Ultrasonographic Evaluation of Solid Organ Sizes in Children with Primary Malnutrition: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of Malnutrition
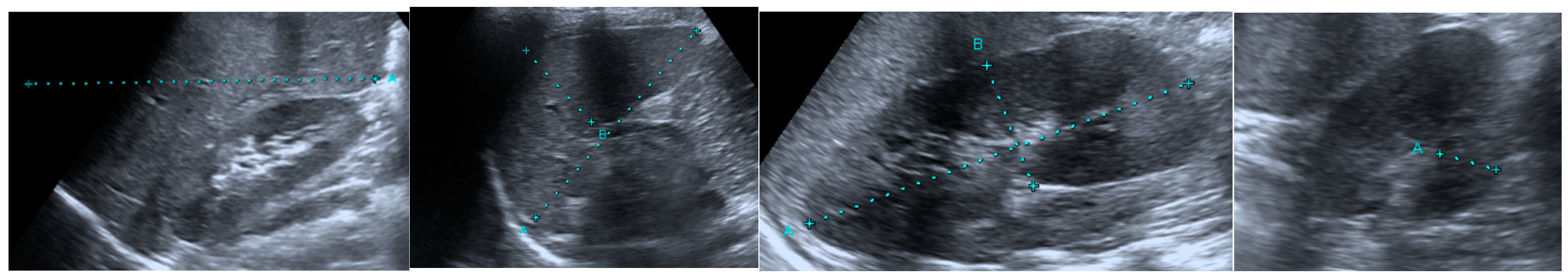
2.2. Ultrasonographic Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Şükrü, G.; Doğan, A. Diaphragm thickness by ultrasound in pediatric patients with primary malnutrition. Eur. J. Pediatr. 2023, 182, 3347–3354. [Google Scholar] [CrossRef]
- Raj, K.O. Determinants of malnutrition among children: A systematic review. Nutrition 2022, 96, 111565. [Google Scholar] [CrossRef]
- Koletzko, B. Pediatric Nutrition in Practice. In World Review Nutrition Dietetics; Karger: Basel, Switzerland, 2015; Volume 113, pp. 139–146. [Google Scholar]
- Valeria, D.; Cucinotta, U.; Romano, C. Acute malnutrition in children: Pathophysiology, clinical effects and treatment. Nutrients 2020, 12, 2413. [Google Scholar] [CrossRef] [PubMed]
- Mamoun, N.; Homedia, S.; Mabyou, M.; Hussan, M.; Muntasir, A.; Salah, T.; Adam, I. Prevalence, types and risk factors for malnutrition in displaced Sudanese children. Am. J. Infect. Dis. 2005, 1, 84–86. [Google Scholar] [CrossRef]
- Grover, Z.; Ee, L.C. Protein energy malnutrition. Pediatr. Clin. N. Am. 2009, 56, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Nalwanga, D.; Musiime, V.; Kizito, S.; Kiggundu, J.B.; Batte, A.; Musoke, P.; Tumwine, J.K. Mortality among children under five years admitted for routine care of severe acute malnutrition: A prospective cohort study from Kampala, Uganda. BMC Pediatr. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rytter, M.J.; Cichon, B.; Fabiansen, C.; Yameogo, C.W.; Windinmi, S.Z.; Michaelsen, K.F.; Filteau, S.; Jeppesen, D.L.; Friis, H.; Briend, A. Thymus size in children with moderate malnutrition: A cohort study from Burkina Faso. Pediatr. Res. 2021, 89, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Luk, W.H.; Lo, A.X.N.; Au-Yeung, A.W.S.; Liu, K.K.Y.; Woo, Y.H.; Chiang, C.C.L.; Lo, K.K.L. Renal length nomogram in Hong Kong Asian children: Sonographic measurement and multivariable approach. J. Paediatr. Child Health 2010, 46, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Coombs, P.R.; Lavender, I.; Leung, M.Y.; Woods, J.C.; Paul, E.; Webb, N.; Ditchfield, M. Normal sonographic renal length measurements in an Australian pediatric population. Pediatr. Radiol. 2019, 49, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Bouma, S. Diagnosing Pediatric Malnutrition. Nutr. Clin. Pract. 2017, 32, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Corkins, M.R.; Lyman, B.; Malone, A.; Goday, P.S.; Carney, L.; Monczka, J.L.; Plogsted, S.W.; Schwenk, W.F.; American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors. Defining pediatric malnutrition: A paradigm shift toward etiology-related definitions. J. Parenter. Enter. Nutr. 2013, 37, 460–481. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Berkley, J.A.; Bandsma, R.H.; Kerac, M.; Trehan, I.; Briend, A. Severe childhood malnutrition. Nat. Rev. Dis. Primers 2017, 3, 1–18. [Google Scholar] [CrossRef]
- Wu, W.-T.; Chang, K.-V.; Hsu, Y.-C.; Hsu, P.-C.; Ricci, V.; Özçakar, L. Artifacts in musculoskeletal ultrasonography: From physics to clinics. Diagnostics 2020, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, M.; De Robertis, R.; Demozzi, E.; Crosara, S.; Canestrini, S.; Mucelli, R.P. Liver volumetry: Is imaging reliable? Personal experience and review of the literature. World J. Radiol. 2014, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.T.; Esterman, A.J.; Thoirs, K.A. Ultrasound measurements of the liver: An intra and inter-rater reliability study. Australas. J. Ultrasound Med. 2014, 17, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.T.; Thoirs, K.A.; Esterman, A.J. The development of a practical and uncomplicated predictive equation to determine liver volume from simple linear ultrasound measurements of the liver. Radiography 2016, 22, e125–e130. [Google Scholar] [CrossRef]
- Safak, A.A.; Simsek, E.; Bahcebasi, T. Sonographic assessment of the normal limits and percentile curves of liver, spleen, and kidney dimensions in healthy school-aged children. J. Ultrasound Med. 2005, 24, 1359–1364. [Google Scholar] [CrossRef]
- Mohtasib, R.S.; Alshamiri, K.M.; Jobeir, A.A.; Saidi, F.M.A.; Masawi, A.M.; Alabdulaziz, L.S.; Hussain, F.Z.B. Sonographic measurements for kidney length in normal Saudi children: Correlation with other body parameters. Ann. Saudi Med. 2019, 39, 143–154. [Google Scholar] [CrossRef]
- Santos, E.W.B.; Dias, C.; Silva, G.B.; Oliveira, D.C.; Beltran, J.S.O.; Hastreiter, A.B.; Paredes-Gamero, E.J.; Borelli, P.B. Study of hematopoiesis in protein restriction: Characterization of bone marrow stromal cells. FASEB J. 2016, 30, 915–923. [Google Scholar] [CrossRef]
- Prasetyo, R.H.; Hestianah, E.P. Honey can repairing damage of liver tissue due to protein energy malnutrition through induction of endogenous stem cells. Vet. World 2017, 10, 711–715. [Google Scholar] [CrossRef]
- Ortiz, R.; Cortes, L.; Cortes, E.; Medina, H. Malnutrition alters the rates of apoptosis in splenocytes and thymocyte subpopulations of rats. Clin. Exp. Immunol. 2009, 155, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.S.; de Oliveira, D.C.; Bizzarro, B.; Sá-Nunes, A.; Hastreiter, A.A.; de Oliveira Beltran, J.S.; Xavier, J.G.; Borelli, P.; Fock, R.A. Protein malnutrition alters spleen cell proliferation and IL-2 and IL-10 production by affecting the STAT-1 and STAT-3 balance. Inflammation 2014, 37, 2125–2138. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Koda, M.; Sugihara, T.; Sugihara, S.; Okamoto, T.; Miyoshi, K.; Hodotsuka, M.; Fujise, Y.; Matono, T.; Okano, J. Left renal cortical thickness measured by ultrasound can predict early progression of chronic kidney disease. Nephron 2016, 132, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bartels, R.H.; Meyer, S.L.; Stehmann, T.A.; Bourdon, C.; Bandsma, R.H.J.; Voskuijl, W.P. Both Exocrine Pancreatic Insufficiency and Signs of Pancreatic Inflammation Are Prevalent in Children with Complicated Severe Acute Malnutrition: An Observational Study. J. Pediatr. 2016, 174, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Campanozzi, A.; Russo, M.; Catucci, A.; Rutigliano, I.; Canestrino, G.; Giardino, I.; Romondia, A.; Pettoello-Mantovani, M. Hospital-acquired malnutrition in children with mild clinical conditions. Nutrition 2009, 25, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Calle-Toro, J.S.; Back, S.J.; Viteri, B.; Andronikou, S.; Kaplan, S.L. Liver, spleen, and kidney size in children as measured by ultrasound: A systematic review. J. Ultrasound Med. 2020, 39, 223–230. [Google Scholar] [CrossRef]
- Fatunla, O.A.; Olatunya, O.S.; Ogundare, E.O.; Fatunla, T.O.; Olatayo, A.S.; Taiwo, A.B.; Ipinnimo, T.M.; Oyebanji, A.H.; Akanbi, G.O.; Oyelami, O.A. Relationship between ultrasound-measured spleen, liver and anthropometry of children living in a rural community in southwest Nigeria: A cross-sectional study. Pediatr. Radiol. 2022, 52, 1–8. [Google Scholar] [CrossRef]
- Saari, A.; Sankilampi, U.; Hannila, M.-L.; Kiviniemi, V.; Kesseli, K.; Dunkel, L. New Finnish growth references for children and adolescents aged 0 to 20 years: Length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann. Med. 2011, 43, 235–248. [Google Scholar] [CrossRef]
- Phillips, C.A.; Bailer, J.; Foster, E.; Li, Y.; Dogan, P.; Smith, E.; Reilly, A.; Freedman, J. Evaluation of an automated pediatric malnutrition screen using anthropometric measurements in the electronic health record: A quality improvement initiative. Support. Care Cancer 2020, 28, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Konrad, J.; Grand, D.; Lourenco, A. MRI: First-line imaging modality for pregnant patients with suspected appendicitis. Abdom. Imaging 2015, 40, 3359–3364. [Google Scholar] [CrossRef]

| Control (68) | Patient (122) | p | ||
|---|---|---|---|---|
| Right kidney | Length | 85.58 ± 15.98 | 71.95 ± 19.58 | <0.001 |
| Thickness | 11.35 ± 3.33 | 9.93 ± 2.97 | 0.004 | |
| Left kidney | Length | 89.24 ± 16.37 | 76.75 ± 19.30 | <0.001 |
| Thickness | 12.68 ± 5.23 | 11.57 ± 3.56 | 0.095 | |
| Spleen | Length | 94.72 ± 20.53 | 75.58 ± 19.01 | <0.001 |
| Liver | Length | 119.92 ± 26.29 | 99.84 ± 19.99 | <0.001 |
| Healthy | Malnutrition | p | ||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| Age | 9.35 ± 4.62 | 8.81 ± 5.31 | 8.98 ± 5.18 | 9.45 ± 5.67 | 0.941 | |
| Z-score | Weight | 0.29 ± 0.92 | −1.56 ± 0.28 | −2.45 ± 0.28 | −3.84 ± 0.85 | <0.001 |
| Height | 0.25 ± 0.88 | −0.98 ± 1.04 | −1.45 ± 1.10 | −2.66 ± 1.23 | <0.001 | |
| BMI | 0.14 ± 1.18 | −1.37 ± 0.83 | −2.15 ± 1.31 | −2.96 ± 1.76 | <0.001 | |
| Right kidney | Length | 85.57 ± 15.98 | 74.09 ± 21.12 | 72.26 ± 18.07 | 68.13 ± 21.67 | <0.001 |
| Thickness | 11.34 ± 3.33 | 9.75 ± 2.95 | 10.11 ± 3.19 | 9.69 ± 2.39 | 0.033 | |
| Left kidney | Length | 89.23 ± 16.36 | 78.53 ± 19.51 | 76.76 ± 18.44 | 74.14 ± 21.96 | <0.001 |
| Thickness | 12.68 ± 5.23 | 11.23 ± 3.28 | 11.90 ± 3.92 | 11.11 ± 2.82 | 0.303 | |
| Spleen | Length | 94.73 ± 20.53 | 77.65 ± 19.35 | 76.90 ± 18.78 | 69.04 ± 18.60 | <0.001 |
| Liver | Length | 119.92 ± 26.29 | 97.68 ± 21.32 | 101.34 ± 19.76 | 98.65 ± 19.26 | <0.001 |
| Z-Score | |||||
|---|---|---|---|---|---|
| Weight | Height | BMI | |||
| N | 190 | 190 | 190 | ||
| Right kidney | Length | Pearson Correlation | 0.387 ** | 0.466 ** | 0.219 ** |
| p | 0.000 | 0.000 | 0.003 | ||
| Thickness | Pearson Correlation | 0.270 ** | 0.274 ** | 0.205 ** | |
| p | 0.000 | 0.000 | 0.005 | ||
| Left kidney | Length | Pearson Correlation | 0.372 ** | 0.461 ** | 0.178 * |
| p | 0.000 | 0.000 | 0.016 | ||
| Thickness | Pearson Correlation | 0.176 * | 0.237 ** | 0.073 | |
| p | 0.018 | 0.001 | 0.326 | ||
| Spleen | Length | Pearson Correlation | 0.500 ** | 0.463 ** | 0.352 ** |
| p | 0.000 | 0.000 | 0.000 | ||
| Liver | Length | Pearson Correlation | 0.426 ** | 0.441 ** | 0.232 ** |
| p | 0.000 | 0.000 | 0.002 | ||
| Cut-Off Point for Malnutrition | Sensitivity | Specificity | AUC | 95% C.I. | p | |
|---|---|---|---|---|---|---|
| Right kidney length * | ≤65.5 | 0.846 | 0.488 | 0.687 | 0.609–0.765 | <0.001 |
| Left kidney length * | ≤72.5 | 0.785 | 0.492 | 0.666 | 0.585–0.747 | <0.001 |
| Spleen length * | ≤84.5 | 0.692 | 0.727 | 0.723 | 0.648–0.797 | <0.001 |
| Liver length * | ≤110.5 | 0.600 | 0.719 | 0.681 | 0.599–0.762 | <0.001 |
| Right kidney parenchyme thicknes * | ≤7.75 | 0.892 | 0.314 | 0.604 | 0.519–0.689 | 0.019 |
| Risk Factors | OD | 95% C.I. | p | |
|---|---|---|---|---|
| Lower | Upper | |||
| Right kidney length (≤65.5 mm) | 5.323 | 2.485 | 11.399 | <0.001 |
| Left kidney length (≤72.5 mm) | 3.643 | 1.828 | 7.261 | <0.001 |
| Spleen lenght (≤84.5 mm | 6.068 | 3.133 | 11.753 | <0.001 |
| Liver lenght (≤110.5 mm) | 3.882 | 2.058 | 7.324 | <0.001 |
| Right kidney parenchyme thicknes (≤7.75 mm) | 3.893 | 1.628 | 9.308 | 0.002 |
| Statistics: Binary Logistic regression analysis | ||||
| Risk Factors | OD | 95% C.I. | p | |
| Lower | Lower | |||
| Right kidney length (≤65.5 mm) | 3.237 | 0.910 | 0.910 | 0.070 |
| Left kidney length (≤72.5 mm) | 0.426 | 0.113 | 0.113 | 0.207 |
| Spleen lenght (≤84.5 mm | 4.058 | 1.565 | 1.565 | 0.004 |
| Liver lenght (≤110.5 mm) | 1.403 | 0.581 | 0.581 | 0.452 |
| Right kidney parenchyme thicknes (≤7.75 mm) | 1.332 | 0.466 | 0.466 | 0.592 |
| Statistics: Multivariate Logistic Regression analysis | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doğan, K.; Güngör, Ş.; Doğan, A.; Karaküçük, S.N. Ultrasonographic Evaluation of Solid Organ Sizes in Children with Primary Malnutrition: A Preliminary Study. J. Clin. Med. 2025, 14, 169. https://doi.org/10.3390/jcm14010169
Doğan K, Güngör Ş, Doğan A, Karaküçük SN. Ultrasonographic Evaluation of Solid Organ Sizes in Children with Primary Malnutrition: A Preliminary Study. Journal of Clinical Medicine. 2025; 14(1):169. https://doi.org/10.3390/jcm14010169
Chicago/Turabian StyleDoğan, Kamil, Şükrü Güngör, Adil Doğan, and Seda Nida Karaküçük. 2025. "Ultrasonographic Evaluation of Solid Organ Sizes in Children with Primary Malnutrition: A Preliminary Study" Journal of Clinical Medicine 14, no. 1: 169. https://doi.org/10.3390/jcm14010169
APA StyleDoğan, K., Güngör, Ş., Doğan, A., & Karaküçük, S. N. (2025). Ultrasonographic Evaluation of Solid Organ Sizes in Children with Primary Malnutrition: A Preliminary Study. Journal of Clinical Medicine, 14(1), 169. https://doi.org/10.3390/jcm14010169







