Neonatal Feeding Practices and SARS-CoV-2 Transmission in Neonates with Perinatal SARS-CoV-2 Exposure: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search STRATEGY
2.3. Data Extraction
2.4. Outcomes
2.5. Risk of Bias Assessment Approach
2.6. Synthesis Methods
2.7. GRADE Assessment
2.8. Statistical Analysis
3. Results
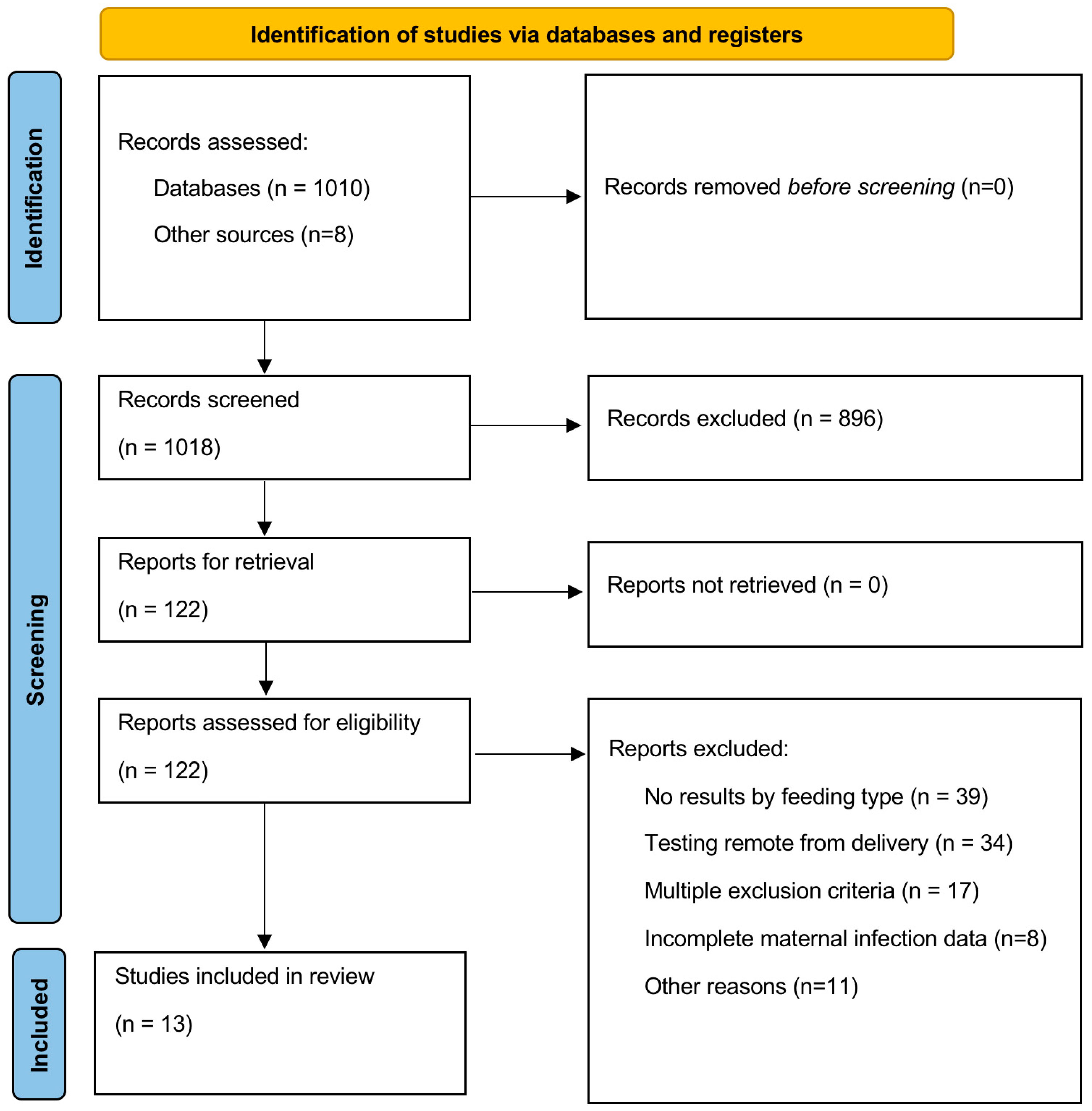
3.1. Selection of Studies
3.2. Study/Participant Characteristics
3.3. Dyad Handling in the Hospital
- Full Isolation: Two studies reported the use of full isolation protocols, with mothers being completely isolated from their newborns.
- Some Precautions: Seven studies (four of these included in the meta-analysis) adopted varying levels of precautionary measures. These likely included the use of masks, different hygiene measures, and placement of the neonate in a bassinet or isolette 6 feet away from the mother.
- Variable Practices: Two studies described variable precaution/isolation practices, indicating multiple different infection control measures.
- Not Available or Unclear: Two studies either did not specify the type of precautionary measures or isolation practices used or did not report whether the recommended precautions were effectively implemented. Only one of those two was included in the meta-analysis.
3.4. Timing of PCR Testing for SARS-CoV-2 in the Pregnant Person
3.5. Risk of Bias Assessment
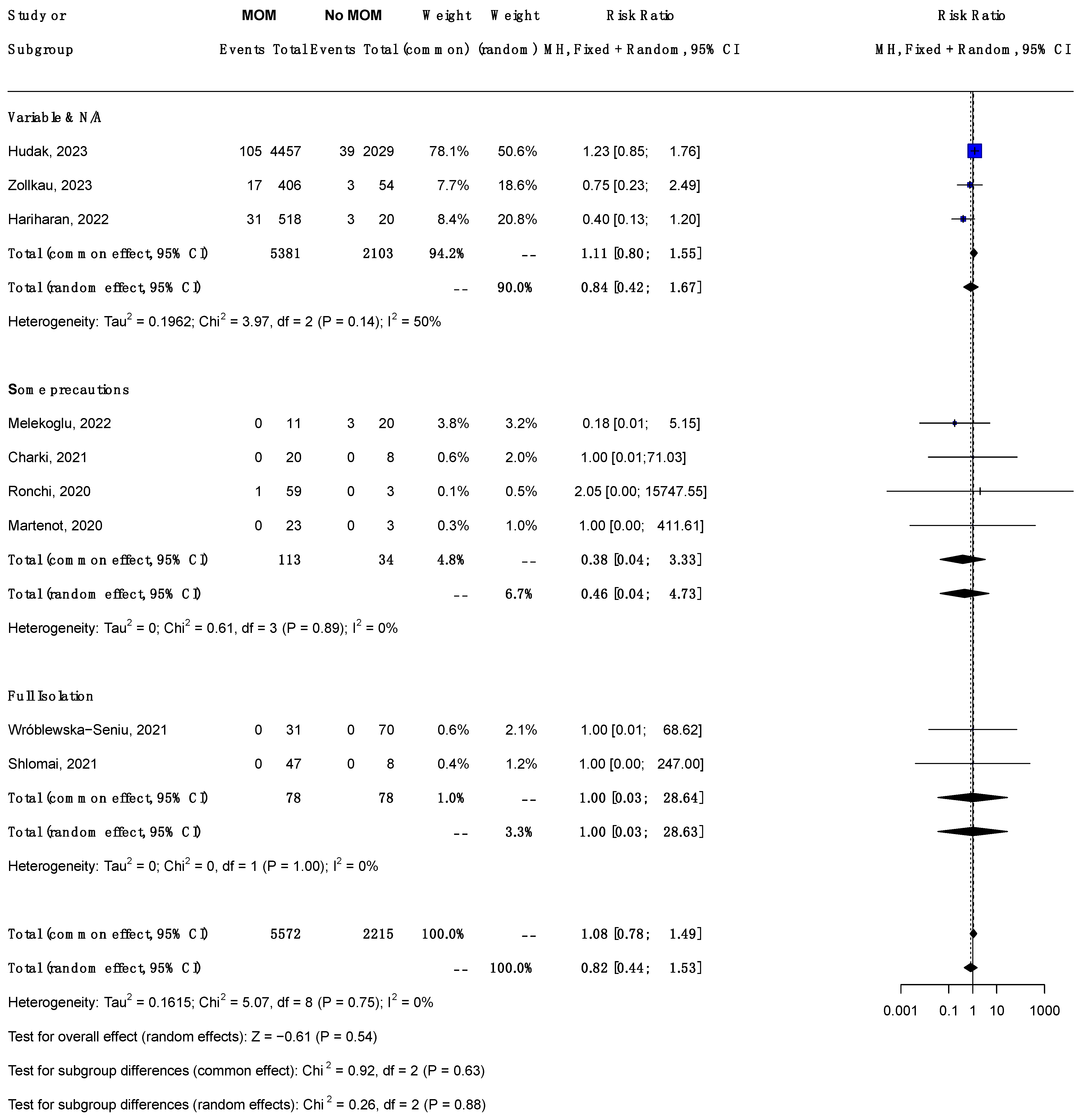
3.6. Association of MoM with SARS-CoV-2 Infection Risk
3.6.1. Title Overall Analysis
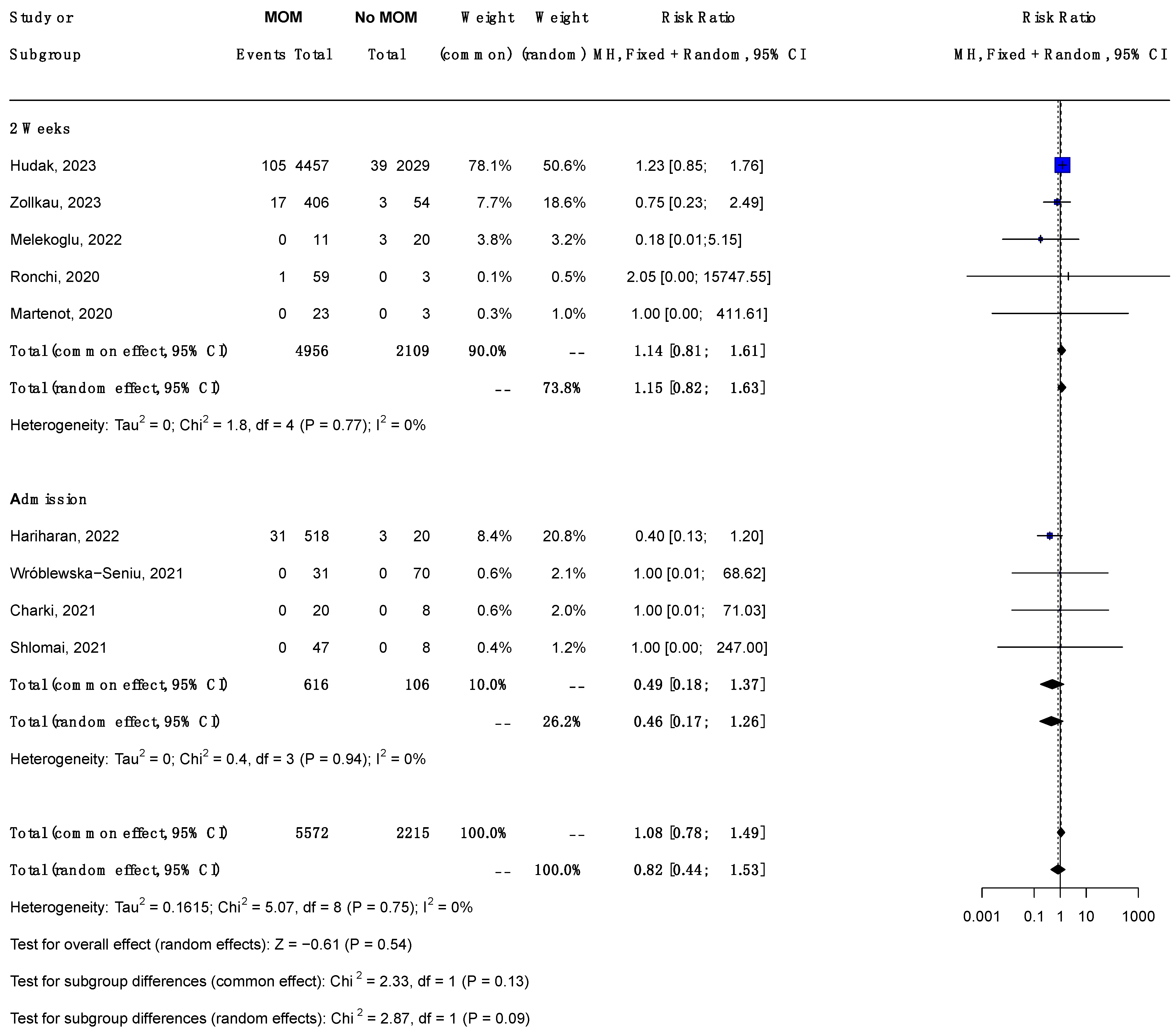
3.6.2. Subgroup Analysis by Timing of Maternal PCR (Figure 2)
3.6.3. Subgroup Analysis by Dyad Handling (Figure 3)
3.7. Sensitivity Analysis
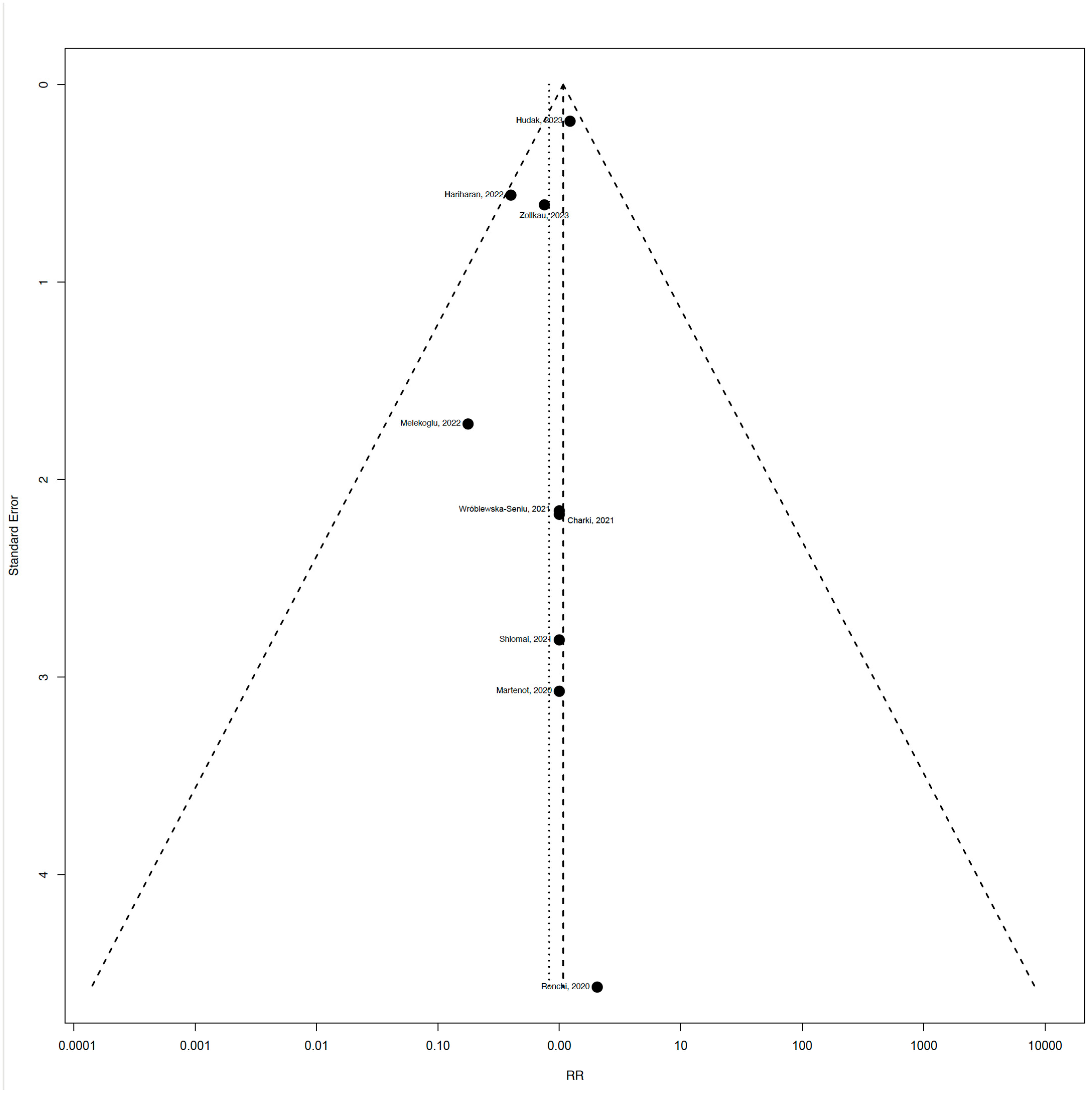
3.8. Publication Bias
3.9. GRADE Rating
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- WHO. Timeline: WHO’s COVID-19 Response 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline#! (accessed on 16 November 2022).
- WHO Coronavirus Disease (COVID-19) Burden n.d. Available online: https://www.who.int/news-room/fact-sheets/detail/coronavirus-disease-(COVID-19) (accessed on 16 October 2024).
- Dumpa, V.; Kamity, R.; Vinci, A.N.; Noyola, E.; Noor, A. Neonatal Coronavirus 2019 (COVID-19) Infection: A Case Report and Review of Literature. Cureus 2020, 12, e8165. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, J.; Du, L. Neonatal Management During the Coronavirus Disease (COVID-19) Outbreak: The Chinese Experience. NeoReviews 2020, 21, e293–e297. [Google Scholar] [CrossRef]
- White, A.; Mukherjee, P.; Stremming, J.; Sherlock, L.G.; Reynolds, R.M.; Smith, D.; Asturias, E.J.; Grover, T.R.; Dietz, R.M. Neonates Hospitalized with Community-Acquired SARS-CoV-2 in a Colorado Neonatal Intensive Care Unit. Neonatology 2020, 117, 641–645. [Google Scholar] [CrossRef]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef]
- Coronado Munoz, A.; Nawaratne, U.; McMann, D.; Ellsworth, M.; Meliones, J.; Boukas, K. Late-Onset Neonatal Sepsis in a Patient with COVID-19. N. Engl. J. Med. 2020, 382, e49. [Google Scholar] [CrossRef]
- Gupta, M.; Zupancic, J.A.; Pursley, D. Caring for Newborns Born to Mothers with COVID-19: More Questions than Answers. Pediatrics 2020, 146, e2020001842. [Google Scholar] [CrossRef]
- Stuebe, A. The Risks of Not Breastfeeding for Mothers and Infants. Rev. Obstet. Gynecol. 2009, 2, 222–231. [Google Scholar]
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I.; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2020, 115, 496–506. [Google Scholar] [CrossRef]
- Fucile, S.; Heath, J.; Dow, K. Impact of the COVID-19 Pandemic on Breastfeeding Establishment in Preterm Infants: An Exploratory Study. Neonatal Netw. 2023, 42, 7–12. [Google Scholar] [CrossRef]
- Ruiz, M.T.; de Oliveira, K.F.; Azevedo, N.F.; Paschoini, M.C.; Rodrigues, W.F.; de Oliveira, C.J.F.; de Oliveira, J.F.; Fonseca, L.M.M.; Wernet, M. Breastfeeding prevalence in newborns of mothers with COVID-19: A systematic review. Rev. Bras. Enferm. 2023, 76 (Suppl. S1), e20220173. [Google Scholar] [CrossRef] [PubMed]
- Preszler, J.; Schriever, M.; Terveen, M. Effects of the COVID-19 Pandemic on Breastfeeding Rates in a Single Tertiary Health Center. S. D. Med. 2022, 75, 263–267. [Google Scholar]
- Marín Gabriel, M.Á.; Martín Lozoya, S.; de Las Heras Ibarra, S.; Domingo Comeche, L.; González Carrasco, E.; Lalaguna Mallada, P.; Sirerol, N.V.; Fernández, L.G.; Martínez, J.J.; Vicente, A.R. Association of the presence of a COVID-19 infection at the time of birth and the rates of exclusive breastfeeding upon discharge in BFHI hospitals: A multicenter, prospective cohort study. Int. Breastfeed. J. 2023, 18, 54. [Google Scholar] [CrossRef]
- Shah, P.S.; Joynt, C.; Håkansson, S.; Narvey, M.; Navér, L.; Söderling, J.; Yang, J.; Beltempo, M.; Stephansson, O.; Fell, D.B.; et al. Infants Born to Mothers Who Were SARS-CoV-2 Positive during Pregnancy and Admitted to Neonatal Intensive Care Unit. Neonatology 2022, 119, 619–628. [Google Scholar] [CrossRef]
- Li, R.; Ware, J.; Chen, A.; Nelson, J.M.; Kmet, J.M.; Parks, S.E.; Morrow, A.L.; Chen, J.; Perrine, C.G. Breastfeeding and post-perinatal infant deaths in the United States, A national prospective cohort analysis. Lancet Reg. Health—Am. 2022, 5, 100094. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.L.R. Safety of breast/chest-feeding by those infected by SARS-CoV-2. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 129. [Google Scholar] [CrossRef]
- Golan, Y.; Prahl, M.; Cassidy, A.G.; Gay, C.; Wu, A.H.B.; Jigmeddagva, U.; Lin, C.Y.; Gonzalez, V.J.; Basilio, E.; Chidboy, M.A.; et al. COVID-19 mRNA Vaccination in Lactation: Assessment of Adverse Events and Vaccine Related Antibodies in Mother-Infant Dyads. Front. Immunol. 2021, 12, 777103. [Google Scholar] [CrossRef]
- Szczygioł, P.; Łukianowski, B.; Kościelska-Kasprzak, K.; Jakuszko, K.; Bartoszek, D.; Krajewska, M.; Królak-Olejnik, B. Antibodies in the breastmilk of COVID-19 recovered women. BMC Pregnancy Childbirth 2022, 22, 635. [Google Scholar] [CrossRef] [PubMed]
- FAQs: Management of Infants Born to Mothers with Suspected or Confirmed COVID-19 n.d. Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/faqs-management-of-infants-born-to-covid-19-mothers/ (accessed on 11 February 2024).
- Liu, X.; Chen, H.; An, M.; Yang, W.; Wen, Y.; Cai, Z.; Wang, L.; Zhou, Q. Recommendations for breastfeeding during Coronavirus Disease 2019 (COVID-19) pandemic. Int. Breastfeed. J. 2022, 17, 28. [Google Scholar] [CrossRef]
- Chan, C.; Kong, J.Y.; Sultana, R.; Mundra, V.; Babata, K.; Mazzarella, K.; Adhikari, E.H.; Yeo, K.T.; Hascoët, J.-M.; Brion, L.P. Optimal Delivery Management for the Prevention of Early Neonatal SARS-CoV-2 Infection: Systematic review and Meta-analysis. Am. J. Perinatol. 2024, 41, 1625–1633. [Google Scholar] [CrossRef]
- Edwards, E.M.; Ehret, D.E.Y.; Soll, R.F.; Horbar, J.D. Vermont Oxford Network: A Worldwide Learning Community. Transl. Pediatr. 2019, 8, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Hudak, M.L.; Flannery, D.D.; Barnette, K.; Getzlaff, T.; Gautam, S.; Dhudasia, M.B.; Mukhopadhyay, S.; Pfeifer, M.R.; Ellington, S.R.; Galang, R.R.; et al. Maternal and Newborn Hospital Outcomes of Perinatal SARS-CoV-2 Infection: A National Registry. Pediatrics 2023, 151, e2022059595. [Google Scholar] [CrossRef]
- Zöllkau, J.; Heimann, Y.; Hagenbeck, C.; Pecks, U.; Abou-Dakn, M.; Schlösser, R.; Schohe, A.; Dressler-Steinbach, I.; Manz, M.; Banz-Jansen, C.; et al. Breastfeeding Behavior Within the COVID-19 Related Obstetric and Neonatal Outcome Study (CRONOS). J. Hum. Lact. 2023, 39, 625–635. [Google Scholar] [CrossRef]
- Singh, N.; Jaiswal, J.; Sherwani, N.; Nagaria, T.; Khandwal, O.; Neral, A. Maternal and Neonatal Outcomes Associated With COVID-19 Infection in Pregnant Mothers Admitted in Tertiary Care Hospital in Central State of India. Cureus 2023, 15, e38235. [Google Scholar] [CrossRef] [PubMed]
- Melekoglu, N.A.; Ozdemir, H.; Yasar, S. Neonatal Outcomes of Pregnant Women With Confirmed Coronavirus Disease 2019: One-Year Experience of a Tertiary Care Center. Clin. Pediatr. 2022, 61, 177–183. [Google Scholar] [CrossRef]
- Hariharan, S.; Manikumar, S.; Muthukumaran, N.; Ramya, S. Outcome of Neonates Born to COVID-19 Positive Mothers at a Tertiary Centre: A Retrospective Cohort Study. J. Neonatol. 2022, 36, 177–183. [Google Scholar] [CrossRef]
- Wróblewska-Seniuk, K.; Basiukajć, A.; Wojciechowska, D.; Telge, M.; Miechowicz, I.; Mazela, J. Clinical Characteristics of Newborns Born to Mothers with COVID-19. J. Clin. Med. 2021, 10, 4383. [Google Scholar] [CrossRef]
- Ferreira, M.; Garcia, C.; Barroso, R. Characteristics of Newborns from Mothers with SARS-CoV-2 Infection in a Portuguese Hospital. Acta Med. Port. 2021, 34, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Charki, S.; Patil, S.V. Experience of COVID-19 infections in neonates in tertiary care centre in north karnataka, India: A prospective cohort study. Curr. Pediatr. Res. 2021, 25, 421–426. [Google Scholar]
- Shlomai, N.O.; Kasirer, Y.; Strauss, T.; Smolkin, T.; Marom, R.; Shinwell, E.S.; Simmonds, A.; Golan, A.; Morag, I.; Waisman, D.; et al. Neonatal SARS-CoV-2 Infections in Breastfeeding Mothers. Pediatrics 2021, 147, e2020010918. [Google Scholar] [CrossRef]
- Ronchi, A.; Pietrasanta, C.; Zavattoni, M.; Saruggia, M.; Schena, F.; Sinelli, M.T.; Agosti, M.; Tzialla, C.; Varsalone, F.F.; Testa, L.; et al. Evaluation of Rooming-in Practice for Neonates Born to Mothers With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Italy. JAMA Pediatr. 2021, 175, 260. [Google Scholar] [CrossRef] [PubMed]
- Martenot, A.; Labbassi, I.; Delfils-Stern, A.; Monroy, O.; Langlet, C.; Pichault-Klein, V.; Delagreverie, H.; De Marcillac, F.; Fafi-Kremer, S.; Deruelle, P.; et al. Favorable outcomes among neonates not separated from their symptomatic SARS-CoV-2-infected mothers. Pediatr. Res. 2021, 90, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Kalamdani, P.; Kalathingal, T.; Manerkar, S.; Mondkar, J. Clinical Profile of SARS-CoV-2 Infected Neonates From a Tertiary Government Hospital in Mumbai, India. Indian Pediatr. 2020, 57, 1143–1146. [Google Scholar] [CrossRef]
- Khan, M.A.; Kumar, V.; Ali, S.R. Vertical Transmission of Novel Coronavirus (COVID-19) from Mother to Newborn: Experience from a Maternity Unit, The Indus Hospital, Karachi. J. Coll. Physicians Surg. Pak. 2020, 30, 136. [Google Scholar] [CrossRef]
- Tool to Assess Risk of Bias in Cohort Studies DistillerSR. DistillerSR n.d. Available online: https://www.distillersr.com/resources/methodological-resources/tool-to-assess-risk-of-bias-in-cohort-studies-distillersr (accessed on 14 November 2024).
- Angelidou, A.; Sullivan, K.; Melvin, P.R.; Shui, J.E.; Goldfarb, I.T.; Bartolome, R.; Chaudhary, N.; Vaidya, R.; Culic, I.; Singh, R.; et al. Association of Maternal Perinatal SARS-CoV-2 Infection with Neonatal Outcomes During the COVID-19 Pandemic in Massachusetts. JAMA Netw. Open. 2021, 4, e217523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dumitriu, D.; Emeruwa, U.N.; Hanft, E.; Liao, G.V.; Ludwig, E.; Walzer, L.; Arditi, B.; Saslaw, M.; Andrikopoulou, M.; Scripps, T.; et al. Outcomes of Neonates Born to Mothers With Severe Acute Respiratory Syndrome Coronavirus 2 Infection at a Large Medical Center in New York City. JAMA Pediatr. 2021, 175, 157–167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kunjumon, B.; Wachtel, E.V.; Lumba, R.; Quan, M.; Remon, J.; Louie, M.; Verma, S.; Moffat, M.A.; Kouba, I.; Bennett, T.A.; et al. Breast Milk and Breastfeeding of Infants Born to SARS-CoV-2 Positive Mothers: A Prospective Observational Cohort Study. Am. J. Perinatol. 2021, 38, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, C.M.; Han, J.Y.; Acker, K.P.; Tiwari, P.; Jin, J.; Brandler, M.; Cangemi, C.; Gordon, L.; Parow, A.; DiPace, J.; et al. Neonatal management and outcomes during the COVID-19 pandemic: An observation cohort study. Lancet Child Adolesc. Health 2020, 4, 721–727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raschetti, R.; Vivanti, A.J.; Vauloup-Fellous, C.; Loi, B.; Benachi, A.; De Luca, D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat. Commun. 2020, 11, 5164. [Google Scholar] [CrossRef] [PubMed]
- Morniroli, D.; Vizzari, G.; Tosi, M.; Treglia, G.; Corsello, A.; Marchisio, P.; Mosca, F.; Agostoni, C.; Giannì, M.L.; Milani, G.P.; et al. Mother-to-child transmission of SARS-CoV-2 infection in high-income countries: A systematic review and meta-analysis of prospective observational studies. Sci. Rep. 2023, 13, 8813. [Google Scholar] [CrossRef] [PubMed]
- Boukoura, M.E.; Dagla, M.; Gourounti, K.; Nieri, A.S.; Taskou, C.; Tsoukala, E.; Sarantaki, A. Breastfeeding Practices for COVID-19-Infected Mothers: A Systematic Review and Meta-Analysis. Nurs. Rep. 2024, 14, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Centeno-Tablante, E.; Medina-Rivera, M.; Finkelstein, J.L.; Rayco-Solon, P.; Garcia-Casal, M.N.; Rogers, L.; Ghezzi-Kopel, K.; Ridwan, P.; Peña-Rosas, J.P.; Mehta, S. Transmission of SARS-CoV-2 through breast milk and breastfeeding: A living systematic review. Ann. N. Y. Acad. Sci. 2021, 1484, 32–54. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Hu, R.; Tian, L.; Lou, F.; Chen, Y.; Wang, S.; He, S.; Zhu, S.; An, X.; Song, L.; et al. Overview of Breastfeeding Under COVID-19 Pandemic. Front. Immunol. 2022, 13, 896068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krogstad, P.; Contreras, D.; Ng, H.; Tobin, N.; Chambers, C.D.; Bertrand, K.; Bode, L.; Aldrovandi, G.M. No infectious SARS-CoV-2 in breast milk from a cohort of 110 lactating women|Pediatric Research. Pediatr. Res. 2022, 92, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Briana, D.D.; Malamitsi-Puchner, A. Breastfeeding provides a protective hug and the benefits have outweighed the risks during the COVID-19 pandemic. Acta Paediatr. 2023, 112, 1177–1181. [Google Scholar] [CrossRef]
- Whited, N.; Cervantes, J. Antibodies Against SARS-CoV-2 in Human Breast Milk After Vaccination: A Systematic Review and Meta-Analysis. Breastfeed. Med. 2022, 17, 475–483. [Google Scholar] [CrossRef]
- Young, B.E.; Seppo, A.E.; Diaz, N.; Rosen-Carole, C.; Nowak-Wegrzyn, A.; Cruz Vasquez, J.M.; Ferri-Huerta, R.; Nguyen-Contant, P.; Fitzgerald, T.; Sangster, M.Y.; et al. Association of Human Milk Antibody Induction, Persistence, and Neutralizing Capacity With SARS-CoV-2 Infection vs mRNA Vaccination. JAMA Pediatr. 2022, 176, 159–168. [Google Scholar] [CrossRef]
- Perez, S.E.; Luna Centeno, L.D.; Cheng, W.A.; Marentes Ruiz, C.J.; Lee, Y.; Congrave-Wilson, Z.; Powell, R.L.; Stellwagen, L.; Pannaraj, P.S. Human Milk SARS-CoV-2 Antibodies up to 6 Months After Vaccination. Pediatrics 2022, 149, e2021054260. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, H.; Yankey, D.; Vashist, K.; Lu, P.; Kriss, J.L.; Nguyen, K.H.; Lee, J.; Ellington, S.; Polen, K.; Bonner, K.; et al. COVID-19 vaccination coverage and intent among women aged 18–49 years by pregnancy status, United States, April–November 2021. Vaccine 2022, 40, 4554–4563. [Google Scholar] [CrossRef]
- Razzaghi, H.; Meghani, M.; Pingali, C.; Crane, B.; Naleway, A.; Weintraub, E.; Kenigsberg, T.A.; Lamias, M.J.; Irving, S.A.; Kauffman, T.L.; et al. COVID-19 Vaccination Coverage Among Pregnant Women During Pregnancy—Eight Integrated Health Care Organizations, United States, 14 December 2020–8 May 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 895–899. [Google Scholar] [CrossRef] [PubMed]
| Author, Study Year, Country | Subjects | Design | Dyad Handling | Maternal SARS-CoV-2 Test Timing | Neonatal Mortality (N) | Mothers (N) | Neonates (N) | SARS-CoV-2-Positive Neonates (MoM) n/N (%) | SARS-CoV-2-Positive Neonates (No MoM) n/N (%) |
|---|---|---|---|---|---|---|---|---|---|
| Hudak [25] (2023, US) | Term and Preterm | National cohort study | Variable | 14 days | 29 * | 7524 | 6236 | 105/4457 (2.4) | 39/2029 (1.9) |
| Zollkau [26] (2023, Austria & Germany) | Term and Preterm | Prospective cohort study | Variable | 14 days | 7 * | 842 | 460 | 17/406 (4.2) | 3/54 (5.6) |
| & Singh [27] (2023, India) | Term and Preterm | Cohort study | Some precautions | Admission | None reported | 396 | 394 | 0/394 (0) | - |
| Melekoglu [28] (2022, Turkey) | Term and Preterm | Retrospective cohort study | Some precautions | 14 days | None reported | 30 | 31 | 0/11 (0) | 3/20 (15) |
| Hariharan [29] (2022, India) | Term | Retrospective cohort study | NA | Admission | 7 ~ | 556 | 531 | 31/518 (6.0) | 3/20 (15) |
| Wróblewska-Seniu [30] (2021, Poland) | Term and Preterm | Retrospective cohort study | Isolation | Admission | None reported ^ | 101 | 101 | 0/31 (0) | 0/70 (0) |
| & Ferreira [31] (2021, Portugal) | Term and Preterm | Retrospective cohort study | Some precautions | Admission | 1 * | 79 | 81 | 0/81 (0) | - |
| Charki [32] (2021, India) | Term and Preterm | Prospective observational study | Some precautions | Admission | None reported | 26 | 28 | 0/20 (0) | 0/8 (0) |
| Shlomai [33] (2021, Israel) | Term | Retrospective cohort study | Isolation | Admission | 1 * | 55 | 55 | 0/47 (0) | 0/8 (0) |
| Ronchi [34] (2020, Italy) | Term and Preterm | Prospective multi-center cohort | Some precautions | 14 days | None reported | 61 | 62 | 1/59 (1.7) | 0/3 (0) |
| Martenot [35] (2020, France) | Term | Retrospective study | Some precautions | 14 days | None reported | 26 | 26 | 0/23 (0) | 0/3 (0) |
| & Kalamdani [36] (2020, India) | Term | Retrospective cohort study | NA | Admission | None reported | 185 | 185 | 12/185 (6.5) | - |
| & Khan [37] (2020, Pakistan) | NA | Retrospective cohort study | Some precautions | Admission | None reported | 66 | 67 | 0/67 (0) | - |
| Overall Total 8514 | 166/6299 (2.6) | 48/2215 (2.2) | |||||||
| # Meta-analysis Total 7787 | 154/5572 (2.7) | 48/2215 (2.2) |
| Risk of Bias Domain | Question | Approach/Assessment |
|---|---|---|
| 1. Selection of exposed and non-exposed cohorts | Were cohorts drawn from the same population? | Green: Similar care and patients. Yellow–Orange: Same patients, but not all information on care is available. Red: Different care/patient populations. |
| 2. Assessment of exposure | Can we be confident in the assessment of exposure? | Green: PCR—positive pregnant person (included in eligibility criteria). |
| 3. Outcome of interest at study start | Can we be confident that the outcome of interest was not present at the start of the study? | Green: Outcome assessed at birth or within <24 h and repeated after 24 h. Yellow: Outcome assessed at any other time. |
| 4. Matching of cohorts for variables | Did the study match exposed and unexposed cohorts for all variables associated with the outcome? | Green: Matching performed. Red: No matching performed. |
| 5. Prognostic factors | Can we be confident in the assessment of prognostic factors? | Green: Data from validated databases or thorough individual assessments. Yellow: Data collected either prospectively or retrospectively with central queries. Orange: Data collected retrospectively without queries. |
| 6. Outcome assessment | Can we be confident in the assessment of outcomes? | Green: PCR testing was carried out and reported for neonates by exposure. |
| 7. Follow-up of cohorts | Was the follow-up of cohorts adequate? | Green: 100% follow-up. Yellow: 80–99% follow-up. Orange: 50–79% follow-up. Red: Less than 50% follow-up. |
| 8. Co-interventions | Were co-interventions similar between groups? | Green: Co-interventions were similar. Yellow–Orange: Minor differences not fully documented. Red: Few or no relevant co-interventions that might influence the outcome of interest are documented to be similar in the exposed and unexposed groups. |
| Author, Year | Question 1 | Question 2 | Question 3 | Question 4 | Question 5 | Question 6 | Question 7 | Question 8 | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Hudak, 2023 [25] | 🟡 | 🟢 | 🟡 | 🔴 | 🟡 | 🟢 | 🟡 | 🔴 | Moderate risk |
| Zolkau, 2023 [26] | 🔴 | 🟢 | 🟡 | 🔴 | 🔴 | 🟢 | 🔴 | 🔴 | High risk |
| Hariharan, 2022 [29] | 🟢 | 🟢 | 🟡 | 🔴 | 🔴 | 🟢 | 🟡 | 🟠 | Moderate risk |
| Melekoglu, 2022 [28] | 🟢 | 🟢 | 🟡 | 🔴 | 🔴 | 🟢 | 🟢 | 🟢 | Moderate risk |
| Charki, 2021 [32] | 🟢 | 🟢 | 🟡 | 🔴 | 🔴 | 🟢 | 🟢 | 🟢 | Moderate risk |
| Ronchi, 2020 [34] | 🟢 | 🟢 | 🟢 | 🔴 | 🔴 | 🟢 | 🟢 | 🟢 | Moderate risk |
| Martenot, 2020 [35] | 🟢 | 🟢 | 🟡 | 🔴 | 🔴 | 🟢 | 🟢 | 🟢 | Moderate risk |
| Wroblewska, 2021 [30] | 🟢 | 🟢 | 🟡 | 🔴 | 🔴 | 🟢 | 🟢 | 🟢 | Moderate risk |
| Shlomai, 2021 [33] | 🟢 | 🟢 | 🟡 | 🔴 | 🔴 | 🟢 | 🟢 | 🟢 | Moderate risk |
| Rate of SARS-CoV-2 Infection in No MoM Compared to Any MoM | |||||
|---|---|---|---|---|---|
| Outcome | Study Population | No MoM | Any MoM | Relative Effect (95% CI) | Certainty of the Evidence (GRADE) |
| Neonatal SARS-CoV-2 infection | 7787 | 2215 | 5572 | 0.82 (0.44–1.53) | Low certainty |
| Patients or population: Neonates born to SARS-CoV-2-positive Mothers Intervention: Any MoM Comparison: No MoM | |||||
| GRADE Domains Low quality of evidence, as these are cohort studies. Risk of bias: High in one study, moderate in all other studies. Sensitivity analysis: No change in results after removal of high-risk study. Inconsistency: No evidence of this. Indirectness: None. Imprecision: Large CI despite large sample size. Especially large for subgroup analysis by type of dyad handling, due to small sample size. Publication bias: No evidence. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babata, K.; Sultana, R.; Hascoët, J.-M.; Albert, R.; Chan, C.; Mazzarella, K.; Muhamed, T.; Yeo, K.T.; Kong, J.Y.; Brion, L.P. Neonatal Feeding Practices and SARS-CoV-2 Transmission in Neonates with Perinatal SARS-CoV-2 Exposure: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 280. https://doi.org/10.3390/jcm14010280
Babata K, Sultana R, Hascoët J-M, Albert R, Chan C, Mazzarella K, Muhamed T, Yeo KT, Kong JY, Brion LP. Neonatal Feeding Practices and SARS-CoV-2 Transmission in Neonates with Perinatal SARS-CoV-2 Exposure: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(1):280. https://doi.org/10.3390/jcm14010280
Chicago/Turabian StyleBabata, Kikelomo, Rehena Sultana, Jean-Michel Hascoët, Riya Albert, Christina Chan, Kelly Mazzarella, Tanaz Muhamed, Kee Thai Yeo, Juin Yee Kong, and Luc P. Brion. 2025. "Neonatal Feeding Practices and SARS-CoV-2 Transmission in Neonates with Perinatal SARS-CoV-2 Exposure: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 1: 280. https://doi.org/10.3390/jcm14010280
APA StyleBabata, K., Sultana, R., Hascoët, J.-M., Albert, R., Chan, C., Mazzarella, K., Muhamed, T., Yeo, K. T., Kong, J. Y., & Brion, L. P. (2025). Neonatal Feeding Practices and SARS-CoV-2 Transmission in Neonates with Perinatal SARS-CoV-2 Exposure: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(1), 280. https://doi.org/10.3390/jcm14010280








