Cumulative Burden of Fatty Liver and Kidney Cancer in Young Men: A National Population-Based Study
Abstract
1. Introduction
2. Methods
2.1. Data Source
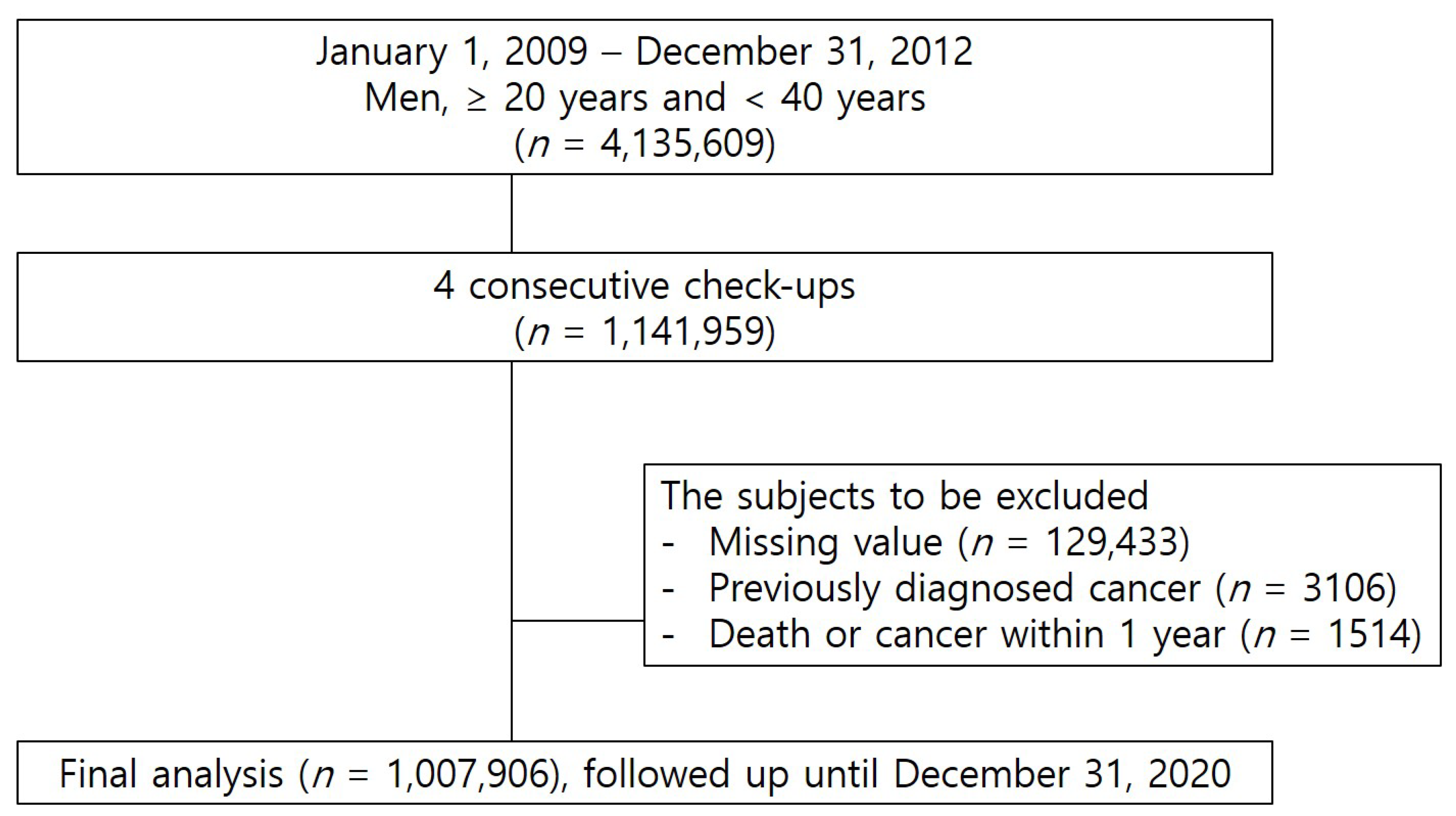
2.2. Study Population
2.3. Definition
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Study Population
3.2. Cumulative Burden of NAFLD and Kidney Cancer
3.3. Changes in NAFLD and Kidney Cancer
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.Y.; Han, K.-D.; Woo, I.S.; Kwon, H.-S. Association of Metabolic Syndrome Components and Nutritional Status with Kidney Cancer in Young Adult Population: A Nationwide Population-Based Cohort Study in Korea. Biomedicines 2023, 11, 1425. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, C.; Suo, C.; Zhao, R.; Jin, L.; Zhang, T.; Chen, X. Metabolic dysfunction-associated fatty liver disease and the risk of 24 specific cancers. Metabolism 2022, 127, 154955. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: A meta-analysis of observational cohort studies. Gut 2022, 71, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, I.S.; Han, K.D.; Park, H.; Kim, K.H.; Kim, J.S. Association Between Fatty Liver Index and Risk of Breast Cancer: A Nationwide Population-Based Study. Clin. Breast Cancer 2020, 20, e450–e457. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.; Lattuada, G.; Ragogna, F.; Garancini, M.P.; Crosignani, P.; Villa, M.; Bosi, E.; Ruotolo, G.; Piemonti, L.; Perseghin, G. Fatty liver index and mortality: The Cremona study in the 15th year of follow-up. Hepatology 2011, 54, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Palombo, C.; Eng, M.P.; Dekker, J.; Flyvbjerg, A.; Mitrakou, A.; Gastaldelli, A.; Ferrannini, E. Fatty liver index, gamma-glutamyltransferase, and early carotid plaques. Hepatology 2012, 55, 1406–1415. [Google Scholar] [CrossRef]
- Lee, Y.H.; Han, K.; Ko, S.H.; Ko, K.S.; Lee, K.U. Data Analytic Process of a Nationwide Population-Based Study Using National Health Information Database Established by National Health Insurance Service. Diabetes Metab. J. 2016, 40, 79–82. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.A.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Sullman, M.J.M.; Bettampadi, D.; Qorbani, M.; Moradi-Lakeh, M.; Ardalan, M.; et al. The burden of kidney cancer and its attributable risk factors in 195 countries and territories, 1990-2017. Sci. Rep. 2020, 10, 13862. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.F.; Leitzmann, M.F.; Albanes, D.; Kipnis, V.; Moore, S.C.; Schatzkin, A.; Chow, W.H. Body size and renal cell cancer incidence in a large US cohort study. Am. J. Epidemiol. 2008, 168, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Du, X.; Zou, S.Y.; Shi, B.M. Blood pressure and kidney cancer risk: Meta-analysis of prospective studies. J. Hypertens. 2017, 35, 1333–1344. [Google Scholar] [CrossRef]
- Neovius, M.; Sundström, J.; Rasmussen, F. Combined effects of overweight and smoking in late adolescence on subsequent mortality: Nationwide cohort study. BMJ 2009, 338, b496. [Google Scholar] [CrossRef] [PubMed]
- Landberg, A.; Fält, A.; Montgomery, S.; Sundqvist, P.; Fall, K. Overweight and obesity during adolescence increases the risk of renal cell carcinoma. Int. J. Cancer 2019, 145, 1232–1237. [Google Scholar] [CrossRef]
- Scelo, G.; Larose, T.L. Epidemiology and Risk Factors for Kidney Cancer. J. Clin. Oncol. 2018, 36, Jco2018791905. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Hong, S.; Ahn, H.Y.; Park, C.Y. Metabolic Dysfunction-Associated Fatty Liver Disease and Mortality: A Population-Based Cohort Study. Diabetes Metab. J. 2023, 47, 220–231. [Google Scholar] [CrossRef]
- Park, J.-H.; Hong, J.Y.; Shen, J.J.; Han, K.; Park, J.O.; Park, Y.S.; Lim, H.Y. Increased Risk of Young-Onset Digestive Tract Cancers Among Young Adults Age 20-39 Years With Nonalcoholic Fatty Liver Disease: A Nationwide Cohort Study. J. Clin. Oncol. 2023, 41, 3363–3373. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Hicks, S.B.; Mara, K.C.; Larson, J.J.; Therneau, T.M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—A longitudinal cohort study. J. Hepatol. 2019, 71, 1229–1236. [Google Scholar] [CrossRef]
- Huang, Y.; He, S.; Li, J.Z.; Seo, Y.K.; Osborne, T.F.; Cohen, J.C.; Hobbs, H.H. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc. Natl. Acad. Sci. USA 2010, 107, 7892–7897. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Manjunath, H.; Yopp, A.C.; Beg, M.S.; Marrero, J.A.; Gopal, P.; Waljee, A.K. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: A meta-analysis. Am. J. Gastroenterol. 2014, 109, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Han, K.D.; Lee, Y.H.; Kim, K.S.; Hong, S.; Park, J.H.; Park, C.Y. Fatty Liver & Diabetes Statistics in Korea: Nationwide Data 2009 to 2017. Diabetes Metab. J. 2023, 47, 347–355. [Google Scholar] [PubMed]
| n (%) | Cumulative Frequency of Fatty Liver Index (FLI) ≥ 60 During 4 Consecutive Check-Ups | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | p | ||
| 723,600 | 86,646 | 58,010 | 53,589 | 86,061 | |||
| Smoker | Non- | 241,154 (33.33) | 21,322 (24.61) | 13,124 (22.62) | 11,613 (21.67) | 16,398 (19.05) | <0.0001 |
| Ex- | 138,444 (19.13) | 17,772 (20.51) | 11,656 (20.09) | 10,298 (19.22) | 14,891 (17.3) | ||
| Current | 344,002 (47.54) | 47,552 (54.88) | 33,230 (57.28) | 31,678 (59.11) | 54,772 (63.64) | ||
| Alcohol consumption | No | 186,117 (25.72) | 16,783 (19.37) | 10,683 (18.42) | 9633 (17.98) | 14,981 (17.41) | <0.0001 |
| Mild | 468,250 (64.71) | 55,822 (64.43) | 37,035 (63.84) | 34,039 (63.52) | 53,410 (62.06) | ||
| Heavy | 69,233 (9.57) | 14,041 (16.21) | 10,292 (17.74) | 9917 (18.51) | 17,670 (20.53) | ||
| Regular exercise (high-intensity) | 138,651 (19.16) | 16,851 (19.45) | 11,215 (19.33) | 10,380 (19.37) | 14,834 (17.24) | <0.0001 | |
| BMI, kg/m2 | 23.09 ± 2.45 | 26.09 ± 2.25 | 27.02 ± 2.39 | 27.93 ± 2.59 | 29.94 ± 3.3 | <0.0001 | |
| WC, cm | 79.87 ± 6.37 | 87.05 ± 5.98 | 89.15 ± 6.32 | 91.08 ± 6.73 | 95.64 ± 8.07 | <0.0001 | |
| Fasting glucose, mg/dL | 91.51 ± 12.94 | 95.05 ± 17.53 | 96.51 ± 19.51 | 98.3 ± 22.61 | 103.35 ± 30.23 | <0.0001 | |
| Total cholesterol, mg/dL | 186.73 ± 31.8 | 202.87 ± 33.99 | 206.32 ± 35.02 | 209.25 ± 36.05 | 214.28 ± 37.43 | <0.0001 | |
| HDL, mg/dL | 54.63 ± 17.61 | 49.66 ± 15.48 | 48.74 ± 16.3 | 47.74 ± 14.39 | 46.38 ± 14.98 | <0.0001 | |
| LDL, mg/dL | 109.63 ± 30.9 | 117.58 ± 35.51 | 119.15 ± 36.34 | 120.23 ± 37.58 | 120.97 ± 40.11 | <0.0001 | |
| Triglyceride, mg/dL | 102.41 (102.29–102.53) | 167.11 (166.52–167.7) | 182.52 (181.76–183.29) | 198.04 (197.18–198.91) | 233.83 (233.05–234.62) | <0.0001 | |
| FLI | n | Event | IR, per 1000 PY | HR (95% CI) | |
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| <30 | 535,979 | 237 | 0.06566 | 1 (Ref.) | 1 (Ref.) |
| 30–60 | 268,573 | 183 | 0.10054 | 1.528 (1.260, 1.853) | 1.447 (1.191, 1.757) |
| ≥60 | 203,354 | 229 | 0.16759 | 2.559 (2.134, 3.069) | 2.391 (1.986, 2.879) |
| p-value | <0.0001 | <0.0001 | |||
| n | Event | IR, per 1000 PY | HR (95% CI) | ||
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| 0 | 723,600 | 354 | 0.0726 | 1 (Ref.) | 1 (Ref.) |
| 1 | 86,646 | 60 | 0.10261 | 1.414 (1.075, 1.858) | 1.353 (1.028, 1.781) |
| 2 | 58,010 | 60 | 0.15339 | 2.114 (1.608, 2.780) | 2.006 (1.524, 2.642) |
| 3 | 53,589 | 57 | 0.15767 | 2.173 (1.643, 2.875) | 2.029 (1.531, 2.689) |
| 4 | 86,061 | 118 | 0.2025 | 2.788 (2.264, 3.434) | 2.541 (2.055, 3.142) |
| p-value | <0.0001 | <0.0001 | |||
| FLI Score | n | Event | IR, per 1000 PY | HR (95% CI) | |
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| 0 | 421,811 | 164 | 0.05793 | 1 (Ref.) | 1 (Ref.) |
| 1 | 109,655 | 61 | 0.08278 | 1.429 (1.065, 1.917) | 1.394 (1.038, 1.871) |
| 2 | 83,075 | 38 | 0.06776 | 1.168 (0.820, 1.662) | 1.120 (0.786, 1.595) |
| 3 | 76,315 | 54 | 0.10447 | 1.798 (1.322, 2.445) | 1.693 (1.244, 2.306) |
| 4 | 75,577 | 63 | 0.12222 | 2.097 (1.568, 2.804) | 1.930 (1.440, 2.585) |
| 5 | 56,192 | 49 | 0.1284 | 2.207 (1.604, 3.037) | 2.038 (1.478, 2.811) |
| 6 | 49,218 | 47 | 0.14109 | 2.430 (1.757, 3.361) | 2.247 (1.621, 3.115) |
| 7 | 50,002 | 55 | 0.16284 | 2.807 (2.068, 3.810) | 2.575 (1.892, 3.505) |
| 8 | 86,061 | 118 | 0.2025 | 3.489 (2.754, 4.420) | 3.143 (2.467, 4.004) |
| p-value | <0.0001 | <0.0001 | |||
| Initial FLI/Last FLI | n | Event | IR, per 1000 PY | HR (95% CI) | |
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| <60/<60 | 768,443 | 391 | 0.07547 | 1 (Ref.) | 1 (Ref.) |
| <60/≥60 | 91,112 | 76 | 0.12553 | 1.676 (1.311, 2.143) | 1.653 (1.291, 2.117) |
| ≥60/<60 | 36,109 | 29 | 0.1165 | 1.529 (1.049, 2.230) | 1.374 (0.941, 2.007) |
| ≥60/≥60 | 112,242 | 153 | 0.20105 | 2.662 (2.208, 3.209) | 2.430 (2.008, 2.941) |
| p-value | <0.0001 | <0.0001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.Y.; Han, K.D.; Kwon, H.-S. Cumulative Burden of Fatty Liver and Kidney Cancer in Young Men: A National Population-Based Study. J. Clin. Med. 2025, 14, 148. https://doi.org/10.3390/jcm14010148
Lee HY, Han KD, Kwon H-S. Cumulative Burden of Fatty Liver and Kidney Cancer in Young Men: A National Population-Based Study. Journal of Clinical Medicine. 2025; 14(1):148. https://doi.org/10.3390/jcm14010148
Chicago/Turabian StyleLee, Hee Yeon, Kyung Do Han, and Hyuk-Sang Kwon. 2025. "Cumulative Burden of Fatty Liver and Kidney Cancer in Young Men: A National Population-Based Study" Journal of Clinical Medicine 14, no. 1: 148. https://doi.org/10.3390/jcm14010148
APA StyleLee, H. Y., Han, K. D., & Kwon, H.-S. (2025). Cumulative Burden of Fatty Liver and Kidney Cancer in Young Men: A National Population-Based Study. Journal of Clinical Medicine, 14(1), 148. https://doi.org/10.3390/jcm14010148





