Retinal Displacement Following Vitrectomy for Rhegmatogenous Retinal Detachment: A Systematic Review of Surgical Techniques, Tamponade Agents, and Outcomes
Abstract
1. Introduction
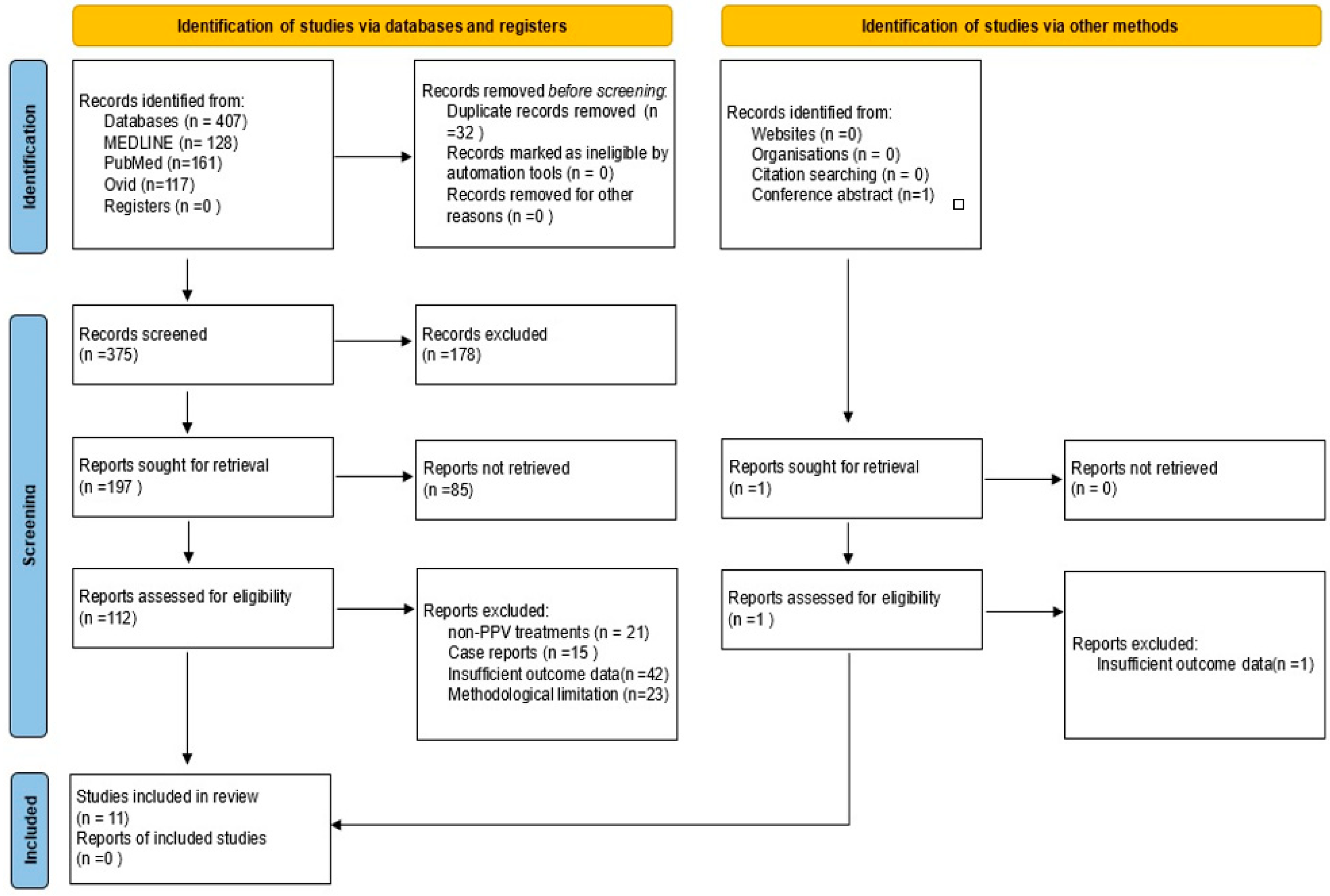
2. Methods
3. Results
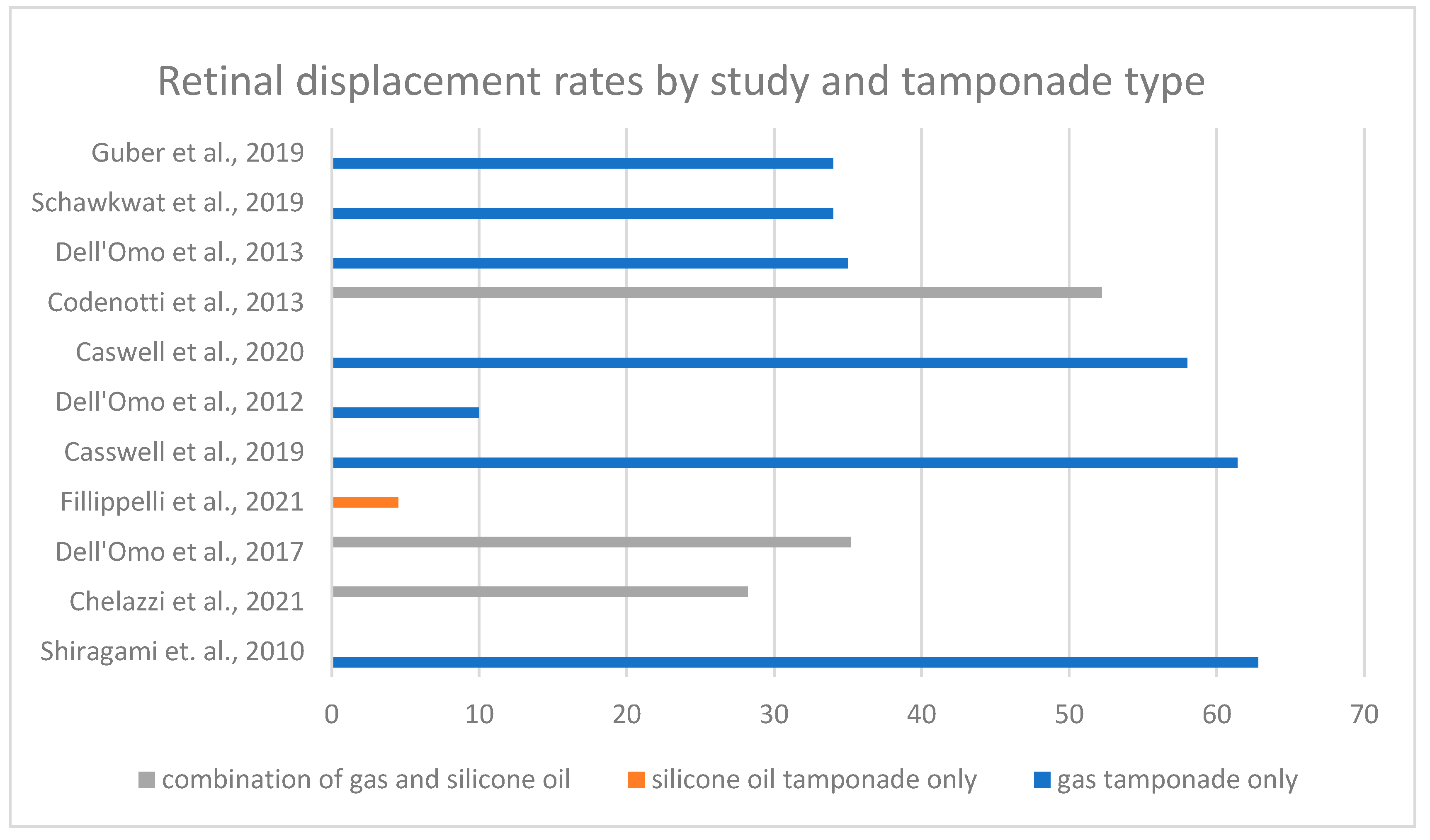
3.1. Presence of Retinal Displacement
3.2. Impact of Tamponade Agents
3.3. Preoperative Macula Status
3.4. Extent of Detachment in Quadrants
3.5. Proliferative Vitreoretinopathy
3.6. Effectiveness of Perfluorocarbon Liquid
3.7. Age Effect
3.8. Effect of Detachment Duration
3.9. Postoperative Management and Head Positioning
3.10. Image Modality
3.11. Postoperative Subretinal Fluid
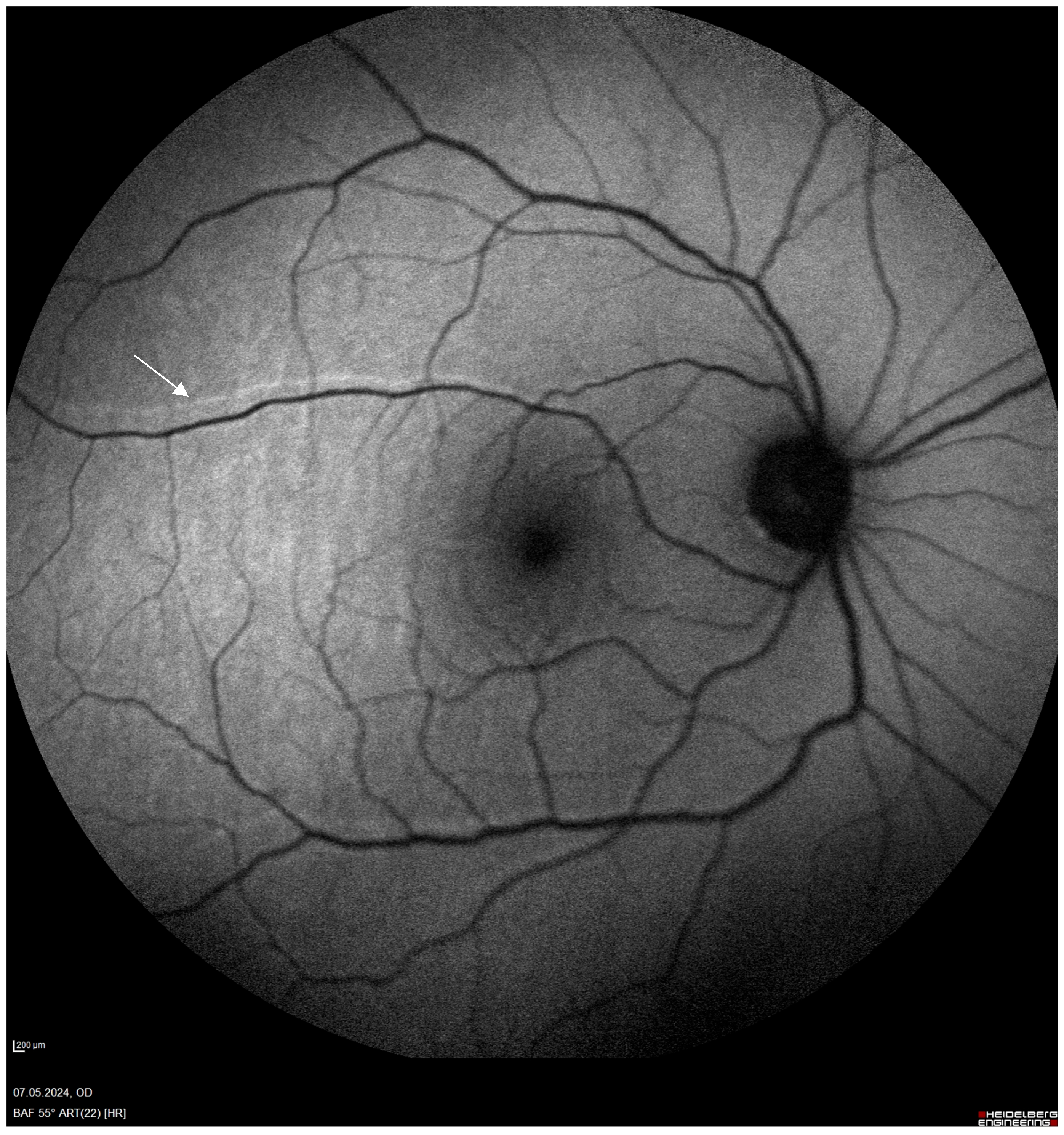
3.12. Distortion
3.13. Postoperative Best Corrected Visual Acuity
3.14. Direction of Displacement
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
Correction Statement
Abbreviations
| RRD | rhegmatogenous retinal detachment |
| PFCL | perfluorocarbon liquid |
| SO | silicone oil |
| OCT | optical coherence tomography |
| PPV | pars plana vitrectomy |
| PVR | proliferative vitreoretinopathy |
| FAF | fundus autofluorescence imaging |
| BCVA | best corrected visual acuity |
| FC | fundus camera |
| cSLO | confocal scanning laser ophthalmoscopy |
| RPE | retinal pigment epithelium |
| SRF | subretinal fluid |
| N/R | not reported |
| C3F8 | perfluoropropane |
| SF6 | Sulfur Hexafluoride |
References
- Cejudo, R.M.; Marchite, C.B.; Morán, T.P.; Piqueras, S.C. Comparison between standard and wide-field autofluorescence in detection of retinal displacement after rhegmatogenous retinal detachment surgery. Adv. Ophthalmol. Pract. Res. 2022, 2, 100076. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Iqbal, K.; Iqbal, S.M.; Ghaffar, Z.; Tariq, M.; Tahir, M.J.; Rahman, F.U.; Raheem, U.; Butt, J.B.; Abbas, K. Risk factors associated with rhegmatogenous retinal detachment. Cureus 2022, 14, e23201. [Google Scholar] [CrossRef] [PubMed]
- Dell’Omo, R.; Semeraro, F.; Guerra, G.; Verolino, M.; Cinelli, M.; Montagnani, S.; Costagliola, C. Short-time prone posturing is well-tolerated and reduces the rate of unintentional retinal displacement in elderly patients operated on for retinal detachment. BMC Surg. 2013, 13 (Suppl. S2), S55. [Google Scholar] [CrossRef] [PubMed]
- Chelazzi, P.; Azzolini, C.; Bellina, C.; Cappelli, F.; Del Genovese, I.; Caraffa, L.; Scullica, F. Efficacy and safety of vitrectomy without using perfluorocarbon liquids and drainage retinotomy associated with postoperative positioning based on residual subretinal fluid for rhegmatogenous retinal detachment. J. Ophthalmol. 2021, 2021, 5588479. [Google Scholar] [CrossRef] [PubMed]
- Dell’Omo, R.; Mura, M.; Oberstein, S.Y.L.; Bijl, H.; Tan, H.S. Early simultaneous fundus autofluorescence and optical coherence tomography features after pars plana vitrectomy for primary rhegmatogenous retinal detachment. Retina 2012, 32, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Williamson, T.H.; Hysi, P.; Shunmugam, M.; Dogramaci, M.; Wong, R.; Laidlaw, D.A. Macular displacement following rhegmatogenous retinal detachment repair. Br. J. Ophthalmol. 2013, 97, 1297–1302. [Google Scholar] [CrossRef]
- Codenotti, M.; Fogliato, G.; Iuliano, L.; Querques, G.; Maestranzi, G.; Prati, M.; Ramoni, A.; De Benedetto, U.; Bandello, F. Influence of intraocular tamponade on unintentional retinal displacement after vitrectomy for rhegmatogenous retinal detachment. Retina 2013, 33, 349–355. [Google Scholar] [CrossRef]
- Dell’Omo, R.; Scupola, A.; Viggiano, D.; Sammarco, M.G.; De Turris, S.; Romano, M.R.; Grimaldi, G.; Dell’Omo, E.; Costagliola, C. Incidence and factors influencing retinal displacement in eyes treated for rhegmatogenous retinal detachment with vitrectomy and gas or silicone oil. Investig. Opthalmol. Vis. Sci. 2017, 58, BIO191–BIO199. [Google Scholar] [CrossRef]
- Chen, H.J.; Tsai, Y.L.; Hsiao, C.H.; Chang, C.J. Air versus gas tamponade for primary rhegmatogenous retinal detachment: A systematic review and meta-analysis. Ophthalmic Res. 2023, 66, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Eibenberger, K.; Sacu, S.; Rezar-Dreindl, S.; Schmidt-Erfurth, U.; Georgopoulos, M. Silicone oil tamponade in rhegmatogenous retinal detachment: Functional and morphological results. Curr. Eye Res. 2020, 45, 38–45. [Google Scholar] [CrossRef]
- Zenoni, S.; Romano, M.R.; Palmieri, S.; Comi, N.; Fiorentini, E.; Fontana, P. Ocular tolerance and efficacy of short-term tamponade with double filling of polydimethyloxane and perfluoro-n-octane. Clin. Ophthalmol. 2011, 5, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Shiragami, C.; Shiraga, F.; Yamaji, H.; Fukuda, K.; Takagishi, M.; Morita, M.; Kishikami, T. Unintentional displacement of the retina after standard vitrectomy for rhegmatogenous intentional displacement of the retina after standard vitrectomy for rhegmatogenous retinal detachment. Ophthalmology 2010, 117, 86–92.e1. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, M.; Napolitano, P.; Costagliola, C.; Rinaldi, M.; Chiosi, F.; Dell’Omo, R. Unintentional retinal displacement in eyes treated for rhegmatogenous retinal detachment complicated by proliferative vitreoretinopathy with pars plana vitrectomy and silicone oil. J. Ophthalmol. 2021, 2021, 5532787. [Google Scholar] [CrossRef]
- Casswell, E.J.; Yorston, D.; Lee, E.; Heeren, T.F.C.; Harris, N.; Zvobgo, T.M.; Tarafdar, S.; Xing, W.; Bourmpaki, E.; Bunce, C.; et al. Effect of face-down positioning vs support-the-break positioning after macula-involving retinal detachment repair: The PostRD randomized clinical trial. JAMA Ophthalmol. 2019, 138, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Casswell, E.J.; Heeren, T.F.C.; Lee, E.; Khabra, K.; Yorston, D.; Charteris, D.G. Postretinal detachment retinal displacement: How best to detect it? Ophthalmologica 2020, 243, 280–287. [Google Scholar] [CrossRef]
- Schawkat, M.; Valmaggia, C.; Lang, C.; Scholl, H.P.; Harsum, S.; Guber, I.; Guber, J. Influence of postoperative posture on macular slippage after macula off retinal detachment, a randomized controlled trial. Adis J. 2019, 8, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Guber, J.; Schawkat, M.; Lang, C.; Scholl, H.P.N.; Valmaggia, C. How to prevent retinal shift after rhegmatogenous retinal detachment repair: A prospective, randomized study. Ophthalmol. Retin. 2019, 3, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lin, Q.; Chen, F. Prevalence and predictors of metamorphopsia after successful rhegmatogenous retinal detachment surgery: A cross-sectional, comparative study. Br. J. Ophthalmol. 2017, 101, 725–729. [Google Scholar] [CrossRef]
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative vitreoretinopathy: A review. Int. Ophthalmol. Clin. 2019, 59, 221–240. [Google Scholar] [CrossRef]
- Pandya, V.B.; Ho, I.V.; Hunyor, A.P. Does unintentional macular translocation after retinal detachment repair influence visual outcome? Clin. Exp. Ophthalmol. 2012, 40, 88–92. [Google Scholar] [CrossRef]
- Kurt, R.A.; Kapran, Z. Heavy silicone oil as an endotamponade in recurrent or complicated retinal detachment and macular hole. Turk. J. Ophthalmol. 2022, 52, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Baino, F. The use of polymers in the treatment of retinal detachment: Current trends and future perspectives. Polymers 2010, 2, 286–322. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment. Polym. Chem. 2020, 11, 6988–7008. [Google Scholar] [CrossRef]
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall | |
|---|---|---|---|---|---|---|---|---|
| Shiragami et al., 2010 [14] | Moderate | Low | Moderate | Serious | Low | Moderate | Moderate | Serious |
| Chelazzi et al., 2021 [4] | Serious | Moderate | Low | Serious | Moderate | Serious | Moderate | Serious |
| Dell’Omo et al., 2017 [8] | Moderate | Low | Low | Moderate | Low | Moderate | Low | Moderate |
| Fillippelli et al., 2021 [15] | Serious | Moderate | Low | Moderate | Moderate | Serious | Moderate | Serious |
| Casswell et al., 2019 [16] | Moderate | Low | Low | Serious | Moderate | Moderate | Low | Serious |
| Dell’Omo et al., 2012 [5] | Moderate | Low | Low | Moderate | Low | Moderate | Low | Moderate |
| Caswell et al., 2020 [17] | Moderate | Low | Low | Serious | Moderate | Moderate | Low | Serious |
| Codenotti et al., 2013 [7] | Moderate | Moderate | Low | Serious | Moderate | Serious | Moderate | Serious |
| Dell’Omo et al., 2013 [3] | Moderate | Low | Low | Moderate | Low | Moderate | Low | Moderate |
| D1 | D2 | D3 | D4 | D5 | Overall | |
|---|---|---|---|---|---|---|
| Schawkwat et al., 2021 [18] | High risk | Some concerns | High risk | Low risk | Some concerns | High risk |
| Guber et al., 2019 [19] | High risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Outcome | No. of Studies | Result | Quality (GRADE) | Comments |
|---|---|---|---|---|
| Retinal Displacement | 11 | Reported rates of retinal displacement vary significantly, ranging from 4.5% to 62.8%. Gas tamponade generally had higher displacement rates than silicone oil. | Low | Although widely reported, moderate-to-serious bias in study designs and inconsistency in reported rates reduce confidence in these findings. |
| Direction of RD | 4 | Downward displacement is the most common pattern (Dell’Omo et al., 2017 [8]: 88.6%). Some upward displacement reported, but no consistent correlation with detachment location. | Moderate | Findings are consistent across studies, but limited sample sizes prevent higher confidence. |
| Impact of Tamponade Agents | 4 | Silicone oil had significantly lower displacement rates (Filippelli et al.: 4.5% [15]; Dell’Omo et al., 2017: 14.3% [8]) compared to gas tamponade. | Moderate | Consistent across studies, with clear statistical support. |
| Preoperative Macula Status | 7 | Macula-off status associated with higher risk of displacement. Patients with macula-on detachments had minimal displacement. | Low | Moderate bias in most studies assessing macula-off impacts, though findings are consistent. |
| BCVA | 4 | Retinal displacement did not consistently correlate with postoperative BCVA. | Low | Imprecision due to underpowered studies limits reliability of findings. |
| Visual Distortion | 4 | Associated with retinal displacement. | Moderate | Consistently reported but lacks uniform definitions and measurement tools across studies. |
| Extent of Detachment | 7 | Quadrants involved in detachment did not consistently impact displacement (significant in only 1 study: p = 0.019). | Low | Data inconsistency and lack of focus on this factor limit confidence. |
| PVR | 4 | PVR grading inconsistently reported; one study (Filippelli et al. [15]) noted retinal displacement in cases with PVR B but lacked statistical power. | Very low | Insufficient data to draw robust conclusions. |
| Postoperative Positioning | 4 | Face-down positioning reduces displacement in gas tamponade cases (Chelazzi et al. [4], Casswell et al. [16]) | Moderate | Findings are consistent but based on limited studies. |
| PFCL | 4 | PFCL use associated with smaller macular shifts in two studies. | Low | Limited and inconsistent evidence on PFCL’s effects. |
| Study Type | Author, Year | Number of Centers | Follow-Up Duration | Age in Years (Mean SD) | PVR Grade | Number of Pseudophakic (%) | Number of Men (%) | Presence of Retinal Displacement (%) | Total Number of Patients | Macular Status | Use of Tamponade Agent | Postoperative Posture | Number of Quadrants of RRD Affected | Use of PFCL | Imaging Modality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective randomized study | Casswell et al., 2020 [17] | Multiple | 8 weeks and 6 months | 60.8 | None-230 PVR B 7 PVR C 2 | N/R | 71.5 | 42–58% | 239 | Off | SF6-110 C2F6-69 C3F8-32 | Face down or support the break for 24 h followed by support-the-break positioning for 6 days | In hours 6–5.5 | 3 | FC |
| Prospective randomized controlled trial | Schawkat et al., 2019 [18] | Single | 3 weeks with SF6 6 weeks with C3F8 | 69 | excluded | N/R | 72 | 34% | 50 | Off | C3F8-88% SF6-12% | Log-roll with face-down position or lying flat on back for 6 h, then support-the-break end position | 1—2 2—31 3—17 4—8 | 25 | cSLO |
| Prospective observational study | dell’Omo et al., 2017 [8] | Multiple | 12 months | 60.9 ± 11.7 | A-B | 63.2 | 68 | 35.2% | 125 | Off | SF6-77.6% SO-22.4% | Prone 24 h after surgery | 2—53.6% 3—20.8% 4—25.6% | 80% | cSLO |
| Prospective observational study | dell’Omo et al. 2013 [3] | Single | 4 weeks | 64.3 ±3.5 | N/R | 80 | 60 | 35% | 20 | Off | SF6 20% | Face-down positioning for 2 h immediately after surgery | Mean 2.95 ± 0.75 | No | cSLO |
| Retrospective Study | Chelazzi et al., 2021 [4] | Multiple | 3 weeks SF6 6 weeks C3F8 1 month SO | 59.1 ± 10.3 | A-B | 77.3 | 59 | 28.2% | 75 | On: 45.3% Off: 54.7% | 20%SF6-20%/12%C3F8-58.7% Silicone Oil 8% Air 13.3% | Supine in macula-off In case of SRF, prone for few hours and then supine or support the break | Superior 45.3% inferior 18.7% superior + inferior 36% | No | cSLO |
| Retrospective observational Study | Filippelli et al., 2021 [15] | Single | 3 months | 65.8 + 11.4 | A: 15.9% B: 63.6% C: 20.5% | 34 | 66 | 4.5% | 44 | Off | Silicone Oil | Face-down position for 24 h | 3: 38.6% 4: 61.4% | 68.2% | cSLO |
| Prospective interventional case series | Shiragami et al., 2010 [14] | Single | 6 months | 60 | Excluded | N/R | 60.5 | 62.8% | 43 | On: 44% Off: 56% | 20% SF6 | Patients sat for a few minutes postoperatively before assuming a prone position for 7 to 10 days | 1—2 2—31 2—8 4—2 | 30 | FC |
| Prospective randomized controlled study | Guber et al., 2019 [19] | Single | 6 months | 69 | Excluded | 32% | 72 | 34.00% | 49 | Off | SF6-88, C3F8-12 | Log-roll 52% or Flat position: 24% | 1—2, 2—23 3—17 4—8 | 25 | cSLO |
| Prospective study | dell’Omo et al., 2012 [5] | Single | 1 month | 59.9 ± 8.5 | N/R | 27 | 82 | 10% | 33 | On: 27 Off: 73 | SF6 | N/R | 4—3 3—9 2—18 1—3 | 45% | cSLO |
| Prospective study | Casswell 2019 [16] | Single | 8 weeks | 60.3 ± 7.7 | N/R | 30 | 75.7 | 61.4% (43/70) in FC 52.8% (37/70) cSLO | 70 | Off | Gas | Face-down 45% or bubble to break 55% (randomized) for 24 h | N/R | No | FC and cSLO |
| Prospective study | Codenotti 2013 [7] | Single | 3 moths | 57.1 ± 9.4 | N/R | 39% | 65.2 | 52.2% | 23 | Off: 10 On: 13 | C3F8 6% 14 eyes SO-9 eyes | 1 week | 1—6 2—10 3—3 4—4 | Yes | FC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwik, P.; Chudoba, T.; Cisiecki, S. Retinal Displacement Following Vitrectomy for Rhegmatogenous Retinal Detachment: A Systematic Review of Surgical Techniques, Tamponade Agents, and Outcomes. J. Clin. Med. 2025, 14, 250. https://doi.org/10.3390/jcm14010250
Siwik P, Chudoba T, Cisiecki S. Retinal Displacement Following Vitrectomy for Rhegmatogenous Retinal Detachment: A Systematic Review of Surgical Techniques, Tamponade Agents, and Outcomes. Journal of Clinical Medicine. 2025; 14(1):250. https://doi.org/10.3390/jcm14010250
Chicago/Turabian StyleSiwik, Paulina, Tomasz Chudoba, and Sławomir Cisiecki. 2025. "Retinal Displacement Following Vitrectomy for Rhegmatogenous Retinal Detachment: A Systematic Review of Surgical Techniques, Tamponade Agents, and Outcomes" Journal of Clinical Medicine 14, no. 1: 250. https://doi.org/10.3390/jcm14010250
APA StyleSiwik, P., Chudoba, T., & Cisiecki, S. (2025). Retinal Displacement Following Vitrectomy for Rhegmatogenous Retinal Detachment: A Systematic Review of Surgical Techniques, Tamponade Agents, and Outcomes. Journal of Clinical Medicine, 14(1), 250. https://doi.org/10.3390/jcm14010250







