Interstitial Lung Disease Associated with Anti-Ku Antibodies: A Case Series of 19 Patients
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Eligibility Criteria
2.2. Antibody Testing
2.3. Clinical and Radiological Data Collection
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Patient Characteristics
3.3. Characteristics of Autoimmune Disease
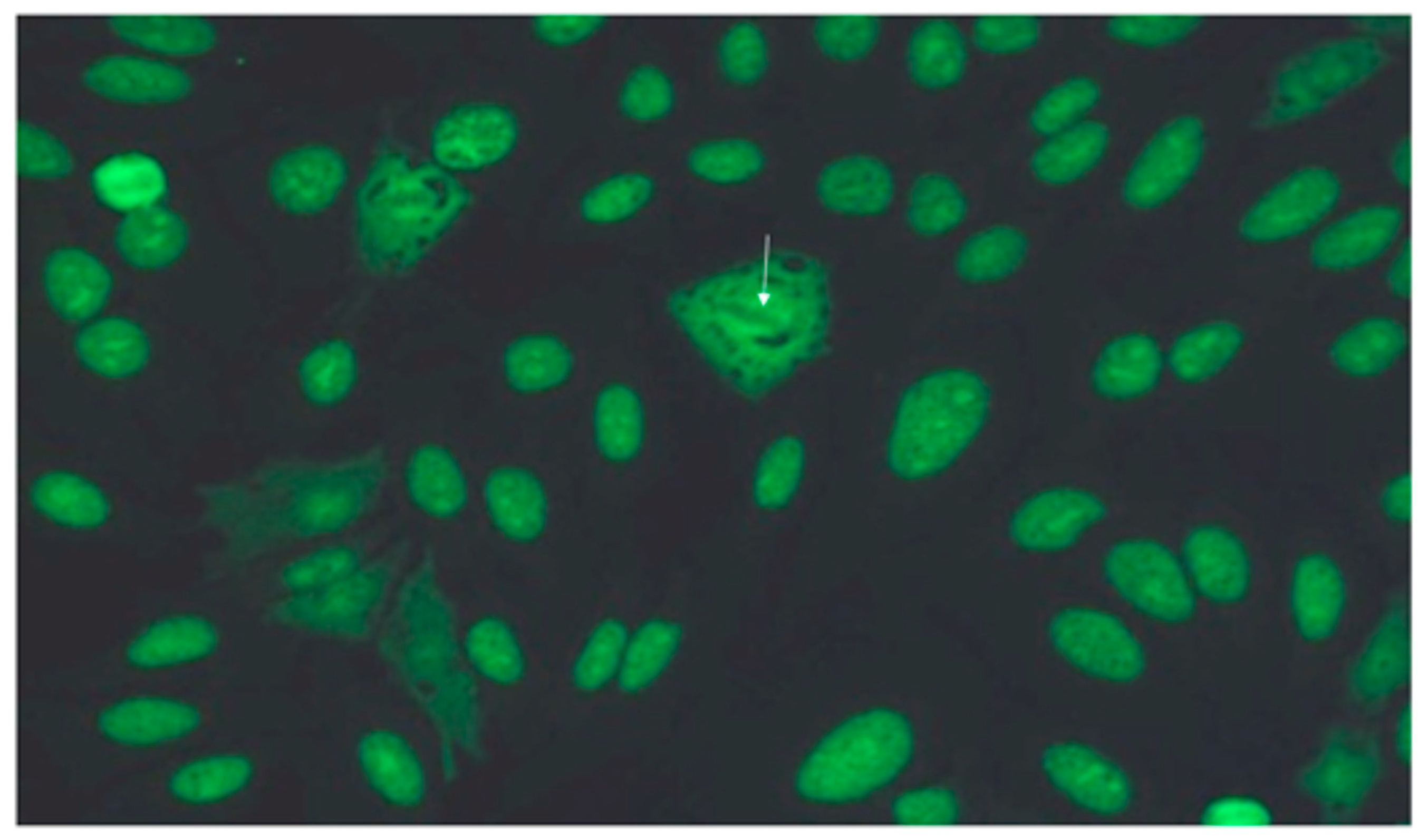
3.4. Immunology
3.5. Pulmonary Function Tests
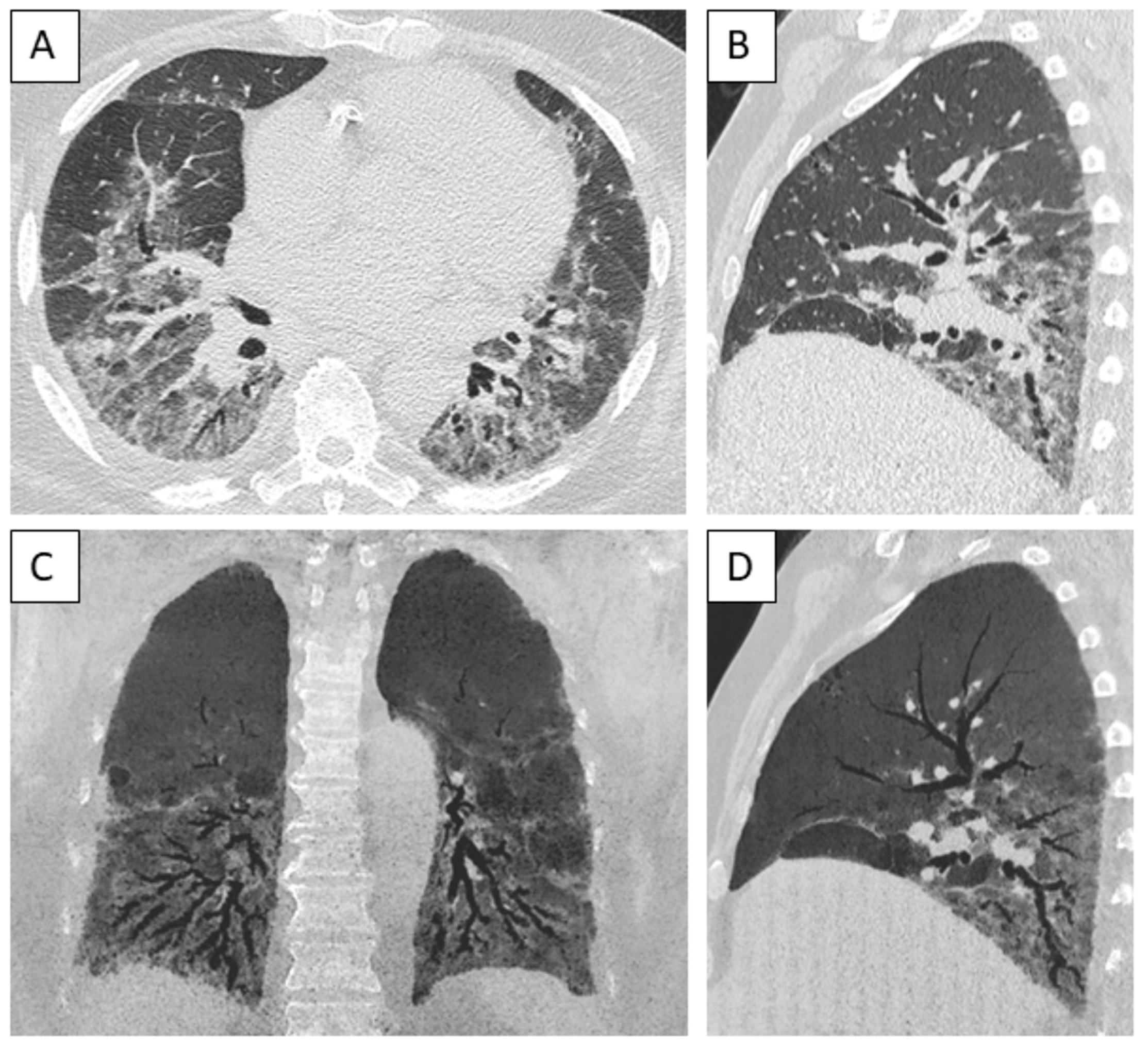
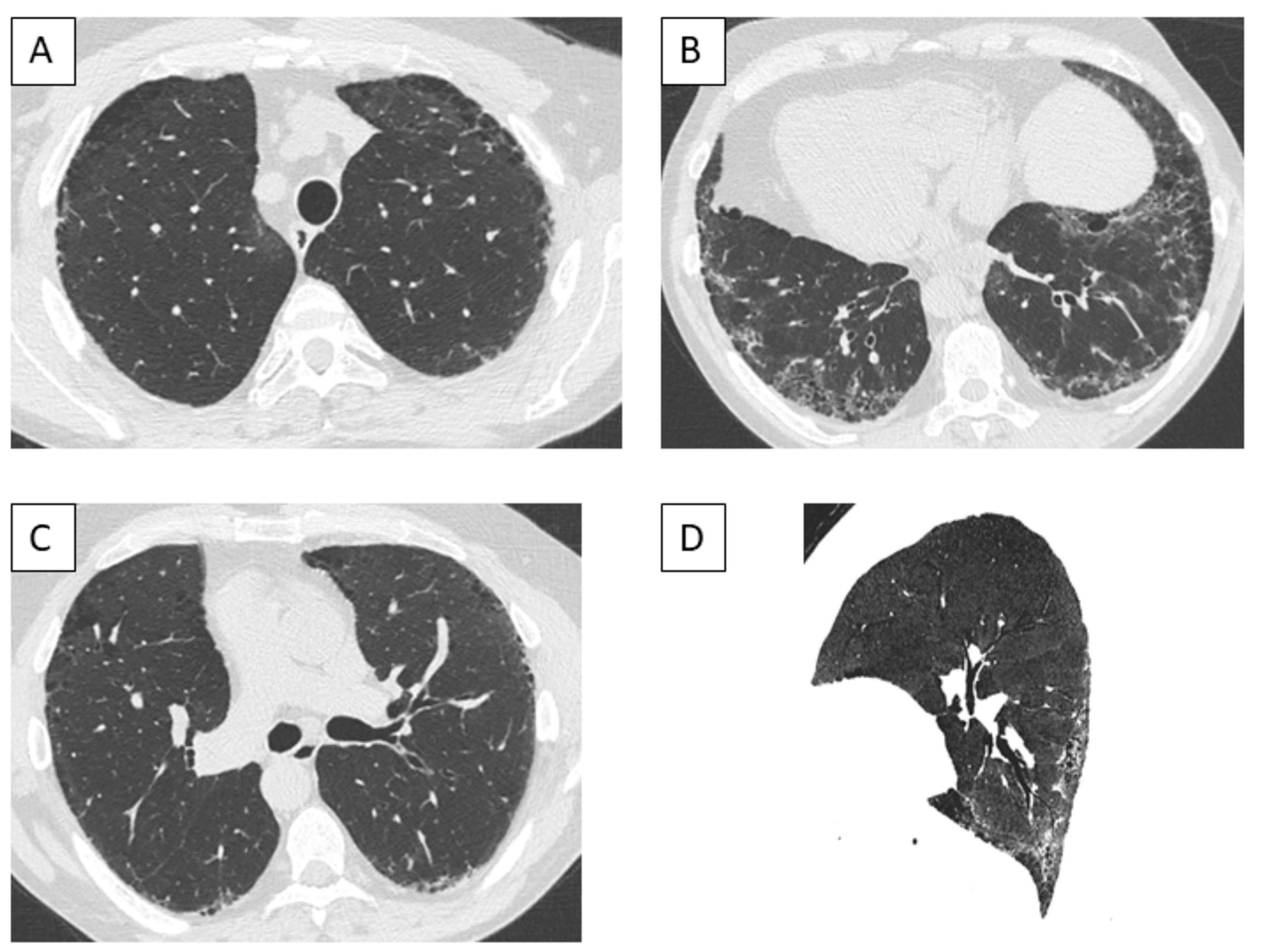
3.6. Imaging Findings
3.7. Bronchoalveolar Lavage
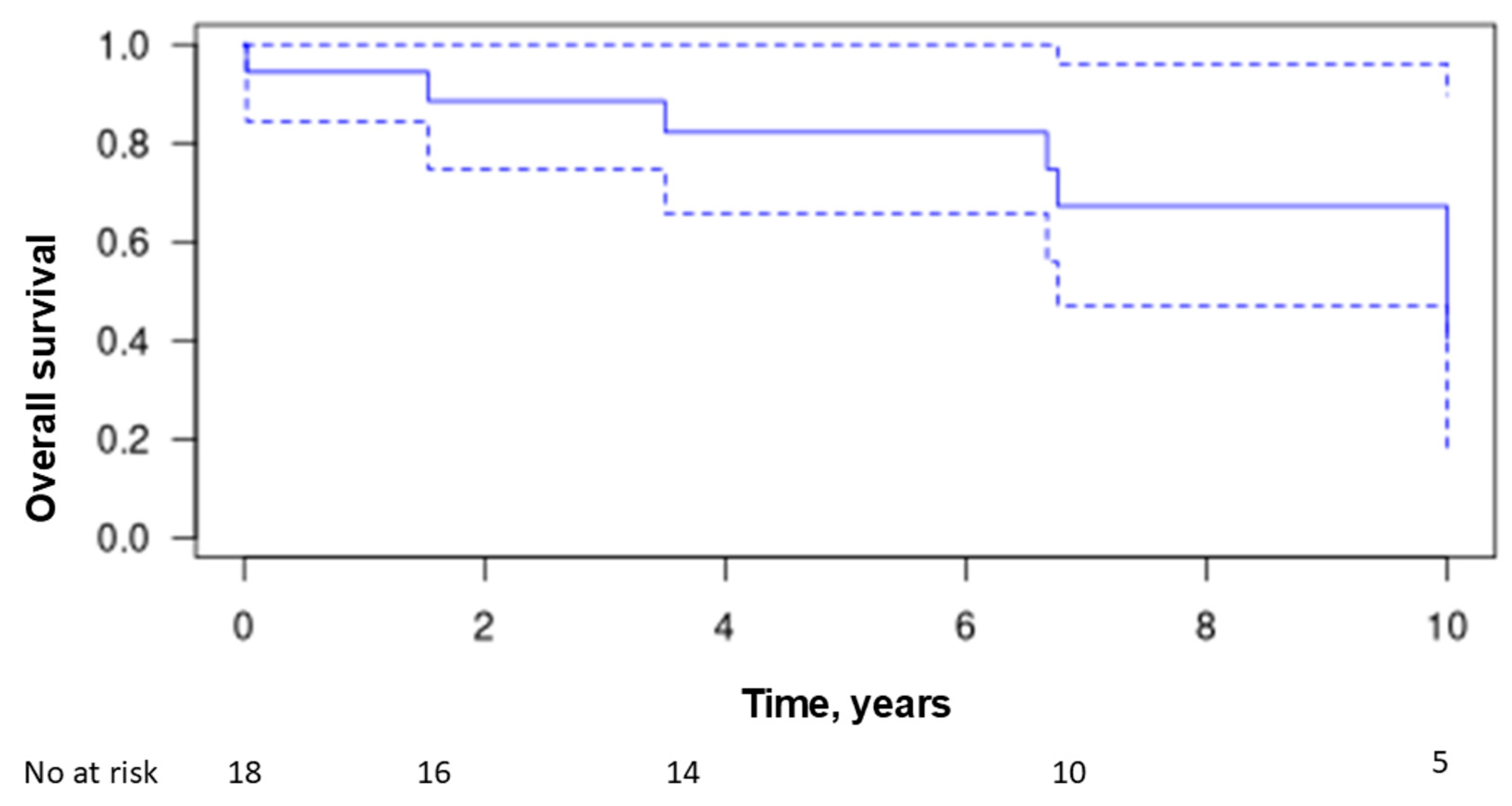
3.8. Treatment and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wijsenbeek, M.; Cottin, V. Spectrum of Fibrotic Lung Diseases. N. Engl. J. Med. 2020, 383, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Joy, G.M.; Arbiv, O.A.; Wong, C.K.; Lok, S.D.; Adderley, N.A.; Dobosz, K.M.; Johannson, K.A.; Ryerson, C.J. Prevalence, imaging patterns and risk factors of interstitial lung disease in connective tissue disease: A systematic review and meta-analysis. Eur. Respir. Rev. 2023, 32, 220210. [Google Scholar] [CrossRef] [PubMed]
- Wijsenbeek, M.; Suzuki, A.; Maher, T.M. Interstitial lung diseases. Lancet 2022, 400, 769–786. [Google Scholar] [CrossRef]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Brown, K.K.; Colby, T.V.; Galvin, J.R.; Goldin, J.G.; Hansell, D.M.; Inoue, Y.; Johkoh, T.; et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Mariampillai, K.; Granger, B.; Amelin, D.; Guiguet, M.; Hachulla, E.; Maurier, F.; Meyer, A.; Tohme, A.; Charuel, J.L.; Musset, L.; et al. Development of a New Classification System for Idiopathic Inflammatory Myopathies Based on Clinical Manifestations and Myositis-Specific Autoantibodies. JAMA Neurol. 2018, 75, 1528–1537. [Google Scholar] [CrossRef]
- Patterson, K.A.; Roberts-Thomson, P.J.; Lester, S.; Tan, J.A.; Hakendorf, P.; Rischmueller, M.; Zochling, J.; Sahhar, J.; Nash, P.; Roddy, J.; et al. Interpretation of an Extended Autoantibody Profile in a Well-Characterized Australian Systemic Sclerosis (Scleroderma) Cohort Using Principal Components Analysis. Arthritis Rheumatol. 2015, 67, 3234–3244. [Google Scholar] [CrossRef]
- Cottin, V.; Thivolet-Béjui, F.; Reynaud-Gaubert, M.; Cadranel, J.; Delaval, P.; Ternamian, P.J.; Cordier, J.F. Interstitial lung disease in amyopathic dermatomyositis, dermatomyositis and polymyositis. Eur. Respir. J. 2003, 22, 245–250. [Google Scholar] [CrossRef]
- Hervier, B.; Devilliers, H.; Stanciu, R.; Meyer, A.; Uzunhan, Y.; Masseau, A.; Dubucquoi, S.; Hatron, P.Y.; Musset, L.; Wallaert, B.; et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: Phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun. Rev. 2012, 12, 210–217. [Google Scholar] [CrossRef]
- Lega, J.C.; Cottin, V.; Fabien, N.; Thivolet-Béjui, F.; Cordier, J.F. Interstitial Lung Disease Associated with Anti-PM/Scl or Anti-Aminoacyl-tRNA Synthetase Autoantibodies: A Similar Condition? J. Rheumatol. 2010, 37, 1000–1009. [Google Scholar] [CrossRef]
- Mismetti, V.; Si-Mohamed, S.; Cottin, V. Interstitial Lung Disease Associated with Systemic Sclerosis. Semin. Respir. Crit. Care Med. 2024, 45, 342–364. [Google Scholar] [CrossRef]
- Barba, T.; Mainbourg, S.; Nasser, M.; Lega, J.C.; Cottin, V. Lung Diseases in Inflammatory Myopathies. Semin. Respir. Crit. Care. Med. 2019, 40, 255–270. [Google Scholar] [CrossRef]
- Marie, I.; Lahaxe, L.; Benveniste, O.; Delavigne, K.; Adoue, D.; Mouthon, L.; Hachulla, E.; Constans, J.; Tiev, K.; Diot, E.; et al. Long-term outcome of patients with polymyositis/dermatomyositis and anti-PM-Scl antibody. Br. J. Dermatol. 2010, 162, 337–344. [Google Scholar] [CrossRef]
- Guillen-Del Castillo, A.; Pilar Simeon-Aznar, C.; Fonollosa-Pla, V.; Alonso-Vila, S.; Reverte-Vinaixa, M.M.; Munoz, X.; Pallisa, E.; Selva-O’allaghan, A.; Fernandez-Codina, A.; Vilardell-Tarres, M. Good outcome of interstitial lung disease in patients with scleroderma associated to anti-PM/Scl antibody. Semin. Arthritis Rheum. 2014, 44, 331–337. [Google Scholar] [CrossRef]
- Fell, V.L.; Schild-Poulter, C. The Ku heterodimer: Function in DNA repair and beyond. Mutat. Res. Rev. Mutat. Res. 2015, 763, 15–29. [Google Scholar] [CrossRef]
- Borie, R.; Kannengiesser, C.; Antoniou, K.; Bonella, F.; Crestani, B.; Fabre, A.; Froidure, A.; Galvin, L.; Griese, M.; Grutters, J.C.; et al. European Respiratory Society statement on familial pulmonary fibrosis. Eur. Respir. J. 2023, 61, 2201383. [Google Scholar] [CrossRef]
- Mimori, T.; Akizuki, M.; Yamagata, H.; Inada, S.; Yoshida, S.; Homma, M. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J. Clin. Investig. 1981, 68, 611–620. [Google Scholar] [CrossRef]
- Franceschini, F.; Cavazzana, I.; Generali, D.; Quinzanini, M.; Viardi, L.; Ghirardello, A.; Doria, A.; Cattaneo, R. Anti-Ku antibodies in connective tissue diseases: Clinical and serological evaluation of 14 patients. J. Rheumatol. 2002, 29, 1393–1397. [Google Scholar]
- Hoa, S.; Hudson, M.; Troyanov, Y.; Proudman, S.; Walker, J.; Stevens, W.; Nikpour, M.; Assassi, S.; Mayes, M.D.; Wang, M.; et al. Single-specificity anti-Ku antibodies in an international cohort of 2140 systemic sclerosis subjects: Clinical associations. Medicine 2016, 95, e4713. [Google Scholar] [CrossRef]
- Rozman, B.; Cucnik, S.; Sodin-Semrl, S.; Czirják, L.; Varjú, C.; Distler, O.; Huscher, D.; Aringer, M.; Steiner, G.; Matucci-Cerinic, M.; et al. Prevalence and clinical associations of anti-Ku antibodies in patients with systemic sclerosis: A European EUSTAR-initiated multi-centre case-control study. Ann. Rheum. Dis. 2008, 67, 1282–1286. [Google Scholar] [CrossRef]
- Cruellas, M.G.; Viana Vdos, S.; Levy-Neto, M.; Souza, F.H.; Shinjo, S.K. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics 2013, 68, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana, I.; Ceribelli, A.; Quinzanini, M.; Scarsi, M.; Airò, P.; Cattaneo, R.; Franceschini, F. Prevalence and clinical associations of anti-Ku antibodies in systemic autoimmune diseases. Lupus 2008, 17, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, A.J.; Bannert, B.; Vonk, M.; Airo, P.; Cozzi, F.; Carreira, P.E.; Bancel, D.F.; Allanore, Y.; Muller-Ladner, U.; Distler, O.; et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann. Rheum. Dis. 2010, 69, 1809–1815. [Google Scholar] [CrossRef]
- Belizna, C.; Henrion, D.; Beucher, A.; Lavigne, C.; Ghaali, A.; Lévesque, H. Anti-Ku antibodies: Clinical, genetic and diagnostic insights. Autoimmun. Rev. 2010, 9, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Cooley, H.M.; Melny, B.J.; Gleeson, R.; Greco, T.; Kay, T.W. Clinical and serological associations of anti-Ku antibody. J. Rheumatol. 1999, 26, 563–567. [Google Scholar] [PubMed]
- Spielmann, L.; Nespola, B.; Séverac, F.; Andres, E.; Kessler, R.; Guffroy, A.; Poindron, V.; Martin, T.; Geny, B.; Sibilia, J.; et al. Anti-Ku syndrome with elevated CK and anti-Ku syndrome with anti-dsDNA are two distinct entities with different outcomes. Ann. Rheum. Dis. 2019, 78, 1101–1106. [Google Scholar] [CrossRef]
- Yang, H.; Li, W.; Tian, X.; Wang, G.; Shu, X.; Peng, Q.; Lu, X. Immune-mediated necrotizing myopathies and interstitial lung disease are predominant characteristics in anti-Ku positive patients with idiopathic inflammatory myopathies. Ann. Rheum. Dis. 2022, 81, e48. [Google Scholar] [CrossRef]
- Rigolet, A.; Musset, L.; Dubourg, O.; Maisonobe, T.; Grenier, P.; Charuel, J.-L.; Behin, A.; Herson, S.; Amoura, Z.; Benveniste, O. Inflammatory Myopathies With Anti-Ku Antibodies. Medicine 2012, 91, 95–102. [Google Scholar] [CrossRef]
- Casal-Dominguez, M.; Pinal-Fernandez, I.; Derfoul, A.; Graf, R.; Michelle, H.; Albayda, J.; Tiniakou, E.; Adler, B.; Danoff, S.K.; Lloyd, T.E.; et al. The phenotype of myositis patients with anti-Ku autoantibodies. Semin. Arthritis Rheum. 2021, 51, 728–734. [Google Scholar] [CrossRef]
- Holzer, M.T.; Uruha, A.; Roos, A.; Hentschel, A.; Schänzer, A.; Weis, J.; Claeys, K.G.; Schoser, B.; Montagnese, F.; Goebel, H.H.; et al. Anti-Ku + myositis: An acquired inflammatory protein-aggregate myopathy. Acta Neuropathol. 2024, 148, 6. [Google Scholar] [CrossRef]
- Oyama, M.; Holzer, M.T.; Ohnuki, Y.; Saito, Y.; Nishimori, Y.; Suzuki, S.; Shiina, T.; Leonard-Louis, S.; Benveniste, O.; Schneider, U.; et al. Pathologic Features of Anti-Ku Myositis. Neurology 2024, 102, e209268. [Google Scholar] [CrossRef] [PubMed]
- Bhalodia, A.; Bermea, K.; Schmidt, J.; Gilotra, N.; Barth, A.S.; Adamo, L.; Paik, J.J. Increased risk of myocarditis and arrythmias in anti-Ku-positive scleroderma-myositis overlap patients: A case series. Rheumatology 2024, 63, e268–e269. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Martins, P.; Santos, B.; Costa, E.; Santos, F.C.; Freitas, R.; Faria, M.; Martins, F.; Rodrigues, T.; Santiago, T.; et al. Anti-Ku antibody syndrome: Is it a distinct clinical entity? A cross-sectional study of 75 patients. Rheumatology 2023, 62, e213–e215. [Google Scholar] [CrossRef] [PubMed]
- Pugashetti, J.V.; Lee, J.S. Overview of Rheumatoid Arthritis-Associated Interstitial Lung Disease and Its Treatment. Semin. Respir. Crit. Care Med. 2024, 45, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Kambouchner, M.; Levy, P.; Nicholson, A.G.; Schubel, K.; Magois, E.; Feuillet, S.; Valeyre, D.; Bernaudin, J.F.; Nunes, H. Prognostic relevance of histological variants in nonspecific interstitial pneumonia. Histopathology 2014, 65, 549–560. [Google Scholar] [CrossRef]
- Enomoto, N.; Sumikawa, H.; Sugiura, H.; Kitani, M.; Tanaka, T.; Hozumi, H.; Fujisawa, T.; Suda, T. Clinical, radiological, and pathological evaluation of "NSIP with OP overlap" pattern compared with NSIP in patients with idiopathic interstitial pneumonias. Respir. Med. 2020, 174, 106201. [Google Scholar] [CrossRef]
- Desai, S.R.; Veeraraghavan, S.; Hansell, D.M.; Nikolakopolou, A.; Goh, N.S.; Nicholson, A.G.; Colby, T.V.; Denton, C.P.; Black, C.M.; du Bois, R.M.; et al. CT features of lung disease in patients with systemic sclerosis: Comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004, 232, 560–567. [Google Scholar] [CrossRef]
- Tansley, S.L.; Li, D.; Betteridge, Z.E.; McHugh, N.J. The reliability of immunoassays to detect autoantibodies in patients with myositis is dependent on autoantibody specificity. Rheumatology 2020, 59, 2109–2114. [Google Scholar] [CrossRef]
- Infantino, M.; Tampoia, M.; Fabris, M.; Alessio, M.G.; Previtali, G.; Pesce, G.; Deleonardi, G.; Porcelli, B.; Musso, M.; Grossi, V.; et al. Combining immunofluorescence with immunoblot assay improves the specificity of autoantibody testing for myositis. Rheumatology 2019, 58, 1239–1244. [Google Scholar] [CrossRef]
- Damoiseaux, J.; Vulsteke, J.B.; Tseng, C.W.; Platteel, A.C.M.; Piette, Y.; Shovman, O.; Bonroy, C.; Hamann, D.; De Langhe, E.; Musset, L.; et al. Autoantibodies in idiopathic inflammatory myopathies: Clinical associations and laboratory evaluation by mono- and multispecific immunoassays. Autoimmun. Rev. 2019, 18, 293–305. [Google Scholar] [CrossRef]
- Picard, C.; Vincent, T.; Lega, J.C.; Hue, S.; Fortenfant, F.; Lakomy, D.; Humbel, R.L.; Goetz, J.; Molinari, N.; Bardin, N.; et al. Heterogeneous clinical spectrum of anti-SRP myositis and importance of the methods of detection of anti-SRP autoantibodies: A multicentric study. Immunol. Res. 2016, 64, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Satoh, M.; Fritzler, M.J. Anti-Ku antibodies: Important points to consider. Ann. Rheum. Dis. 2021, 80, e182. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Allanore, Y.; Alves, M.; Brunborg, C.; Airó, P.; Ananieva, L.P.; Czirják, L.; Guiducci, S.; Hachulla, E.; Li, M.; et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann. Rheum. Dis. 2021, 80, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Montesi, S.B.; Silver, R.M.; Hossain, T.; Macrea, M.; Herman, D.; Barnes, H.; Adegunsoye, A.; Azuma, A.; Chung, L.; et al. Treatment of Systemic Sclerosis-associated Interstitial Lung Disease: Evidence-based Recommendations. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2024, 209, 137–152. [Google Scholar] [CrossRef]
- Johnson, S.R.; Bernstein, E.J.; Bolster, M.B.; Chung, J.H.; Danoff, S.K.; George, M.D.; Khanna, D.; Guyatt, G.; Mirza, R.D.; Aggarwal, R.; et al. 2023 American College of Rheumatology (ACR)/American College of Chest Physicians (CHEST) Guideline for the Treatment of Interstitial Lung Disease in People with Systemic Autoimmune Rheumatic Diseases. Arthritis Rheumatol. 2024, 76, 1182–1200. [Google Scholar] [CrossRef]
- Khanna, D.; Lin, C.J.F.; Furst, D.E.; Goldin, J.; Kim, G.; Kuwana, M.; Allanore, Y.; Matucci-Cerinic, M.; Distler, O.; Shima, Y.; et al. Tocilizumab in systemic sclerosis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2020, 8, 963–974. [Google Scholar] [CrossRef]
- Ebata, E.; Yoshizaki, A.; Oba, K.; Kashiwabara, K.; Ueda, K.; Uemura, Y.; Watadani, T.; Fukasawa, T.; Miura, S.; Yoshizaki-Ogawa, A.; et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): A double-blind, investigator-initiated, randomised, placebo-controlled trial. Lancet Rheumatol. 2021, 3, e489–e497. [Google Scholar] [CrossRef]
- Cottin, V.; Martinez, F.J.; Smith, V.; Walsh, S.L. Multidisciplinary teams in the clinical care of fibrotic interstitial lung disease: Current perspectives. Eur. Respir. Rev. 2022, 31, 220003. [Google Scholar] [CrossRef]

| Sex | Age | Tobacco Smoking | CTD | ANA Titer | ANA Pattern | FVC, % | DLCO, % | CT Pattern | Follow-Up, Yrs | Death | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 62 | 1 | IIM | 1280 | Speckled | 96 | 52 | Indeterm. UIP | 1.1 | N |
| 2 | M | 68 | 1 | SSc | 160 | Speckled | 48 | 28 | NSIP | 0.0 | N |
| 3 | F | 61 | 0 | SM, RA, SjS | 1280 | Speckled | 50 | N/A | Indeterm. UIP | 6.8 | Y |
| 4 | F | 61 | 1 | SSc, RA, SjS | 1280 | Speckled | 106 | 51 | Indeterm. UIP | 11.1 | N |
| 5 | F | 65 | 0 | SSc | 1280 | Speckled | 81 | 23 | NSIP | 11.6 | Y |
| 6 | F | 40 | 1 | SSc | 1280 | Speckled | 96 | 75 | Indeterm. UIP | 9.6 | N |
| 7 | M | 76 | 0 | UCTD, PMR | 1280 | Speckled | 61 | N/A | NSIP/OP | 6.7 | Y |
| 8 | F | 69 | 0 | UCTD | 1600 | Speckled | 81 | 54 | NSIP | 3.5 | Y |
| 9 | M | 59 | 1 | IIM | 1280 | Speckled | 76 | 70 | Indeterm. UIP | 1.7 | N |
| 10 | M | 73 | 0 | UCTD | 1280 | Speckled | 86 | 75 | Probable UIP | 9.9 | N |
| 11 | F | 26 | 0 | RA | 1280 | Speckled | 75 | 77 | Probable UIP | 15.2 | N |
| 12 | F | 55 | 0 | UCTD, SjS | 1280 | Speckled | 55 | 70 | NSIP/OP | 11.8 | N |
| 13 | M | 61 | 0 | SSc | 1280 | Speckled | 33 | 24 | NSIP | 0.0 | Y |
| 14 | F | 66 | 0 | SM | 1600 | Speckled | 87 | 35 | NSIP | 15.3 | Y |
| 15 | M | 45 | 1 | SSc | 1280 | Speckled | 99 | 58 | NSIP | 5.4 | N |
| 16 | M | 75 | 1 | SM | 1280 | Speckled | 90 | 26 | NSIP | 1.5 | Y |
| 17 | M | 50 | 0 | SSc, RA | 1280 | Speckled | 119 | 81 | NSIP | 7.6 | N |
| 18 | F | 66 | 0 | RA, SjS | 1280 | Homogeneous | 133 | 82 | NSIP | 9.2 | N |
| 19 | F | 53 | 1 | SSc | 1280 | Speckled | 79 | 47 | NSIP | 4.1 | N |
| Clinical Autoimmune Disease Diagnosis | N (%) |
| Systemic sclerosis | 8 (42) |
| Rheumatoid arthritis | 5 (26) |
| Sjögren syndrome | 4 (21) |
| Undifferentiated connective tissue disease | 4 (21) |
| Scleromyositis | 3 (14) |
| Idiopathic inflammatory myopathy | 2 (10) |
| Polymyalgia rheumatica | 1 (5) |
| Autoantibodies | N (%) |
| Anti-Ku | 19 (100) |
| Antinuclear antibodies | 19 (100) |
| Typical speckled pattern | 18 (95) |
| Homogeneous pattern | 1 (5) |
| Other autoantibodies | |
| Rheumatoid factor | 4 (21) |
| Anti-SSA | 2 (11) |
| Anti-TRIM21 | 2 (11) |
| Anti-PMScl | 1 (5) |
| Anti-RNA-polymerase-3 | 1 (5) |
| Anti-JO1 | 1 (5) |
| Anti-EJ | 1 (5) |
| Anti-PL7 | 1 (5) |
| Anti-fibrillarin | 1 (5) |
| Anti-chromatin | 1 (5) |
| Anti-NOR90 | 1 (5) |
| Anti-cyclic citrullinated peptide | 1 (5) |
| Mean ± SD or N (%) | |
|---|---|
| Age at ILD diagnosis | 61 ± 11.7 |
| Sex, female | 10 (53%) |
| Medical history | |
| Hypertension | 5 (26%) |
| Atrial fibrillation | 5 (26%) |
| Venous thromboembolism | 4 (21%) |
| Cancer | 4 (21%) |
| Hypothyroidism | 3 (16%) |
| Type 2 diabetes | 2 (11%) |
| Ischemic cardiomyopathy | 3 (16%) |
| Chronic renal failure | 1 (5%) |
| Obstructive sleep apnea syndrome | 1 (5%) |
| Respiratory manifestations | |
| Shortness of breath | 6 (32%) |
| Cough | 4 (21%) |
| Crackles | 10 (53%) |
| Finger clubbing | 1 (5%) |
| Other organ manifestations | |
| Arthralgia | 12 (63%) |
| Myalgia | 6 (32%) |
| Muscle weakness | 6 (32%) |
| Skin ulcers or necrosis | 3 (16%) |
| Calcinosis | 1 (5%) |
| Raynaud’s phenomenon | 11 (58%) |
| Sclerodactyly | 8 (42%) |
| Mouth-opening limitation | 5 (26%) |
| Telangiectasiae | 1 (5%) |
| Gastroesophageal reflux | 7 (37%) |
| Epigastric pain | 3 (16%) |
| Dysphagia | 3 (16%) |
| Pericarditis | 2 (11%) |
| Sicca syndrome | 4 (21%) |
| Peripheral neuropathy | 5 (26%) |
| Parameter | Mean ± SD |
|---|---|
| Forced vital capacity (L) | 3.04 ± 0.82 |
| Forced vital capacity, % of predicted | 82 ± 26 |
| Forced expiratory volume in 1 s | 2.22 L ± 0.89 |
| Forced expiratory volume in 1 s, % of predicted | 80 ± 26 |
| Forced expiratory volume in 1 s/forced vital capacity, absolute | 0.80 ± 0.096 |
| Total lung capacity (L) | 4.27 L ± 1.59 |
| Total lung capacity, % of predicted | 77 ± 23 |
| Diffusion capacity of carbon monoxide, % of predicted | 55 ± 21 |
| Diffusion coefficient, % of predicted | 80 ± 22 |
| CT Features | n (%) |
| Reticulation | 16 (84) |
| Intralobular reticulation | 14 (74) |
| Ground-glass opacities | 9 (68) |
| Traction bronchiectasis | 10 (53) |
| Traction bronchiolectasis | 10 (53) |
| Honeycombing | 3 (16) |
| Cysts | 3 (16) |
| Emphysema | 3 (16) |
| Micronodules | 1 (5) |
| Consolidations | 1 (5) |
| Mediastinal lymphadenopathy | 7 (37) |
| Subpleural sparing | 2 (11) |
| CT patterns | |
| Nonspecific interstitial pneumonia | 10 (53) |
| Nonspecific interstitial pneumonia/organizing pneumonia | 2 (11) |
| Indeterminate for usual interstitial pneumonia | 5 (26) |
| Probable usual interstitial pneumonia | 2 (11) |
| Definite usual interstitial pneumonia | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petitgrand, L.; Ahmad, K.; Gamondès, D.; Diesler, R.; Fabien, N.; Gallay, L.; Fort, R.; Traclet, J.; Lestelle, F.; Chapurlat, R.; et al. Interstitial Lung Disease Associated with Anti-Ku Antibodies: A Case Series of 19 Patients. J. Clin. Med. 2025, 14, 247. https://doi.org/10.3390/jcm14010247
Petitgrand L, Ahmad K, Gamondès D, Diesler R, Fabien N, Gallay L, Fort R, Traclet J, Lestelle F, Chapurlat R, et al. Interstitial Lung Disease Associated with Anti-Ku Antibodies: A Case Series of 19 Patients. Journal of Clinical Medicine. 2025; 14(1):247. https://doi.org/10.3390/jcm14010247
Chicago/Turabian StylePetitgrand, Laure, Kaïs Ahmad, Delphine Gamondès, Rémi Diesler, Nicole Fabien, Laure Gallay, Romain Fort, Julie Traclet, François Lestelle, Roland Chapurlat, and et al. 2025. "Interstitial Lung Disease Associated with Anti-Ku Antibodies: A Case Series of 19 Patients" Journal of Clinical Medicine 14, no. 1: 247. https://doi.org/10.3390/jcm14010247
APA StylePetitgrand, L., Ahmad, K., Gamondès, D., Diesler, R., Fabien, N., Gallay, L., Fort, R., Traclet, J., Lestelle, F., Chapurlat, R., Confavreux, C. B., Durupt, S., Turquier, S., Si-Mohamed, S. A., Coutant, F., & Cottin, V. (2025). Interstitial Lung Disease Associated with Anti-Ku Antibodies: A Case Series of 19 Patients. Journal of Clinical Medicine, 14(1), 247. https://doi.org/10.3390/jcm14010247







