Evaluating Anesthesia Guidance for Rescue Analgesia in Awake Patients Undergoing Carotid Endarterectomy with Cervical Plexus Blocks: Preliminary Findings from a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ultrasound Examination of Cerebral Circulation
2.3. Eversion Carotid Endarterectomy
2.3.1. STAGE 1
SUPERFICIAL CERVICAL PLEXUS BLOCK
INTERMEDIATE CERVICAL PLEXUS BLOCK
DEEP CERVICAL PLEXUS BLOCK
CASI
SKIN INFILTRATION
2.3.2. STAGE 2
2.3.3. STAGE 3 (INTRAOPERATIVELY)
2.3.4. STAGE 4
2.4. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirchhoff, F.; Eckstein, H.-H. Munich carotid investigation group Locoregional Anaesthesia and Intra-Operative Angiography in Carotid Endarterectomy: 16 Year Results of a Consecutive Single Centre Series. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sidawy, A.; Nguyen, B.-N. Locoregional Versus General Anesthesia in Prolonged Carotid Endarterectomy: A Propensity Score-Matched Study from the American College of Surgeons National Surgical Quality Improvement Program Database from 2011 to 2022. Ann. Vasc. Surg. 2025, 110, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Stoneham, M.D.; Stamou, D.; Mason, J. Regional Anaesthesia for Carotid Endarterectomy. Br. J. Anaesth. 2015, 114, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Mądro, P.; Dąbrowska, A.; Jarecki, J.; Garba, P. Anaesthesia for Carotid Endarterectomy. Ultrasound-Guided Superficial/Intermediate Cervical Plexus Block Combined with Carotid Sheath Infiltration. Anaesthesiol. Intensive Ther. 2016, 48, 234–238. [Google Scholar] [CrossRef]
- Lamperti, M.; Romero, C.S.; Guarracino, F.; Cammarota, G.; Vetrugno, L.; Tufegdzic, B.; Lozsan, F.; Macias Frias, J.J.; Duma, A.; Bock, M.; et al. Preoperative Assessment of Adults Undergoing Elective Noncardiac Surgery: Updated Guidelines from the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2025, 42, 1. [Google Scholar] [CrossRef]
- Vega Colón, M.; López González, J.M.; Jiménez Gómez, B.M.; Pico Veloso, J.; Fernández Mendez, M.; Fernández Suárez, F.E.; Del Castro Madrazo, J.A.; Álvarez Marcos, F.; Fajardo Pérez, M.; Lin, J.-A.; et al. Prospective Observational Study after Eversion Carotid Endarterectomy with Ultrasound-Guided Deep-Intermediate Cervical Plexus Blockade. Healthcare 2022, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.; Missir, A.; Pluta, A.; Szumera, I.; Stasiak, M.; Szopa, W.; Błaszczyk, B.; Możdżyński, B.; Majchrzak, K.; Tymowski, M.; et al. Influence of Infiltration Anaesthesia on Perioperative Outcomes Following Lumbar Discectomy under Surgical Pleth Index-Guided General Anaesthesia: A Preliminary Report from a Randomised Controlled Prospective Trial. Adv. Med. Sci. 2020, 65, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Kalgudi, P.; Sriganesh, K. Ultrasound-Guided Erector Spinae Plane Block for Perioperative Analgesia in Cervical and Thoracic Spine Surgeries—A Case Series. Neurol. India 2021, 69, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.D.; Lee, Y.; Choi, S.; Lee, H.; Ohn, C.; Kwon, W. Abdominal Wall Block Decreases Intraoperative Opioid Con-Sumption by Surgical Pleth Index-Guided Remifentanil Administration in Single-Port Laparoscopic Herniorrhaphy: A Prospective Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 16012. [Google Scholar] [CrossRef]
- Choi, J.J.; Jo, Y.Y.; Kim, S.H.; Jung, W.S.; Lee, D.; Kim, K.Y.; Kwak, H.J. Remifentanil-Sparing Effect of Pectoral Nerve Block Type II in Breast Surgery under Surgical Pleth Index-Guided Analgesia during Total Intravenous Anesthesia. J. Clin. Med. 2019, 8, 1181. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Kawka, M.; Krawczyk, L.; Niewiadomska, E.; Dobrowolski, D.; Rejdak, R.; Król, S.; Żak, J.; et al. Preventive Analgesia, Hemodynamic Stability, and Pain in Vitreoretinal Surgery. Medicina 2021, 57, 262. [Google Scholar] [CrossRef] [PubMed]
- Magyar-Stang, R.; Pál, H.; Csányi, B.; Gaál, A.; Mihály, Z.; Czinege, Z.; Csipo, T.; Ungvari, Z.; Sótonyi, P.; Varga, A.; et al. Assessment of Cerebral Autoregulatory Function and Inter-Hemispheric Blood Flow in Older Adults with Internal Carotid Artery Stenosis Using Transcranial Doppler Sonography-Based Measurement of Transient Hyperemic Response after Carotid Artery Compression. Geroscience 2023, 45, 3333–3357. [Google Scholar] [CrossRef]
- Franjić, B.D.; Lovričević, I.; Brkić, P.; Dobrota, D.; Aždajić, S.; Hranjec, J. Role of Doppler Ultrasound Analysis of Blood Flow Through the Ophthalmic and Intracranial Arteries in Predicting Neurologic Symptoms During Carotid Endarterectomy. J. Ultrasound Med. 2021, 40, 2141–2156. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.A.F.; Fahmy, A.M.; Fathi, H.M.; Elmesallamy, W.A.E.A.; Khalifa, O.Y.A. Regional Analgesia Using Ultrasound-Guided Intermediate Cervical Plexus Block versus Cervical Erector Spinae Block for Anterior Cervical Spine Surgery: A Randomized Trial. BMC Anesth. Anesthesiol. 2024, 24, 153. [Google Scholar] [CrossRef]
- Misiołek, H.; Cettler, M.; Woroń, J.; Wordliczek, J.; Dobrogowski, J.; Mayzner-Zawadzka, E. The 2014 Guidelines for Post-Operative Pain Management. Anaesthesiol. Intensive Ther. 2014, 46, 221–244. [Google Scholar] [CrossRef]
- Viderman, D.; Ben-David, B.; Sarria-Santamera, A. Analysis of Bupivacaine and Ropivacaine-Related Cardiac Arrests in Regional Anesthesia: A Systematic Review of Case Reports. Rev. Esp. Anestesiol. Reanim. (Engl. Ed.) 2021, 68, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.S.; Sundara Rajan, R.; Roberts, A.M. The Cervical Plexus. BJA Educ. 2023, 23, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Martusevicius, R.; Swiatek, F.; Joergensen, L.G.; Nielsen, H.B. Ultrasound-Guided Locoregional Anaesthesia for Carotid Endarterectomy: A Prospective Observational Study. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 27–30. [Google Scholar] [CrossRef]
- Raithel, D. Carotid Eversion Endarterectomy: A Better Technique than the Standard Operation? Cardiovasc. Surg. 1997, 5, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, M.; Ilies, C. Monitoring the Nociception-Anti-Nociception Balance. Best. Pract. Res. Clin. Anaesthesiol. 2013, 27, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Park, M.H.; Kim, D.K.; Kim, B.J. Prediction of Postoperative Pain and Opioid Consumption Using Intraoperative Surgical Pleth Index After Surgical Incision: An Observational Study. J. Pain. Res. 2020, 13, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Misiołek, H.; Zajączkowska, R.; Daszkiewicz, A.; Woroń, J.; Dobrogowski, J.; Wordliczek, J.; Owczuk, R. Postoperative Pain Management—2018 Consensus Statement of the Section of Regional Anaesthesia and Pain Therapy of the Polish Society of Anaesthesiology and Intensive Therapy, the Polish Society of Regional Anaesthesia and Pain Therapy, the Polish Association for the Study of Pain and the National Consultant in Anaesthesiology and Intensive Therapy. Anaesthesiol. Intensive Ther. 2018, 50, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Tomić Mahečić, T.; Malojčić, B.; Tonković, D.; Mažar, M.; Baronica, R.; Juren Meaški, S.; Crkvenac Gregorek, A.; Meier, J.; Dünser, M.W. Near-Infrared Spectroscopy-Guided, Individualized Arterial Blood Pressure Management for Carotid Endarterectomy under General Anesthesia: A Randomized, Controlled Trial. J. Clin. Med. 2023, 12, 4885. [Google Scholar] [CrossRef]
- Rerkasem, A.; Orrapin, S.; Howard, D.P.; Nantakool, S.; Rerkasem, K. Local versus General Anaesthesia for Carotid Endarterectomy. Cochrane Database Syst. Rev. 2021, 10, CD000126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Hua, Y.; Ji, X.; Jia, L.; Zhang, K.; Li, Q.; Li, Q.; Yang, J.; Li, J.; Jiao, L. Ultrasound-Based Carotid Plaque Characteristics Help Predict New Cerebral Ischemic Lesions after Endarterectomy. Ultrasound Med. Biol. 2021, 47, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Taşar, M.; Kalender, M.; Karaca, O.G.; Ecevit, A.N.; Salihi, S.; Adademir, T.; Darçın, O.T. Regional Cervical Plexus Blockage for Carotid Endarterectomy in Patients with Cardiovascular Risk Factors. Heart Surg. Forum 2015, 18, E140–E142. [Google Scholar] [CrossRef][Green Version]
- Shao, P.; Li, H.; Shi, R.; Li, J.; Wang, Y. Understanding Fascial Anatomy and Interfascial Communication: Implications in Regional Anesthesia. J. Anesth. 2022, 36, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Sait Kavaklı, A.; Kavrut Öztürk, N.; Umut Ayoğlu, R.; Sağdıç, K.; Çakmak, G.; İnanoğlu, K.; Emmiler, M. Comparison of Combined (Deep and Superficial) and Intermediate Cervical Plexus Block by Use of Ultrasound Guidance for Carotid Endarterectomy. J. Cardiothorac. Vasc. Anesth. 2016, 30, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Soh, E.Y.; Lee, J.; Kwon, S.H.; Hur, M.; Min, S.-K.; Kim, J.-S. Incidence of Hemi-Diaphragmatic Paresis after Ultrasound-Guided Intermediate Cervical Plexus Block: A Prospective Observational Study. J. Anesth. 2020, 34, 483–490. [Google Scholar] [CrossRef]
- Do, W.; Cho, A.-R.; Kim, E.-J.; Kim, H.-J.; Kim, E.; Lee, H.-J. Ultrasound-Guided Superficial Cervical Plexus Block under Dexmedetomidine Sedation versus General Anesthesia for Carotid Endarterectomy: A Retrospective Pilot Study. Yeungnam Univ. J. Med. 2018, 35, 45–53. [Google Scholar] [CrossRef]
- Skrtic, M.; Lijovic, L.; Pazur, I.; Perisa, N.; Radocaj, T. Hemodynamic Safety and Effect of Dexmedetomidine on Superficial Cervical Block Quality for Carotid Endarterectomy: A Prospective Study. J. Cardiothorac. Vasc. Anesth. 2023, 37, 2006–2011. [Google Scholar] [CrossRef]
- Sindjelic, R.P.; Vlajkovic, G.P.; Davidovic, L.B.; Markovic, D.Z.; Markovic, M.D. The Addition of Fentanyl to Local Anesthetics Affects the Quality and Duration of Cervical Plexus Block: A Randomized, Controlled Trial. Anesth. Analg. 2010, 111, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Kavrut Ozturk, N.; Kavakli, A.S.; Sagdic, K.; Inanoglu, K.; Umot Ayoglu, R. A Randomized Controlled Trial Examining the Effect of the Addition of the Mandibular Block to Cervical Plexus Block for Carotid Endarterectomy. J. Cardiothorac. Vasc. Anesth. 2018, 32, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Kavakli, A.S.; Kavrut Ozturk, N.; Yavuzel Adas, H.; Kudsioglu, S.T.; Ayoglu, R.U.; Özmen, S.; Sagdic, K.; Yapici, N. The Effects of Music on Anxiety and Pain in Patients during Carotid Endarterectomy under Regional Anesthesia: A Randomized Controlled Trial. Complement. Ther. Med. 2019, 44, 94–101. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Chabierska, E.; Marciniak, R.; Kolny, M.; Zuber, M.; Jałowiecki, P.; Pluta, A.; Szumera, I. Risk Factors for Occurrence of Failed Interscalene Brachial Plexus Blocks for Shoulder Arthroscopy Using 20 mL 0.5% Ropivacaine: A Randomised Trial. Anaesthesiol. Intensive Ther. 2018, 50, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Funcke, S.; Pinnschmidt, H.O.; Wesseler, S.; Brinkmann, C.; Beyer, B.; Jazbutyte, V.; Behem, C.R.; Trepte, C.; Nitzschke, R. Guiding Opioid Administration by 3 Different Analgesia Nociception Monitoring Indices During General Anesthesia Alters Intraoperative Sufentanil Consumption and Stress Hormone Release: A Randomized Controlled Pilot Study. Anesth. Analg. 2020, 130, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Seidel, R.; Zukowski, K.; Wree, A.; Schulze, M. Ultrasound-Guided Intermediate Cervical Plexus Block and Perivascular Local Anesthetic Infiltration for Carotid Endarterectomy: A Randomized Controlled Trial. Anaesthesist 2016, 65, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Alilet, A.; Petit, P.; Devaux, B.; Joly, C.; Samain, E.; Pili-Floury, S.; Besch, G. Ultrasound-Guided Intermediate Cervical Block versus Superficial Cervical Block for Carotid Artery Endarterectomy: The Randomized-Controlled CERVECHO Trial. Anaesth. Crit. Care Pain. Med. 2017, 36, 91–95. [Google Scholar] [CrossRef]
- Koköfer, A.; Nawratil, J.; Felder, T.K.; Stundner, O.; Mader, N.; Gerner, P. Ropivacaine 0.375% vs. 0.75% with Prilocaine for Intermediate Cervical Plexus Block for Carotid Endarterectomy: A Randomised Trial. Eur. J. Anaesthesiol. 2015, 32, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Torp, K.D.; Metheny, E.; Simon, L.V. Lidocaine Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chamseddine, H.; Chahrour, M.; Aboul Hosn, M.; Kabbani, L. In Patients with Heart Failure Undergoing Carotid Endarterectomy, Locoregional Anesthesia Is Not Associated with Decreased Mortality, Stroke, or Myocardial Infarction Compared to General Anesthesia. Ann. Vasc. Surg. 2024, 106, 189–195. [Google Scholar] [CrossRef]
- Marcucci, M.; Painter, T.W.; Conen, D.; Lomivorotov, V.; Sessler, D.I.; Chan, M.T.V.; Borges, F.K.; Leslie, K.; Duceppe, E.; Martínez-Zapata, M.J.; et al. Hypotension-Avoidance Versus Hypertension-Avoidance Strategies in Noncardiac Surgery: An International Randomized Controlled Trial. Ann. Intern. Med. 2023, 176, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Wachtendorf, L.J.; Azimaraghi, O.; Santer, P.; Linhardt, F.C.; Blank, M.; Suleiman, A.; Ahn, C.; Low, Y.H.; Teja, B.; Kendale, S.M.; et al. Association Between Intraoperative Arterial Hypotension and Postoperative Delirium After Noncardiac Surgery: A Retrospective Multicenter Cohort Study. Anesth. Analg. 2022, 134, 822–833. [Google Scholar] [CrossRef]
- Awad, H.; Alcodray, G.; Raza, A.; Boulos, R.; Essandoh, M.; Bhandary, S.; Dalton, R. Intraoperative Hypotension-Physiologic Basis and Future Directions. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hu, P.; Wang, C.-F.; Yu, C.-Q.; Sheng, J.; Ma, S.-J. Prediction Model of Cardiac Risk for Dental Extraction in Elderly Patients with Cardiovascular Diseases. Gerontology 2019, 65, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Acharya, Y.; Barrett, N.; Hynes, N. A Pilot Protocol and Review of Triple Neuroprotection with Targeted Hypothermia, Controlled Induced Hypertension, and Barbiturate Infusion during Emergency Carotid Endarterectomy for Acute Stroke after Failed tPA or beyond 24-Hour Window of Opportunity. Ann. Transl. Med. 2020, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
- van Gils, M.; Korhonen, I.; Yli-Hankala, A. Procedures for Evaluating the Adequacy of Anesthesia. Crit. Rev. Biomed. Eng. 2017, 45, 187–218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Deng, H.; Zeng, J.; Ran, H.; Yu, C. Unconscious Classification of Quantitative Electroencephalogram Features from Propofol versus Propofol Combined with Etomidate Anesthesia Using One-Dimensional Convolutional Neural Network. Front. Med. 2024, 11, 1447951. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Starzewska, M.; Niewiadomska, E.; Król, S.; Marczak, K.; Żak, J.; Pluta, A.; Eszyk, J.; Grabarek, B.O.; Szumera, I.; et al. Adequacy of Anesthesia Guidance for Colonoscopy Procedures. Pharmaceuticals 2021, 14, 464. [Google Scholar] [CrossRef]
- De Jonckheere, J.; Bonhomme, V.; Jeanne, M.; Boselli, E.; Gruenewald, M.; Logier, R.; Richebé, P. Physiological Signal Processing for Individualized Anti-Nociception Management During General Anesthesia: A Review. Yearb. Med. Inf. Inform. 2015, 10, 95–101. [Google Scholar] [CrossRef]
- Ilies, C.; Gruenewald, M.; Ludwigs, J.; Thee, C.; Höcker, J.; Hanss, R.; Steinfath, M.; Bein, B. Evaluation of the Surgical Stress Index during Spinal and General Anaesthesia. Br. J. Anaesth. 2010, 105, 533–537. [Google Scholar] [CrossRef]
- Gruenewald, M.; Meybohm, P.; Ilies, C.; Höcker, J.; Hanss, R.; Scholz, J.; Bein, B. Influence of Different Remifentanil Concentrations on the Performance of the Surgical Stress Index to Detect a Standardized Painful Stimulus during Sevoflurane Anaesthesia. Br. J. Anaesth. 2009, 103, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Laferrière-Langlois, P.; Morisson, L.; Jeffries, S.; Duclos, C.; Espitalier, F.; Richebé, P. Depth of Anesthesia and Nociception Monitoring: Current State and Vision For 2050. Anesth. Analg. 2024, 138, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Niewiadomska, E.; Krawczyk, L.; Dobrowolski, D.; Grabarek, B.O.; Kawka, M.; Rejdak, R.; Szumera, I.; et al. Adequacy of Anaesthesia for Nociception Detection during Vitreoretinal Surgery. Life 2023, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T. Objective Monitoring of Nociception: A Review of Current Commercial Solutions. Br. J. Anaesth. 2019, 123, e312–e321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, M.; Du, Y.; He, J.; Li, J. Ventricular Tachycardia and Acute Heart Failure Induced by Atropine in the Treatment of Bradycardia: A Case Report and Literature Review. Medicine 2023, 102, e34775. [Google Scholar] [CrossRef]
- Praveen, M.; Kumar, A.; Parikh, B.; Sikdar, I. Evaluation of qCON and qNOX Indices in Pediatric Surgery under General Anesthesia. J. Anaesthesiol. Clin. Pharmacol. 2024, 40, 264–270. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, B.-M.; Jung, Y.-R.; Lee, Y.-H.; Bang, J.-Y.; Noh, G.-J. Evaluation of Surgical Pleth Index and Analgesia Nociception Index as Surrogate Pain Measures in Conscious Postoperative Patients: An Observational Study. J. Clin. Monit. Comput. 2020, 34, 1087–1093. [Google Scholar] [CrossRef]
- Hung, K.-C.; Huang, Y.-T.; Kuo, J.-R.; Hsu, C.-W.; Yew, M.; Chen, J.-Y.; Lin, M.-C.; Chen, I.-W.; Sun, C.-K. Elevated Surgical Pleth Index at the End of Surgery Is Associated with Postoperative Moderate-to-Severe Pain: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2167. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T.; Sommerfield, D.; Slevin, L.; Conrad, J.; von Ungern-Sternberg, B.S. Surgical Pleth Index: Prediction of Postoperative Pain in Children? Br. J. Anaesth. 2017, 119, 979–983. [Google Scholar] [CrossRef]
- Ledowski, T.; Schneider, M.; Gruenewald, M.; Goyal, R.K.; Teo, S.R.; Hruby, J. Surgical Pleth Index: Prospective Validation of the Score to Predict Moderate-to-Severe Postoperative Pain. Br. J. Anaesth. 2019, 123, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Lyssek-Boroń, A.; Kawka-Osuch, M.; Niewiadomska, E.; Grabarek, B.O. Possibility of Using Surgical Pleth Index in Predicting Postoperative Pain in Patients after Vitrectomy Performed under General Anesthesia. Diagnostics 2024, 14, 425. [Google Scholar] [CrossRef] [PubMed]
- Ince, B.; Zuhour, M.; Yusifov, M.; Erol, A.; Dadaci, M. The Impact of Surgical Procedures During Septorhinoplasty on the Intraoperative Pain Response. Aesthet. Surg. J. 2021, 41, NP1421–NP1426. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitreoretinal Surgery under Adequacy of Anesthesia Guidance-Risk Factor Analysis. Pharmaceuticals 2022, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Putowski, Z.; Czajka, S.; Krzych, Ł.J. Association between Intraoperative Blood Pressure Drop and Clinically Significant Hypoperfusion in Abdominal Surgery: A Cohort Study. J. Clin. Med. 2021, 10, 5010. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, C.; Vicent, O.; Spieth, S.; Ludwig, S.; Reeps, C.; Heller, A.R.; Thea, K.; Spieth, P.M.; Rössel, T. Influence of Anatomic Conditions on Efficacy and Safety of Combined Intermediate Cervical Plexus Block and Perivascular Infiltration of Internal Carotid Artery in Carotid Endarterectomy: A Prospective Observational Trial. Ultrasound Med. Biol. 2021, 47, 2890–2902. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Szumera, I.; Wardas, P.; Król, S.; Żak, J.; Missir, A.; Pluta, A.; Niewiadomska, E.; Krawczyk, L.; Jałowiecki, P.; et al. Adequacy of Anesthesia and Pupillometry for Endoscopic Sinus Surgery. J. Clin. Med. 2021, 10, 4683. [Google Scholar] [CrossRef]
- Pluta, A.; Stasiowski, M.J.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitrectomy under Adequacy of Anesthesia-An Additional Report. J. Clin. Med. 2021, 10, 4172. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Zmarzły, N.; Grabarek, B.O.; Gąsiorek, J. Postoperative Nausea and Vomiting Following Endoscopic Sinus Surgery under the Guidance of Adequacy of Anesthesia or Pupillometry with Intravenous Propofol/Remifentanil. Pharmaceuticals 2024, 17, 2. [Google Scholar] [CrossRef] [PubMed]
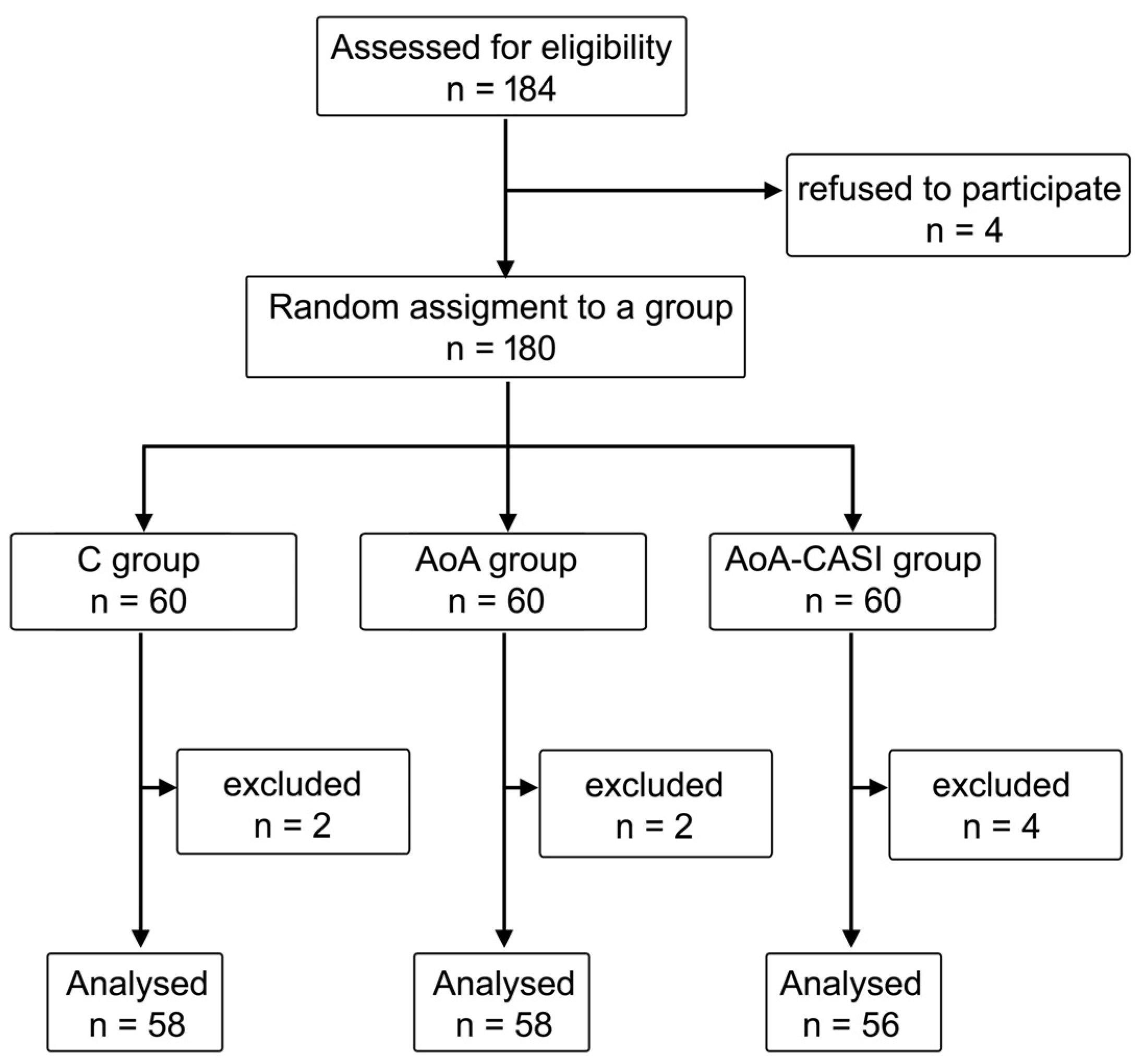
| Anthropometric Data | Total | C Group | AoA Group | Aoa-CASI Group | p-Value | |
|---|---|---|---|---|---|---|
| n = 172 (100%) | n = 58 (33.7%) | n = 58 (33.7%) | n = 56 (32.6%) | |||
| Age X ± Sd Me (IQR) | years | 69.3 ± 7.1 69 (9) | 69.4 ± 7.8 69.5 (11) | 69.4 ± 7.9 70 (12) | 69 ± 5.4 69 (7) | 0.90 NS |
| Height X ± Sd Me (IQR) | cm | 167.1 ± 9.3 168 (11) | 167.1 ± 7.9 168 (12) | 167.4 ± 7.4 168 (8) | 166.8 ± 12.1 168 (14) | 1.0 NS |
| Weight X ± Sd Me (IQR) | kg | 76.2 ± 13.2 75.5 (20) | 75 ± 14.2 77 (22) | 77.8 ± 13.2 77 (16) | 75.8 ± 12.2 75 (18.5) | 0.69 NS |
| BMI X ± Sd Me (IQR) | kg/m2 | 27.4 ± 4.9 274 (5.9) | 26.8 ± 4.2 27.7 (6.9) | 27.8 ± 4.7 27.9 (6.2) | 27.5 ± 5.9 27 (5.1) | 0.72 NS |
| BMI N (%) | underweight | 2 (1.2%) | 2 (3.4%) | 0 (0%) | 0 (0%) | 0.60 NS |
| normal | 53 (30.8%) | 17 (29.3%) | 19 (32.8%) | 17 (30.4%) | ||
| overweight | 73 (42.4%) | 24 (41.4%) | 23 (39.7%) | 26 (46.4%) | ||
| obese | 44 (25.6%) | 15 (25.9%) | 16 (27.6%) | 13 (23.2%) | ||
| Sex N (%) | male | 116 (67.4%) | 42 (72.4%) | 37 (63.8%) | 37 (66%) | 0.59 NS |
| female | 56 (32.6%) | 16 (27.6%) | 21 (36.2%) | 19 (33.9%) | ||
| Parameter | C Group | AoA Group | AoA-CASI Group | p-Value |
|---|---|---|---|---|
| n = 58 (33.7%) | n = 58 (33.7%) | n = 56 (32.6%) | ||
| Stage 1—Onset | ||||
| HR (beats/min) | 68.6 ± 11.3 68 (17) | 68.5 ± 10 66.5 (12) | 71.4 ± 13.2 69 (18) | 0.58 NS |
| SAP (mmHg) | 154.6 ± 24.6 158 (33) | 151.1 ± 27.4 151.5 (32) | 152.5 ± 22.5 154 (37) | 0.73 NS |
| MAP (mmHg) | 106.1 ± 20.2 105.5 (28) | 107.3 ± 15.3 108.5 (17) | 106.9 ± 14.5 105 (23) | 0.69 NS |
| DAP (mmHg) | 76.6 ± 10.8 75.5 (13) | 75 ± 11.6 74 (16) | 76.4 ± 12.3 76 (13) | 0.76 NS |
| SE | 87.7 ± 2.5 89 (2) | 88.4 ± 2.4 89 (2) | 87.7 ± 2 88 (2) | AoA vs. AoA-CASI, p = 0.03 |
| SPI | - | 57 ± 15.2 57 (18) | 59.6 ± 16.8 64 (26) | 1.0 NS |
| Parameter | C Group | AoA Group | AoA-CASI Group | p-Value |
|---|---|---|---|---|
| n = 58 (33.7%) | n = 58 (33.7%) | n = 56 (32.6%) | ||
| Stage 2—After CPB | ||||
| mean HR (beats/min) | 70.3 ± 14 67 (20) | 68.9 ± 12 67.5 (17) | 76.6 ± 14.1 74 (20) | C vs. AoA-CASI, p = 0.03 AoA vs. AoA-CASI, p = 0.01 |
| mean SAP (mmHg) | 148.3 ± 28.3 148.5 (36) | 155.5 ± 26.4 156 (45) | 157.6 ± 26.9 160 (32) | 0.8 NS |
| mean MAP (mmHg) | 101 ± 19.1 100 (28) | 107.6 ± 16 108.5 (19) | 112.8 ± 18.5 113 (20) | C vs. AoA-CASI, p = 0.003 |
| mean DAP (mmHg) | 77.4 ± 14.4 75.5 (14) | 77 ± 14.1 77 (18) | 80.3 ± 13.7 81 (19) | 0.30 NS |
| mean SE | 85.9 ± 6.1 88 (4) | 84.1 ± 9.5 88 (3) | 80.2 ± 12.9 50 (49) | C vs. AoA-CASI, p < 0.001 AoA vs. AoA-CASI, p < 0.001 |
| mean SPI | - | 53.3 ± 23.5 47.3 (42.7) | 79.3 ± 15.8 87 (14) | 1.0 NS |
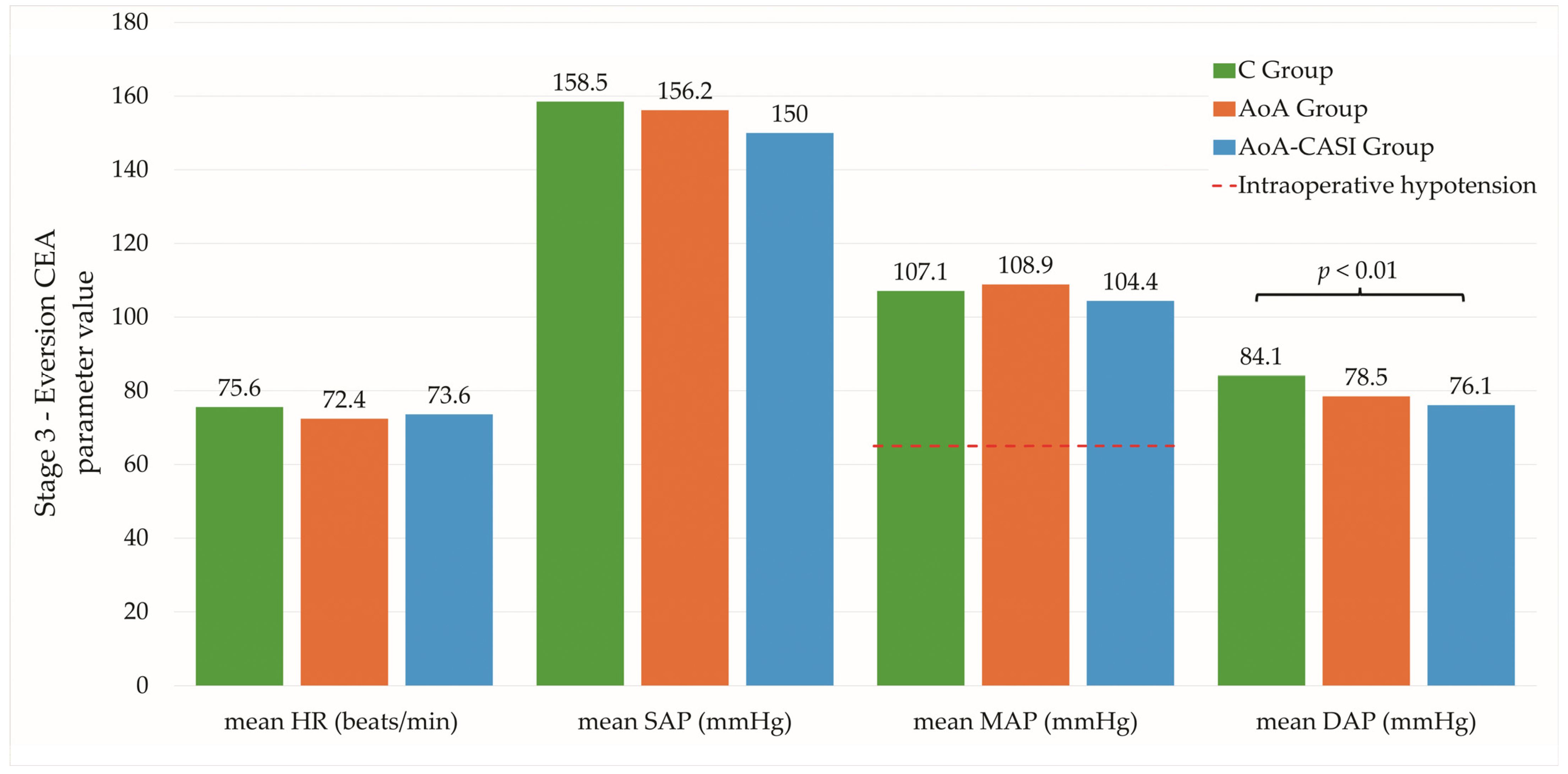
| Stage 3—Eversion CEA | ||||
| mean HR (beats/min) | 75.6 ± 13.4 74.5 (19) | 72.4 ± 12.1 71.1 (18.9) | 73.6 ± 13.3 71.7 (17.1) | 0.48 NS |
| mean SAP (mmHg) | 158.5 ± 23.3 160.1 (35.4) | 156.2 ± 21.1 157 (27.6) | 150 ± 23.7 155 (29.5) | 0.13 NS |
| mean MAP (mmHg) | 107.1 ± 15.1 107.1 (22.5) | 108.9 ± 13 110.7 (16.1) | 104.4 ± 14.6 104.6 (20) | 0.35 NS |
| mean DAP (mmHg) | 84.1 ± 12.8 82.9 (18.3) | 78.5 ± 12 77.3 (16) | 76.1 ± 13.6 76.8 (22.6) | C vs. AoA-CASI, p < 0.01 |
| mean SE | 87.1 ± 2.5 87.4 (3.3) | 86.9 ± 4.2 88 (2) | 88 ± 2.5 88.4 (1.8) | C vs. AoA-CASI, p < 0.02 |
| mean SPI | - | 42.2 ± 10.8 41.4 (14.5) | 44.3 ± 10.8 44.5 (17.6) | 1.0 NS |
| Stage 4—PACU | ||||
| mean HR (beats/min) | 77.2 ± 13.7 77 (15.8) | 71.9 ± 12.3 71.3 (17.7) | 73.2 ± 13.4 72.6 (17.2) | 0.11 NS |
| mean SAP (mmHg) | 156.7 ± 25.5 158 (29) | 153 ± 25.4 154 (22.8) | 159.3 ± 21 162.2 (31.3) | 0.45 NS |
| mean MAP (mmHg) | 105.7 ± 15.7 107.5 (20.5 | 106.4 ± 13.5 109 (15.8) | 110 ± 13.7 112.3 (17.7) | 0.22 NS |
| mean DAP (mmHg) | 83.3 ± 12.8 84 (16) | 75.9 ± 12.5 75 (15.7) | 79.5 ± 12.4 79 (19.7) | C vs. AoA, p = 0.004 |
| mean SPI | - | 45.2 ± 15.4 43.1 (21.9) | 50.6 ± 14.8 52.3 (23.2) | 1.0 NS |
| Parameter | C Group | AoA Group | AoA-CASI Group | p-Value |
|---|---|---|---|---|
| n = 58 (33.7%) | n = 58 (33.7%) | n = 56 (32.6%) | ||
| Stage 3—Eversion CEA | ||||
| max HR (beats/min) | 87.6 ± 18.4 85.5 (27) | 83.8 ± 15.4 83.5 (17) | 85.2 ± 14.6 85 (19) | 0.66 NS |
| max SAP (mmHg) | 189.4 ± 34 189 (36) | 184 ± 25.5 187 (35) | 173.9 ± 26.8 178 (32) | C vs. AoA-CASI, p < 0.03 |
| max MAP (mmHg) | 128 ± 19.7 127 (27) | 125.2 ± 15.7 125 (20) | 119.4 ± 16.3 120 (22) | 0.05 NS |
| max DAP (mmHg) | 98.5 ± 20.4 96.5 (28) | 89.5 ± 12.1 88.5 (16) | 87.1 ± 14.4 89 (21) | C vs. AoA, p = 0.007 C vs. AoA-CASI, p = 0.002 |
| max SE | 89.9 ± 1.7 90 (2) | 90.4 ± 0.8 91 (1) | 90.1 ± 2.6 91 (1) | 0.13 NS |
| max SPI | - | 72.5 ± 15.4 76 (21) | 72.3 ± 14.1 75 (21) | 1.0 NS |
| min HR (beats/min) | 63.8 ± 11.9 64 (18) | 61.9 ± 12.2 61.5 (16) | 64.1 ± 16.4 61 (18) | 0.56 NS |
| min SAP (mmHg) | 130.3 ± 31 130.5 (34) | 128.8 ± 26 130.5 (37) | 125.1 ± 30.1 132 (48) | 0.91 NS |
| min MAP (mmHg) | 85.2 ± 17.9 85 (24) | 91.4 ± 18.5 94 (23) | 89.1 ± 20 92 (32) | 0.21 NS |
| min DAP (mmHg) | 68 ± 12.3 67 (14) | 64.9 ± 13.8 65 (20) | 65.6 ± 16.5 66 (28) | 0.5 NS |
| min SE | 79.6 ± 11.8 84 (5) | 79.9 ± 13.8 85 (5) | 80.3 ± 15.2 85 (6) | 0.1 NS |
| min SPI | - | 19.4 ± 8.7 17 (13) | 22.5 ± 9.2 21 (10) | 1.0 NS |
| Stage 4—PACU | ||||
| max HR (beats/min) | 80.3 ± 14.3 80 (19) | 77.1 ± 14.4 76 (18) | 77.7 ± 13.8 77 (22) | 0.37 NS |
| max SAP (mmHg) | 163.9 ± 25.6 166 (38) | 163.6 ± 23.9 166 (24) | 169.2 ± 22.6 175 (32.5) | 0.35 NS |
| max MAP (mmHg) | 111.3 ± 18.5 111.5 (27) | 112.2 ± 14.1 114 (14) | 116.3 ± 14.5 118.5 (17.5) | 0.19 NS |
| max DAP (mmHg) | 88.6 ± 15.9 89 (22) | 80.6 ± 13 82 (17) | 84.6 ± 14.6 86 (24.5) | C vs. AoA, p = 0.02 |
| max SPI | - | 62.3 ± 17.9 59 (29) | 64.2 ± 14.5 66 (20) | 1.0 NS |
| min HR (beats/min) | 73.7 ± 13.7 | 68.1 ± 11.7 67 (15) | 70.4 ± 13.3 69 (19) | 0.08 NS |
| min SAP (mmHg) | 150 ± 26.1 150 (32) | 144.7 ± 24.9 147 (30) | 149.9 ± 22.1 153.5 (32) | 0.41 NS |
| min MAP (mmHg) | 101.6 ± 13.7 104 (22) | 100.6 ± 14.8 103 (17) | 104. ± 14.7 106 (21) | 0.39 NS |
| min DAP (mmHg) | 79.6 ± 14 77 (19) | 71.1 ± 13.5 69 (18) | 75.3 ± 11.7 75.5 (19.5) | C vs. AoA, p = 0.002 |
| min SPI | - | 31.6 ± 16 31 (20.5) | 36.5 ± 16.3 31 (27.5) | 1.0 NS |
| Complications | Total | C Group | AoA Group | AoA-CASI Group | p-Value |
|---|---|---|---|---|---|
| Postoperative pain perception | |||||
| NPRS | 0.4 ± 1.1 0 (0) | 0.4 ± 1.2 0 (0) | 0.3 ± 0.9 0 (0) | 0.4 ± 1.3 0 (0) | 0.99 NS |
| Number of patients with IPPP (NPRS 4–10) | 7 (4.07%) | 3 (5.17%) | 1 (1.72%) | 3 (5.36%) | 0.5 NS |
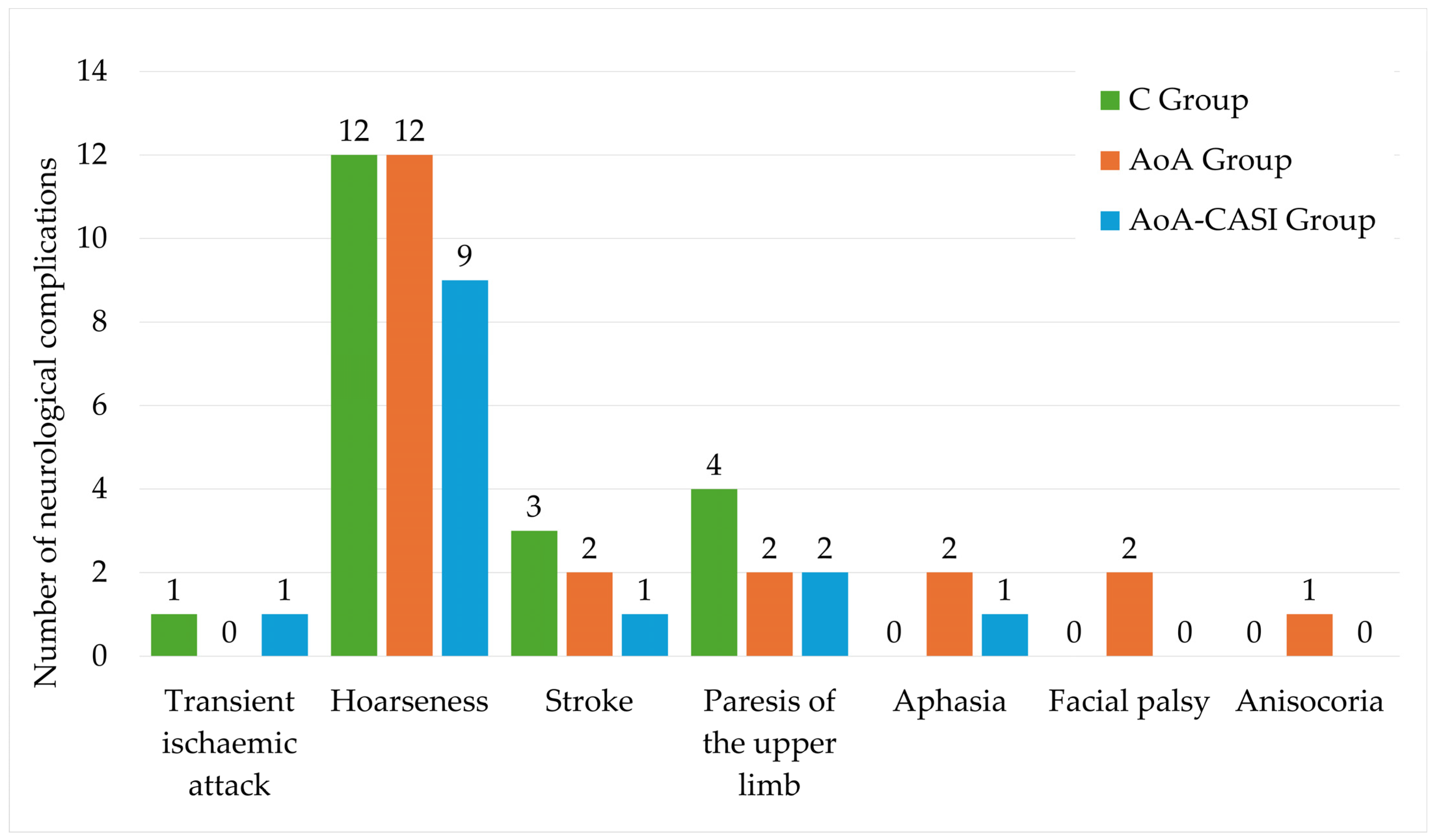
| Neurological complications (% of incidence) | |||||
| Transient ischemic attack | 2 (1.16%) | 1 (1.72%) | 0 (0%) | 1 (1.79%) | 1.0 NS |
| Hoarseness | 33 (19.19%) | 12 (20.69%) | 12 (20.69%) | 9 (16.07%) | 0.8 NS |
| Stroke | 6 (3.49%) | 3 (5.17%) | 2 (3.45%) | 1 (1.79%) | 0.6 NS |
| Paresis of the upper limb | 8 (4.65%) | 4 (6.9%) | 2 (3.45%) | 2 (3.57%) | 0.6 NS |
| Aphasia | 3 (1.74%) | 0 (0%) | 2 3.45%) | 1 (1.79%) | 0.4 NS |
| Facial palsy | 2 (1.16%) | 0 (0%) | 2 (3.45%) | 0 (0%) | 0.1 NS |
| Anisocoria | 1 (0.58%) | 0 (0%) | 1 (1.72%) | 0 (0%) | 0.4 NS |
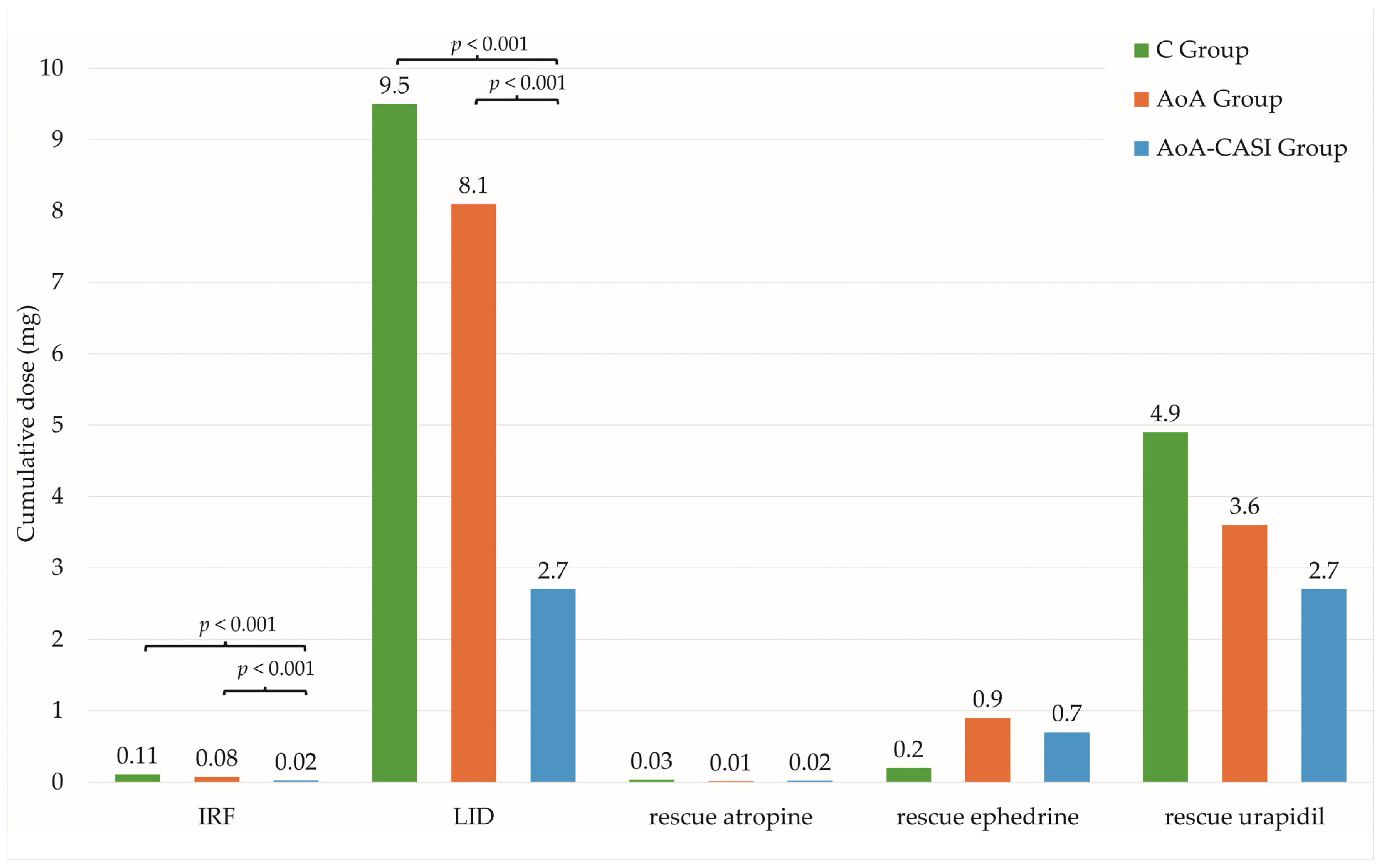
| Intraoperative Rescue Intervention | Total | C Group | AoA Group | AoA-CASI Group | p-Value |
|---|---|---|---|---|---|
| n = 172 (100%) | N = 58 (33.7%) | n = 58 (33.7%) | N = 56 (32.6%) | ||
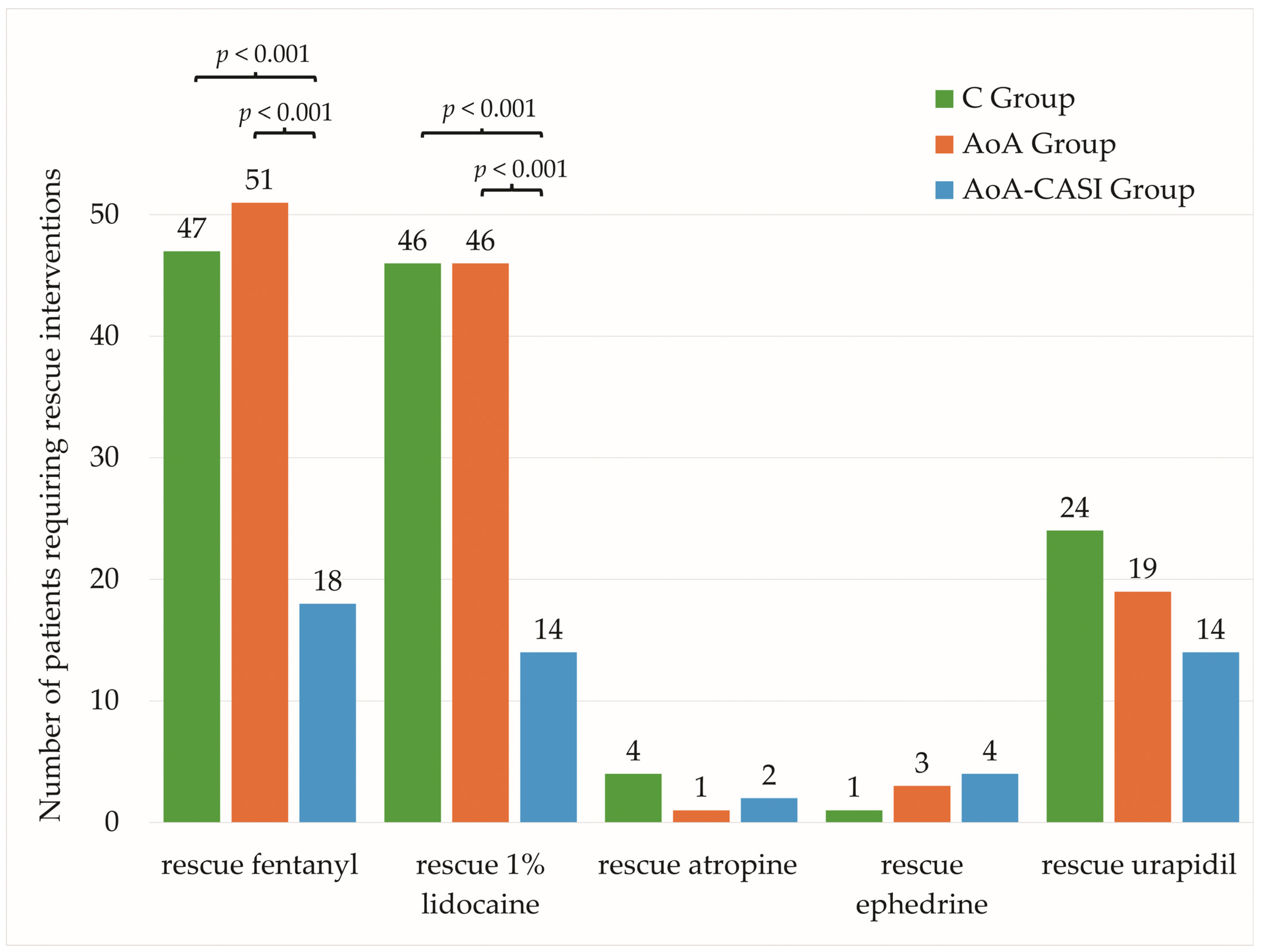
| Number of patients requiring IRF | 116 (67.4%) | 47 (81%) | 51 (87.9%) | 18 (32.1%) | C vs. AoA-CASI, p < 0.001 AoA vs. AoA-CASI, p < 0.001 C vs. AoA, p = 0.4 |
| Cumulative dose of requiring IRF (µg) | 69.9 ± 64.7 50 (100) | 109.5 ± 69.7 100 (100) | 76.2 ± 51 50 (50) | 22.3 ± 35.6 0 (50) | C vs. AoA-CASI, p < 0.001 AoA vs. AoA-CASI, p < 0.001 C vs. AoA, p=0.1 |
| Number of patients requiring rescue LID | 106 (61.6%) | 46 (79.3%) | 46 (79.3%) | 14 (25%) | C vs. AoA-CASI, p < 0.001 AoA vs. AoA-CASI, p < 0.001 C vs. AoA, p = 1.0 |
| Cumulative dose of rescue 1% LID (mg) | 6.8 ± 6.9 5 (10) | 9.5 ± 7 10 (10) | 8.1 ± 6.4 9 (5) | 2.7 ± 5.5 0 (2.5) | C vs. AoA-CASI, p < 0.001 AoA vs. AoA-CASI, p < 0.001 C vs. AoA, p = 1.0 |
| Number of patients requiring rescue atropine | 7 (4.1%) | 4 (6.9%) | 1 (1.7%) | 2 (3.6%) | 0.4 NS |
| Cumulative dose of rescue atropine (µg) | 20.5 ± 113.2 0 (0) | 34.5 ± 158.4 0 (0) | 8.6 ± 65.7 0 (0) | 18.2 ± 94.5 0 (0) | 0.4 NS |
| Number of patients requiring rescue ephedrine | 8 (4.7%) | 1 (1.7%) | 3 (5.2%) | 4 (7.3%) | 0.4 NS |
| Cumulative dose of rescue ephedrine (mg) | 0.6 ± 3 0 (0) | 0.2 ± 1.3 0 (0) | 0.9 ± 4.3 0 (0) | 0.7 ± 2.6 0 (0) | 0.4 NS |
| Number of patients requiring rescue urapidil | 57 (33.3%) | 24 (41.4%) | 19 (32.8%) | 14 (25.5%) | 0.2 NS |
| Cumulative dose of rescue urapidil (mg) | 3.8 ± 6.7 0 (5) | 4.9 ± 7.6 0 (10) | 3.6 ± 6.6 0 (5) | 2.7 ± 5.8 0 (5) | 0.2 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiowski, M.J.; Zmarzły, N.; Grabarek, B.O. Evaluating Anesthesia Guidance for Rescue Analgesia in Awake Patients Undergoing Carotid Endarterectomy with Cervical Plexus Blocks: Preliminary Findings from a Randomized Controlled Trial. J. Clin. Med. 2025, 14, 120. https://doi.org/10.3390/jcm14010120
Stasiowski MJ, Zmarzły N, Grabarek BO. Evaluating Anesthesia Guidance for Rescue Analgesia in Awake Patients Undergoing Carotid Endarterectomy with Cervical Plexus Blocks: Preliminary Findings from a Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(1):120. https://doi.org/10.3390/jcm14010120
Chicago/Turabian StyleStasiowski, Michał Jan, Nikola Zmarzły, and Beniamin Oskar Grabarek. 2025. "Evaluating Anesthesia Guidance for Rescue Analgesia in Awake Patients Undergoing Carotid Endarterectomy with Cervical Plexus Blocks: Preliminary Findings from a Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 1: 120. https://doi.org/10.3390/jcm14010120
APA StyleStasiowski, M. J., Zmarzły, N., & Grabarek, B. O. (2025). Evaluating Anesthesia Guidance for Rescue Analgesia in Awake Patients Undergoing Carotid Endarterectomy with Cervical Plexus Blocks: Preliminary Findings from a Randomized Controlled Trial. Journal of Clinical Medicine, 14(1), 120. https://doi.org/10.3390/jcm14010120





