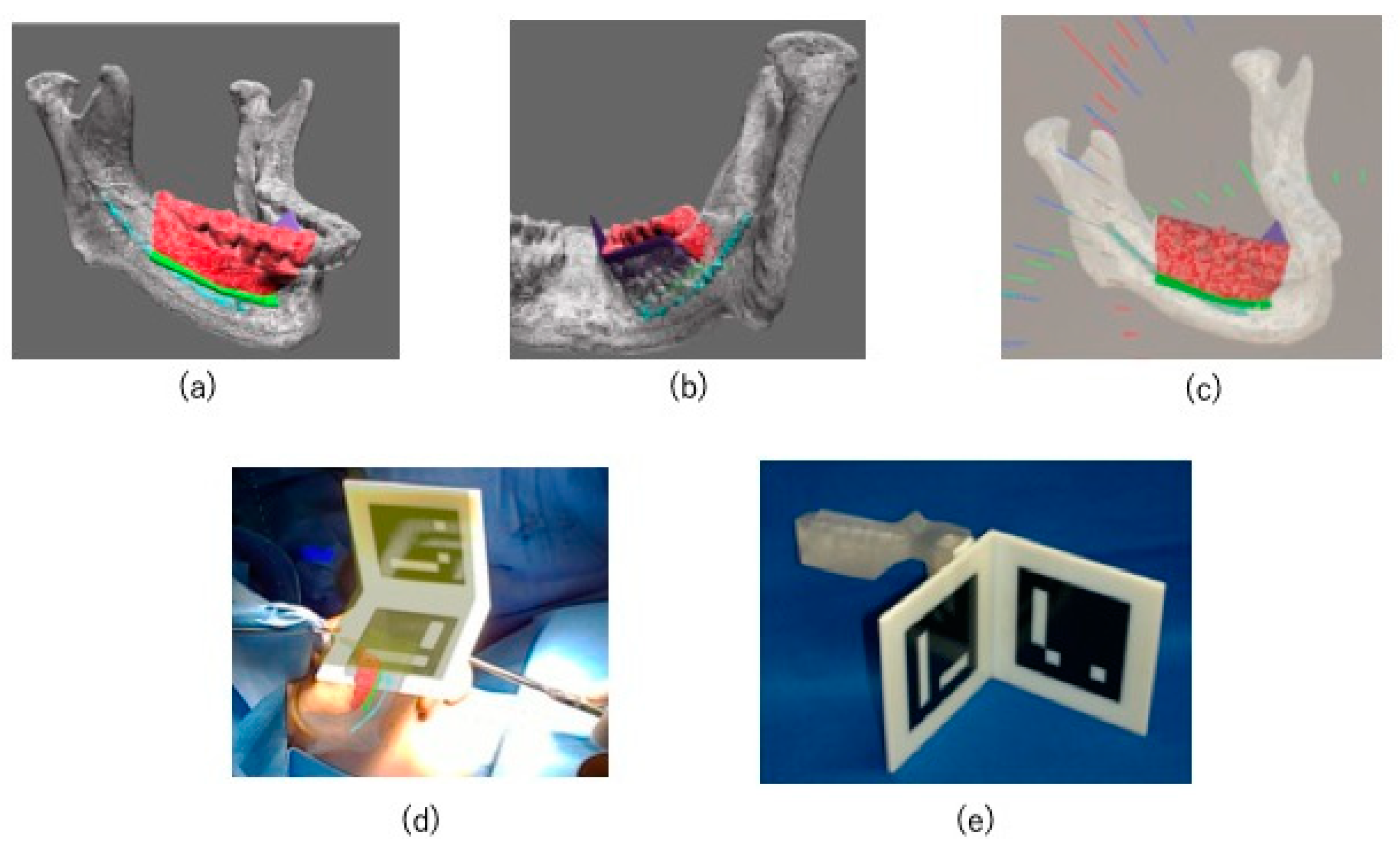
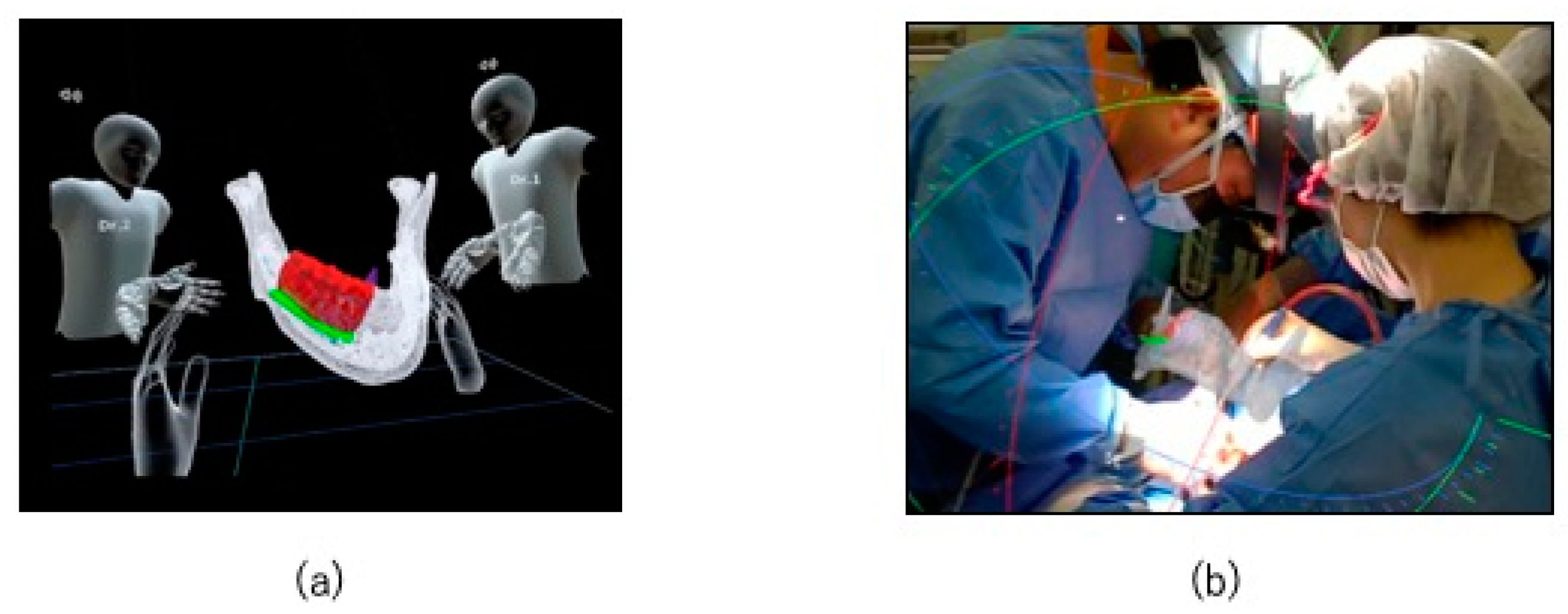
2.2. Preoperative Preparation (CAD/CAM Osteotomy Guide, Virtual Surgery, and Fabrication of XR Application and Registration Marker)
2.3. HoloLens Application
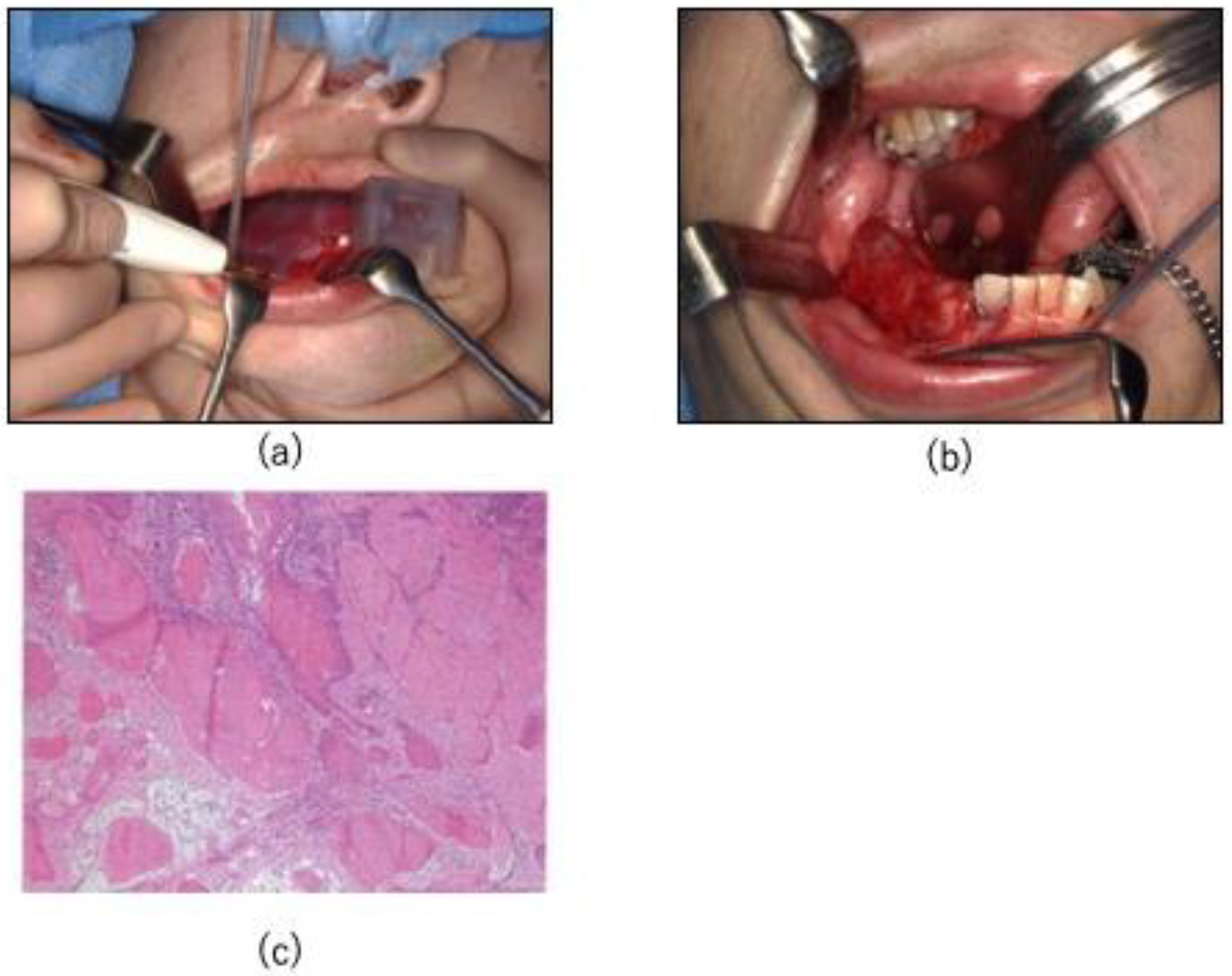
2.4. Surgical Procedure
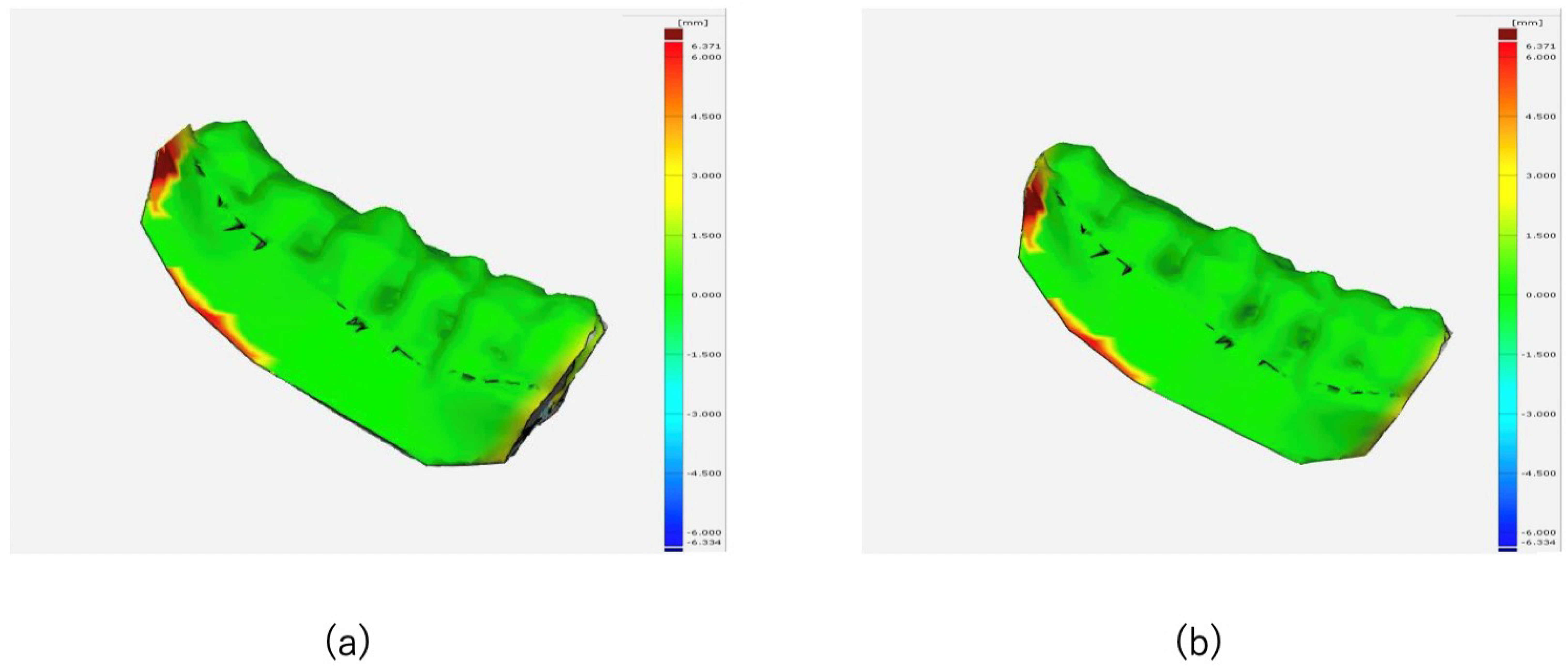
2.5. Evaluation
3. Results
3.1. Surgical Accuracy
3.2. Postoperative Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winkler, D.; Kropla, F.; Busse, M.; Jung, S.; Scholz, S.; Güresir, E.; Gericke, M.; Vychopen, M.; Wach, J.; Grunert, R. Mixed reality for spine surgery: A step into the future with a human cadaveric accuracy study. Neurosurg. Focus 2024, 56, E10. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Koyachi, M.; Koyama, Y.; Sugimoto, M.; Matsunaga, S.; Odaka, K.; Abe, S.; Katakura, A. Mixed reality and three dimensional printed models for resection of maxillary tumor: A case report. Quant. Imaging Med. Surg. 2021, 11, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Koyachi, M.; Sugahara, K.; Odaka, K.; Matsunaga, S.; Abe, S.; Sugimoto, M.; Katakura, A. Accuracy of Le Fort I osteotomy with combined computer-aided design/computer-aided manufacturing technology and mixed reality. Int. J. Oral Maxillofac. Surg. 2021, 50, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Subramanian, T.; Stambuk, H.; Schreyer, M.; Woods, R.; Scholfield, D.; Wong, R.; Cohen, M.A.; Shah, J.; Ganly, I. CAD/CAM-assisted ablative surgery and intraoperative brachytherapy for pediatric skull-base sarcomas. Head Neck 2023, 45, E61–E66. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.H.; Mathari, S.E.; Abjigitova, D.; Maat, A.P.W.M.; Taverne, Y.J.H.J.; Bogers, A.J.J.C.; Mahtab, E.A.F. Current and Future Applications of Virtual, Augmented, and Mixed Reality in Cardiothoracic Surgery. Ann. Thorac. Surg. 2022, 113, 681–691. [Google Scholar] [CrossRef]
- Koyachi, M.; Sugahara, K.; Tachizawa, K.; Nishiyama, A.; Odaka, K.; Matsunaga, S.; Sugimoto, M.; Tachiki, C.; Nishii, Y.; Katakura, A. Enhanced Precision in Genioplasty: A Novel Intraoperative Spatial Repositioning Using Computer-Aided Design and Manufacturing Technology and a Holographic Mixed Reality Application. J. Clin. Med. 2023, 12, 7408. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, F.; Chai, G.; Pan, J.J.; Jiang, T.; Lin, L.; Xin, Y.; Zhang, Y.; Li, Q. A novel augmented reality system for displaying inferior alveolar nerve bundles in maxillofacial surgery. Sci. Rep. 2017, 7, 42365. [Google Scholar] [CrossRef]
- Atallah, S.; Martin-Perez, B.; Larach, S. Image-guided real-time navigation for transanal total mesorectal excision: A pilot study. Tech. Coloproctol. 2015, 19, 679–684. [Google Scholar] [CrossRef]
- Mischkowski, R.A.; Zinser, M.J.; Kübler, A.C.; Krug, B.; Seifert, U.; Zöller, J.E. Application of an augmented reality tool for maxillary positioning in orthognathic surgery—A feasibility study. J. Craniomaxillofac. Surg. 2006, 34, 478–483. [Google Scholar] [CrossRef]
- El-Mahallawy, Y.; Dessoky, N.Y.; Abdelrahman, H.H.; Al-Mahalawy, H. Evaluation of the resection plane three-dimensional positional accuracy using a resection guide directional guidance slot; a randomized clinical trial. BMC Oral Health 2024, 24, 736. [Google Scholar] [CrossRef]
- De BrouwerKoning, S.G.; Ter Braak, T.P.; Geldof, F.; van Veen, R.L.P.; van Alphen, M.J.A.; Karssemakers, L.H.E.; Schreuder, W.H.; Karakullukcu, M.B. Evaluating the accuracy of resection planes in mandibular surgery using a preoperative, intraoperative, and postoperative approach. Int. J. Oral Maxillofac. Surg. 2021, 50, 287–293. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Su, Y.X.; Pow, E.H.N.; Yang, W.F.; Qin, L.; Choi, W.S. “Three-in-one” patient-specific surgical guides for simultaneous dental implants in fibula flap jaw reconstruction: A prospective case series. Clin. Implant. Dent. Relat. Res. 2021, 23, 43–53. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Z.; Pan, W.; Li, H.; Zhao, T.; Meng, Y.; Ma, G.; Ye, H.; Shang, J. Wearing individualized 3D printed oral stent to protect normal tissues in patients with nasopharyngeal carcinoma during radiotherapy. J. Appl. Clin. Med. Phys. 2023, 24, e14145. [Google Scholar] [CrossRef] [PubMed]
- Rios-Vicil, C.I.; Barbery, D.; Dang, P.; Jean, W.C. Single-stage cranioplasty with customized polyetheretherketone implant after tumor resection using virtual reality and augmented reality for precise implant customization and placement: Illustrative case. J. Neurosurg. Case Lessons 2022, 3, CASE2255. [Google Scholar] [CrossRef] [PubMed]
- Lethaus, B.; Safi, Y.; ter Laak-Poort, M.; Kloss-Brandstätter, A.; Banki, F.; Robbenmenke, C.; Steinseifer, U.; Kessler, P. Cranioplasty with customized titanium and PEEK implants in a mechanical stress model. J. Neurotrauma 2012, 29, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- De Barros, A.; Brauge, D.; QuÉhan, R.; Cavallier, Z.; Roux, F.E.; Moyse, E. One-step customized peek cranioplasty after 3D printed resection template assisted surgery for a frontal intraosseous meningioma: A case report. Turk. Neurosurg. 2020, 31, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Hu, L.; Yu, Y.; Zhang, W.; Peng, X. Mixed Reality Combined with Surgical Navigation in Resection of Micro- and Mini-Tumors of the Parotid Gland: A Pilot Study. Laryngoscope 2024, 134, 1670–1678. [Google Scholar] [CrossRef]
- Kashikar, T.S.; Kerwin, T.F.; Moberly, A.C.; Wiet, G.J. A review of simulation applications in temporal bone surgery. Laryngoscope Investig. Otolaryngol. 2019, 4, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Incekara, F.; Smits, M.; Dirven, C.; Vincent, A. Clinical Feasibility of a Wearable Mixed-Reality Device in Neurosurgery. World Neurosurg. 2018, 118, e422–e427. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Sugimoto, M.; Tanaka, Y.; Suetsugu, T.; Imai, T.; Hatanaka, Y.; Matsuhashi, N.; Takahashi, T.; Yamaguchi, K.; Yoshida, K. Holographic image-guided thoracoscopic surgery: Possibility of usefulness for esophageal cancer patients with abnormal artery. Esophagus 2020, 17, 508–511. [Google Scholar] [CrossRef]
- Tu, P.; Qin, C.; Guo, Y.; Li, D.; Lungu, A.J.; Wang, H.; Chen, X. Ultrasound Image Guided and Mixed Reality-Based Surgical System With Real-Time Soft Tissue Deformation Computing for Robotic Cervical Pedicle Screw Placement. IEEE Trans. Biomed. Eng. 2022, 69, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Olexa, J.; Trang, A.; Cohen, J.; Kim, K.; Rakovec, M.; Saadon, J.; Sansur, C.; Woodworth, G.; Schwartzbauer, G.; Cherian, J. The Apple Vision Pro as a Neurosurgical Planning Tool: A Case Report. Cureus 2024, 16, e54205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakada, T.; Koyachi, M.; Sugahara, K.; Nishiyama, A.; Kawakami, M.; Nakajima, S.; Tachizawa, K.; Odaka, K.; Matsunaga, S.; Sugimoto, M.; et al. A Case of Application of Computer-Aided Design and Manufacturing Technology and Extended Reality Surgical Assistance to Marginal Mandibulectomy. J. Clin. Med. 2025, 14, 8. https://doi.org/10.3390/jcm14010008
Nakada T, Koyachi M, Sugahara K, Nishiyama A, Kawakami M, Nakajima S, Tachizawa K, Odaka K, Matsunaga S, Sugimoto M, et al. A Case of Application of Computer-Aided Design and Manufacturing Technology and Extended Reality Surgical Assistance to Marginal Mandibulectomy. Journal of Clinical Medicine. 2025; 14(1):8. https://doi.org/10.3390/jcm14010008
Chicago/Turabian StyleNakada, Takahiro, Masahide Koyachi, Keisuke Sugahara, Akihiro Nishiyama, Mana Kawakami, Shintaro Nakajima, Kotaro Tachizawa, Kento Odaka, Satoru Matsunaga, Maki Sugimoto, and et al. 2025. "A Case of Application of Computer-Aided Design and Manufacturing Technology and Extended Reality Surgical Assistance to Marginal Mandibulectomy" Journal of Clinical Medicine 14, no. 1: 8. https://doi.org/10.3390/jcm14010008
APA StyleNakada, T., Koyachi, M., Sugahara, K., Nishiyama, A., Kawakami, M., Nakajima, S., Tachizawa, K., Odaka, K., Matsunaga, S., Sugimoto, M., & Katakura, A. (2025). A Case of Application of Computer-Aided Design and Manufacturing Technology and Extended Reality Surgical Assistance to Marginal Mandibulectomy. Journal of Clinical Medicine, 14(1), 8. https://doi.org/10.3390/jcm14010008







