Skin Physiological Parameters and Their Association with Severe Atopic Dermatitis in Mongolian Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Study Participants
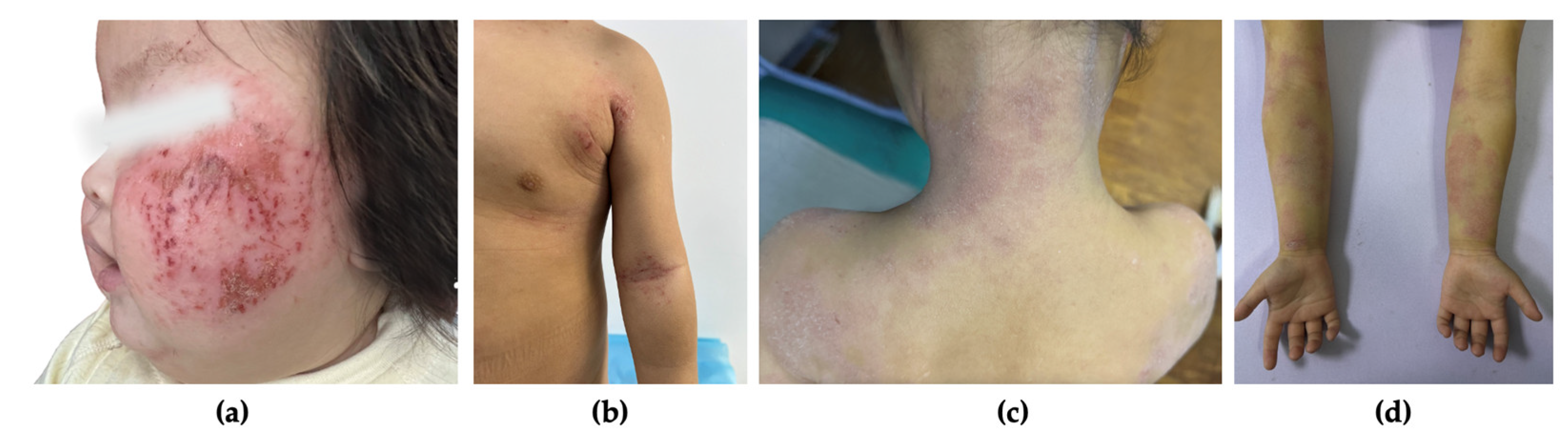
2.2. Atopic Dermatitis Severity
2.3. Skin Parameters
2.4. Other Parameters
2.5. Statistical Analysis
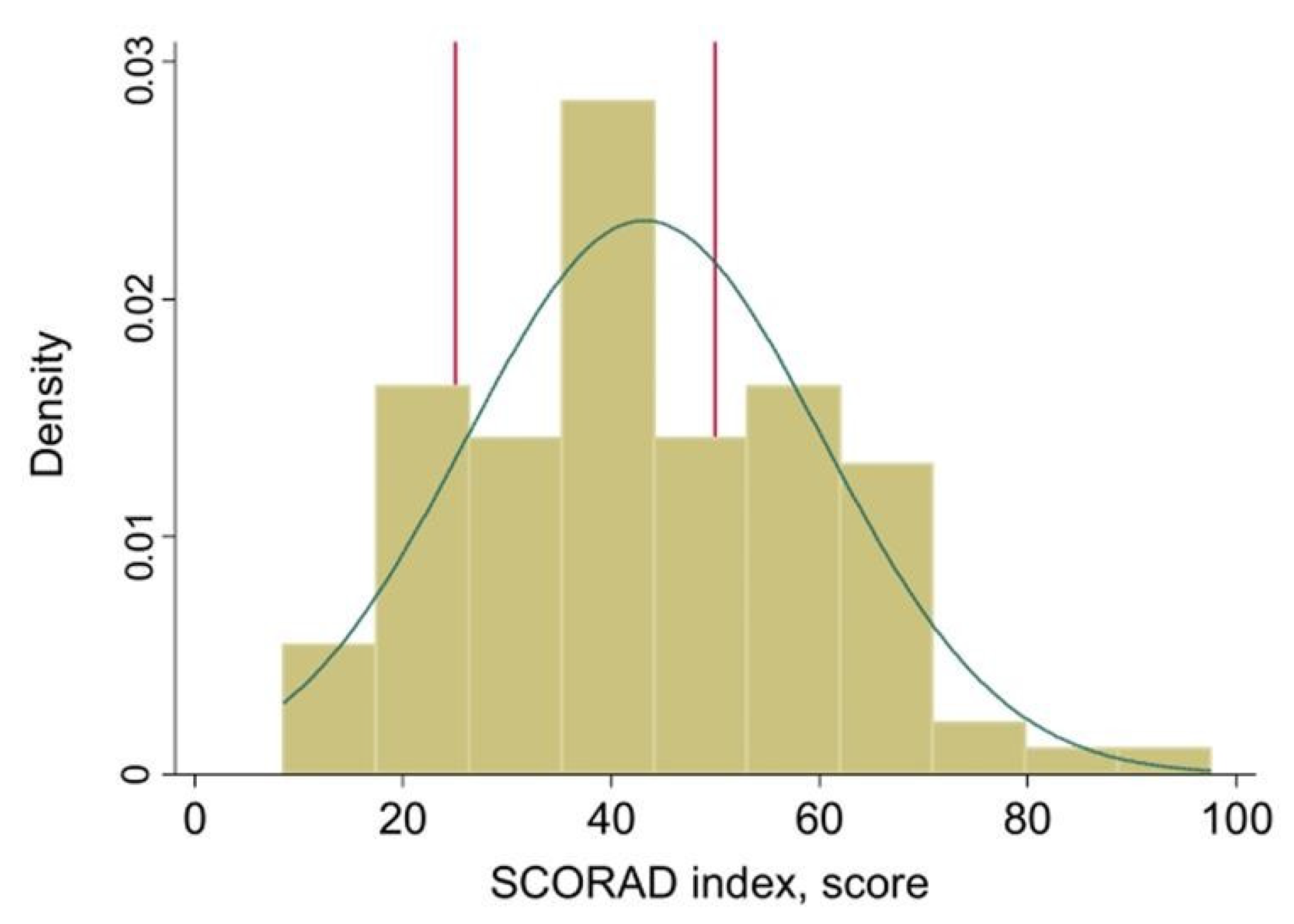
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, H.; Stewart, A.; von Mutius, E.; Cookson, W.; Anderson, H.R.; Asthma, I.S. Is eczema really on the increase worldwide? J. Allergy Clin. Immunol. 2008, 121, 947–954.e15. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. Tion J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Center for Health Development M. Health Indicators. Available online: https://hdc.gov.mn/file-category/56/ (accessed on 1 October 2024).
- Lopez Carrera, Y.I.; Al Hammadi, A.; Huang, Y.H.; Llamado, L.J.; Mahgoub, E.; Tallman, A.M. Epidemiology, Diagnosis, and Treatment of Atopic Dermatitis in the Developing Countries of Asia, Africa, Latin America, and the Middle East: A Review. Dermatol. Ther. 2019, 9, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Mallol, J.; Crane, J.; von Mutius, E.; Odhiambo, J.; Keil, U.; Stewart, A. The International Study of Asthma and Allergies in Child-hood (ISAAC) Phase Three: A global synthesis. Allergol. Et Immunopathol. 2013, 41, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, P.; Tang, J.; Han, X.; Zou, X.; Xu, G.; Xu, Z.; Wei, F.; Liu, Q.; Wang, M.; et al. Prevalence of Atopic Dermatitis in Chinese Children aged 1–7 ys. Sci. Rep. 2016, 6, 29751. [Google Scholar] [CrossRef]
- Goh, Y.Y.; Keshavarzi, F.; Chew, Y.L. Prevalence of Atopic Dermatitis and Pattern of Drug Therapy in Malaysian Children. Dermatitis 2018, 29, 151–161. [Google Scholar] [CrossRef]
- Tan, T.N.; Lim, D.L.; Lee, B.W.; Van Bever, H.P. Prevalence of allergy-related symptoms in Singaporean children in the second year of life. Pediatr. Allergy Immunol. 2005, 16, 151–156. [Google Scholar]
- Losol, P.; Sokolowska, M.; Hwang, Y.-K.; Ogulur, I.; Mitamura, Y.; Yazici, D.; Pat, Y.; Radzikowska, U.; Ardicli, S.; Yoon, J.-E.; et al. Epithelial Barrier Theory: The Role of Exposome, Microbiome, and Barrier Function in Allergic Diseases. Allergy Asthma Immunol. Res. 2023, 15, 705–724. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Barbarot, S.; Gadkari, A.; Simpson, E.L.; Weidinger, S.; Mina-Osorio, P.; Rossi, A.B.; Brignoli, L.; Saba, G.; Guillemin, I.; et al. Atopic dermatitis in the pediatric pop-ulation: A cross-sectional, international epidemiologic study. Ann. Allergy Asthma Immunol. 2021, 126, 417–428.e2. [Google Scholar] [CrossRef]
- Narmandakh, Z.; Tulgaa, K.; Munkhbayar, A.; Dungubat, E.; Lkhamkhuu, A.; Tumurbaatar, O.; Byamba, K.; Sandag, T.; Gunchin, B. The Determination of Filaggrin Gene Single Nucleotides Polymorphisms in Patients with Atopic Dermatitis. Cent. Asian J. Med. Sci. 2017, 3, 31–40. [Google Scholar] [CrossRef]
- Gautam, P.; Chaurasia, A.; Bhattacharya, A.; Grover, R.; Mukerji, M.; Natarajan, V. Population diversity and adaptive evolution in keratinization genes: Impact of environment in shaping skin phenotypes. Mol. Biol. Evol. 2015, 32, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A. Ethnic skin types: Are there differences in skin structure and function? 1. Int. J. Cosmet. Sci. 2006, 28, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Bater, J.; Bromage, S.; Jambal, T.; Tsendjav, E.; Lkhagvasuren, E.; Jutmann, Y.; Martineau, A.R.; Ganmaa, D. Prevalence and Determinants of Vitamin D Deficiency in 9595 Mongolian Schoolchildren: A Cross-Sectional Study. Nutrients 2021, 13, 4175. [Google Scholar] [CrossRef]
- National Agency Meteorology and the Enviromental Monitoring. Available online: http://www.tsag-agaar.gov.mn/ (accessed on 1 October 2024).
- Korea Meteorological Administration. Available online: https://web.kma.go.kr/eng/biz/climate_01.jsp. (accessed on 1 October 2024).
- Japan Meteorological Agency. Available online: https://ds.data.jma.go.jp/tcc/tcc/products/japan/index.html (accessed on 1 October 2024).
- National Centers for Environmental Information (NCEI). Available online: https://www.ncei.noaa.gov/. (accessed on 1 October 2024).
- Meteoblue: Weather and Climate Data Visualization Platform. Available online: https://www.meteoblue.com/en/weather/historyclimate/climatemodelled/ulaanbaatar_mongolia_2028462. (accessed on 17 December 2024).
- Montero-Vilchez, T.; Cuenca-Barrales, C.; Rodriguez-Pozo, J.-A.; Diaz-Calvillo, P.; Tercedor-Sanchez, J.; Martinez-Lopez, A.; Molina-Leyva, A.; Arias-Santiago, S. Epidermal Barrier Function and Skin Homeostasis in Atopic Dermatitis: The Impact of Age. Life 2022, 12, 132. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Feingold, K.R.; Elias, P.M. Transepidermal water loss reflects permeability barrier status: Validation in human and rodent in vivo and ex vivo models. Exp. Dermatol. 2006, 15, 483–492. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef]
- Dikstein, S.; Zlotogorski, A. Measurement of skin pH. Acta Derm. Venereol. Suppl. 1994, 185, 18–20. [Google Scholar]
- Zainal, H.; Jamil, A.; Md Nor, N.; Tang, M.M. Skin pH mapping and its relationship with transepidermal water loss, hydration and disease severity in adult patients with atopic dermatitis. Ski. Res. Technol. 2020, 26, 91–98. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Rajka, G. Diagnostic Features of Atopic Dermatitis. Acta Derm. Venereol. 1980, 60, 44–47. [Google Scholar] [CrossRef]
- Severity scoring of atopic dermatitis: The SCORAD index. Consensus Report of the European Task Force on Atopic Derma-titis. Dermatology 1993, 186, 23–31.
- Parra, J.L.; Paye, M. EEMCO guidance for the in vivo assessment of skin surface pH. Ski. Pharmacol. Appl. Ski. Physiol. 2003, 16, 188–202. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.C.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Ski. Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, A.B.; Plessis, J.; John, S.M.; Eloff, F.; Agner, T.; Chou, T.C.; Nixon, R.; Steiner, M.F.C.; Kudla, I.; Holness, D.L. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 1. pH. Ski. Res. Technol. 2013, 19, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Seidenari, S.; Giusti, G. Objective assessment of the skin of children affected by atopic dermatitis: A study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm. Venereol. 1995, 75, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.E.; Lam, M.C.A.; Leung, T.F.; Wong, K.Y.; Chow, C.M.; Fok, T.F.; Ng, P.C. Are age-specific high serum IgE levels associated with worse symptomatology in children with atopic dermatitis? Int. J. Dermatol. 2007, 46, 1258–1262. [Google Scholar] [CrossRef]
- Raap, U.; Wichmann, K.; Bruder, M.; Ständer, S.; Wedi, B.; Kapp, A.; Werfel, T. Correlation of IL-31 serum levels with severity of atopic dermatitis. J. Allergy Clin. Immunol. 2008, 122, 421–423. [Google Scholar] [CrossRef]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; De Waard-van der Spek, F.B. Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br. J. Dermatol. 2007, 157, 645–648. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.J.; Yang, H.S.; Kim, E.; Huh, I.S.; Yang, J.M. IL-31 Serum Protein and Tissue mRNA Levels in Patients with Atopic Dermatitis. Ann. Dermatol. 2011, 23, 468–473. [Google Scholar] [CrossRef]
- Fang, Z.; Pan, T.; Li, L.; Wang, H.; Zhu, J.; Zhang, H.; Zhao, J.; Chen, W.; Lu, W. Bifidobacterium longum mediated tryptophan metabolism to improve atopic dermatitis via the gut-skin axis. Gut Microbes 2022, 14, 2044723. [Google Scholar] [CrossRef]
- Vakirlis, E.; Lazaridou, E.; Tzellos, T.G.; Gerou, S.; Chatzidimitriou, D.; Ioannides, D. Investigation of cytokine levels and their association with SCORAD index in adults with acute atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 409–416. [Google Scholar] [CrossRef]
- Ulzii, D.; Nakahara, T.; Furue, M.; Byamba, K.; Kido-Nakahara, M. Severity strata of patient-oriented eczema measure scores in patients with atopic dermatitis in Mongolia. Int. J. Dermatol. 2022, 61, e469–e472. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, M.; Lan, T.; Wang, Y.; Zhou, X.; Dong, W.; Cheng, G.; Li, W.; Cheng, L. Effects of ambient temperature on atopic dermatitis and attributable health burden: A 6-year time-series study in Chengdu, China. PeerJ 2023, 11, e15209. [Google Scholar] [CrossRef] [PubMed]
- Engebretsen, K.; Johansen, J.; Kežić, S.; Linneberg, A.; Thyssen, J. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Cork, M.J.; Danby, S.G.; Vasilopoulos, Y.; Hadgraft, J.; Lane, M.E.; Moustafa, M.; Guy, R.H.; MacGowan, A.L.; Tazi-Ahnini, R.; Ward, S.J. Epidermal barrier dysfunction in atopic dermatitis. J. Investig. Dermatol. 2009, 129, 1892–1908. [Google Scholar] [CrossRef]
- Bandier, J.; Johansen, J.D.; Petersen, L.J.; Carlsen, B.C. Skin pH, atopic dermatitis, and filaggrin mutations. Dermatitis 2014, 25, 127–129. [Google Scholar] [CrossRef]
- Panther, D.J.; Jacob, S.E. The Importance of Acidification in Atopic Eczema: An Underexplored Avenue for Treatment. J. Clin. Med. 2015, 4, 970–978. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef]
- Liang, Y.; Chang, C.; Lu, Q. The Genetics and Epigenetics of Atopic Dermatitis-Filaggrin and Other Polymorphisms. Clin. Rev. Allergy Immunol. 2016, 51, 315–328. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]

| Parameters | Total | AD Severity Groups | p-Value | ||
|---|---|---|---|---|---|
| Mild (n = 17) | Moderate (n = 50) | Severe (n = 36) | |||
| Age, years | 5 [0.25–18] | 6 [0.33–18] | 5 [0.5–18] | 5 [0.25–18] | 0.991 ** |
| Gender, % (n) | |||||
| Male | 41.70 (43) | 41.18 (7) | 38.00 (19) | 47.22 (17) | 0.693 *** |
| Female | 58.25 (60) | 58.82 (10) | 62.00 (31) | 52.78 (19) | |
| Age of AD onset, years | 1 [0–17] | 0 [0–4] | 1 [0–17] | 0 [0–13] | 0.027 ** |
| AD duration, years | 3 [0–18] | 4 [0–18] | 3 [0–13] | 4 [0–18] | 0.526 ** |
| Breastfeeding, % (n) | 93.20 (96) | 88.24 (15) | 94.00 (47) | 94.44 (34) | 0.670 *** |
| Birth weight, gram | |||||
| <2500 | 8.74 (9) | 11.76 (2) | 10.00 (5) | 5.56 (2) | 0.915 *** |
| 2500–3500 | 40.78 (42) | 41.18 (7) | 38.00 (19) | 44.44 (16) | |
| >3500 | 50.49 (52) | 47.07 (8) | 52.00 (26) | 50.00 (18) | |
| Housing type: apartment, % (n) | 71.57 (73) | 76.47 (13) | 67.35 (33) | 75 (27) | 0.792 *** |
| Living area: urban, % (n) | 73.29 (76) | 82.35 (14) | 72.00 (36) | 72.22 (26) | 0.679 *** |
| Regional distribution, % (n) | |||||
| Eastern | 29.13 (30) | 35.29 (6) | 30.00 (14) | 25.00 (9) | 0.729 *** |
| Central | 42.72 (44) | 29.41 (5) | 42.00 (21) | 50.00 (18) | |
| Western | 28.16 (29) | 35.29 (6) | 28.00 (14) | 25.00 (9) | |
| Recent moisturizer application history (last 6 months), % (n) | 90.29 (93) | 100 (17) | 88.00 (44) | 88.89 (32) | 0.300 *** |
| Daily moisturizing, % (n) | 51.46 (53) | 35.29 (6) | 54.00 (27) | 55.56 (20) | 0.020 *** |
| Hospital admission, % (n) | 21.36 (22) | 5.88 (1) | 10 (5) | 44.44 (16) | <0.001 *** |
| School absence, % (n) | 46.60 (48) | 17.65 (3) | 42.00 (21) | 66.67 (24) | 0.015 *** |
| Total IgE level, IU/ml | 118.8 [2.3–458] | 60 [7.1–337.5] | 116.2 [2.3–436.4] | 229.45 [6.1–458] | 0.013 ** |
| Parameters | Total | AD Severity Groups | p-Value | ||
|---|---|---|---|---|---|
| Mild (n = 17) | Moderate (n = 50) | Severe (n = 36) | |||
| Non-lesional area | |||||
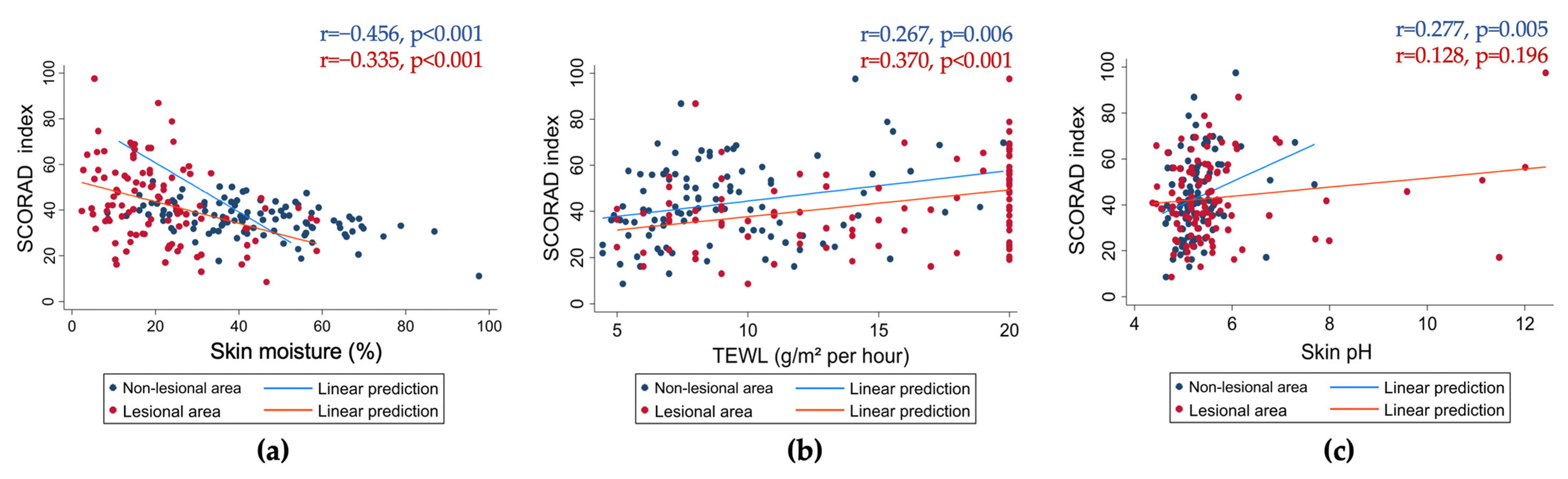
| Skin moisture, % | 36.27 ± 7.25 | 40.14 ± 5.24 | 37.59 ± 6.78 | 32.62 ± 7.28 | <0.001 * |
| TEWL, g/m2/h | 9.01 ± 3.44 | 8.08 ± 3.31 | 8.71 ± 3.35 | 9.89 ± 3.54 | 0.138 * |
| Skin pH | 5.29 ± 0.49 | 5.13 ± 0.44 | 5.24 ± 0.46 | 5.44 ± 0.53 | 0.049 * |
| Lesional area | |||||
| Skin moisture, % | 18.67 [2.33–58.67] | 26 [10.33–58.67] | 18.67 [2.33–58.67] | 15.17 [2.67–33.33] | 0.002 ** |
| TEWL, g/m2/h | 17 [5–20] | 11 [5–20] | 12.5 [5–20] | 20 [7–20] | <0.001 ** |
| Skin pH | 5.42 [4.37–12.43] | 5.36 [4.76–11.48] | 5.31 [4.37–9.59] | 5.50 [4.45–12.43] | 0.185 ** |
| Parameters | Univariate Regression | Multivariate Regression | ||||
|---|---|---|---|---|---|---|
| B Coefficient | 95% CI | p Value | B Coefficient | 95% CI | p Value | |
| Non-lesional area | ||||||
| Skin moisture, % | −0.772 | −1.116; −0.428 | <0.001 | −0.725 | −1.068; −0.381 | <0.001 |
| TEWL, g/m2/h | 0.327 | 0.092; 0.561 | 0.007 | 0.350 | 0.139; 0.561 | 0.001 |
| Skin pH | 1.415 | 0.442; 2.389 | 0.005 | 0.733 | −0.190; 1.657 | 0.118 |
| Lesional area | ||||||
| Skin moisture, % | −0.226 | −0.347; −0.106 | <0.001 | −0.168 | −0.295; −0.040 | 0.011 |
| TEWL, g/m2/h | 0.329 | 0.137; 0.523 | 0.001 | 0.232 | 0.029; 0.435 | 0.025 |
| Skin pH | 0.230 | −0.215; 0.676 | 0.308 | 0.2086 | −0.205; 0.622 | 0.320 |
| (A) | |||
| Parameters | SCORAD Index-Linear Regression | ||
| B Coefficient | 95% CI | p Value | |
| TEWL | |||
| Model 1 | 0.375 | 0.130; 0.621 | 0.003 |
| Model 2A | 0.341 | 0.119; 0.564 | 0.003 |
| Model 2B | 0.248 | 0.027; 0.469 | 0.028 |
| Model 3 | 0.260 | 0.048; 0.473 | 0.017 |
| Model 4 | 0.249 | 0.038; 0.462 | 0.022 |
| Model 5 | 0.263 | 0.046; 0.482 | 0.018 |
| Model 6 | 0.328 | 0.104; 0.551 | 0.004 |
| Skin moisture | |||
| Model 1 | −0.857 | −1.224; −0.490 | <0.001 |
| Model 2A | −0.741 | −1.110; −0.373 | <0.001 |
| Model 2B | −0.532 | −0.887; −0.177 | 0.004 |
| Model 3 | −0.523 | −0.880; −0.166 | 0.005 |
| Model 4 | −0.515 | −0.874; −0.158 | 0.005 |
| Model 5 | −0.513 | −0.881; −0.144 | 0.007 |
| Model 6 | −0.771 | −1.133; −0.411 | <0.001 |
| Skin pH | |||
| Model 1 | 1.410 | 0.431; 2.390 | 0.005 |
| Model 2A | 0.727 | −0.203; 1.656 | 0.124 |
| Model 2B | 0.572 | −0.332; 1.477 | 0.212 |
| Model 3 | 0.277 | −0.600; 1.155 | 0.531 |
| Model 4 | 0.234 | −0.637; 1.104 | 0.596 |
| Model 5 | 0.290 | 0.604; 1.184 | 0.521 |
| Model 6 | 0.984 | −0.031; 1.999 | 0.057 |
| (B) | |||
| Parameters | SCORAD Index-Linear Regression | ||
| B Coefficient | 95% CI | p Value | |
| TEWL | |||
| Model 1 | 0.334 | 0.140; 0.528 | 0.001 |
| Model 2A | 0.231 | 0.025; 0.437 | 0.028 |
| Model 2B | 0.209 | 0.031; 0.386 | 0.021 |
| Model 3 | 0.126 | −0.061; 0.313 | 0.185 |
| Model 4 | 0.118 | −0.069; 0.304 | 0.214 |
| Model 5 | 0.129 | −0.062; 0.321 | 0.183 |
| Model 6 | 0.272 | 0.077; 0.467 | 0.007 |
| Skin moisture | |||
| Model 1 | −0.228 | −0.351; −0.106 | <0.001 |
| Model 2A | −0.169 | −0.300; −0.037 | 0.012 |
| Model 2B | −0.167 | −0.275; −0.059 | 0.003 |
| Model 3 | −0.138 | −0.254; −0.022 | 0.020 |
| Model 4 | −0.140 | −0.257; −0.023 | 0.020 |
| Model 5 | −0.139 | −0.258; −0.019 | 0.023 |
| Model 6 | −0.218 | −0.335; −0.100 | <0.001 |
| Skin pH | |||
| Model 1 | 0.223 | −0.232; 0.678 | 0.334 |
| Model 2A | 0.211 | −0.210; 0.632 | 0.323 |
| Model 2B | −0.104 | −0.507; 0.299 | 0.610 |
| Model 3 | −0.080 | −0.467; 0.306 | 0.681 |
| Model 4 | −0.077 | −0.463; 0.309 | 0.693 |
| Model 5 | −0.086 | −0.482; 0.309 | 0.666 |
| Model 6 | 0.111 | −0.331; 0.553 | 0.618 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batbileg, L.; Baasanjav, S.; Tulgaa, K.; Byambasukh, O.; Naymdavaa, K.; Yadamsuren, E.; Biziya, B. Skin Physiological Parameters and Their Association with Severe Atopic Dermatitis in Mongolian Children. J. Clin. Med. 2025, 14, 112. https://doi.org/10.3390/jcm14010112
Batbileg L, Baasanjav S, Tulgaa K, Byambasukh O, Naymdavaa K, Yadamsuren E, Biziya B. Skin Physiological Parameters and Their Association with Severe Atopic Dermatitis in Mongolian Children. Journal of Clinical Medicine. 2025; 14(1):112. https://doi.org/10.3390/jcm14010112
Chicago/Turabian StyleBatbileg, Lkhamdari, Sevjidmaa Baasanjav, Khosbayar Tulgaa, Oyuntugs Byambasukh, Khurelbaatar Naymdavaa, Enkhtur Yadamsuren, and Baasanjargal Biziya. 2025. "Skin Physiological Parameters and Their Association with Severe Atopic Dermatitis in Mongolian Children" Journal of Clinical Medicine 14, no. 1: 112. https://doi.org/10.3390/jcm14010112
APA StyleBatbileg, L., Baasanjav, S., Tulgaa, K., Byambasukh, O., Naymdavaa, K., Yadamsuren, E., & Biziya, B. (2025). Skin Physiological Parameters and Their Association with Severe Atopic Dermatitis in Mongolian Children. Journal of Clinical Medicine, 14(1), 112. https://doi.org/10.3390/jcm14010112






