Abstract
Introduction: The management of aortic dissection has evolved significantly over the decades, with the frozen elephant trunk (FET) procedure emerging as a key technique for treating complex aortic pathologies. Recent practices involve deploying the FET prosthesis more proximally in the aorta (Zone 0) to reduce complications, leading to questions about its impact on long-term aortic remodeling compared to traditional Zone 2 deployment. Methods: This retrospective analysis utilized 3D segmentation software to assess the volumetric changes in aortic remodeling after acute Type A aortic dissections, comparing FET stent graft deployment in Zone 0 and Zone 2. The study included 27 patients operated on between 2020 and 2022, with volumetric measurements taken from postoperative and 6-month follow-up CT scans. Statistical analyses were performed to evaluate the differences in the aortic true lumen (TL) and the perfused false lumen (PFL) between the two groups. Results: Both Zone 0 and Zone 2 deployments resulted in significant true lumen (TL) increases (Z0 p = 0.001, Z2 p < 0.001) and perfused false lumen (PFL) decreases (Z0 p = 0.02, Z2 p = 0.04), with no significant differences in volumetric changes between the groups (p = 0.7 post op and p = 0.9 after 6 months). The distal anastomosis in Zone 0 did not compromise the aortic remodeling outcomes and was associated with reduced distal ischemia and cerebral perfusion times (p = 0.041). The angle measurements in Zone 0 did not show any significant changes after the 6-month control (p = 0.2). However, in Zone 2, a significant change was detected. (p = 0.022). The part comparison analyses did not indicate significant differences in aortic deviation between the groups (p = 0.62), suggesting comparable effectiveness in aortic remodeling. Conclusions: Performing the distal anastomosis more proximally in Zone 0 offers technical advantages without compromising the effectiveness of aortic remodeling compared to the traditional Zone 2 deployment. This finding supports the continued recommendation of Zone 0 deployment in the management of acute Type A aortic dissections, with ongoing studies being needed to confirm the long-term outcomes and survival benefits.
1. Introduction
Managing aortic dissection remains a challenging endeavor [1]. In the last decades, numerous treatment methodologies have been proposed and established. Most famously, the 2-stage elephant trunk approach was first outlined in 1983 [2]. This method sparked the idea of integrating a hybrid prosthesis featuring a distal stent in the descending aorta. Over time, this concept underwent significant evolution, until the first description of the frozen elephant trunk (FET) procedure in 1996 [3,4].
The FET technique changed the treatment landscape for complex aortic pathologies. Compared to the traditional 2-stage approach, or total arch replacement, the FET procedure simplified the therapeutic process significantly [1]. In recent times, the FET method has solidified its position as an invaluable tool in the management of a spectrum of aortic pathologies, particularly in the management of the acute aortic Type A dissection [4,5].
Despite this considerable advancement, FET remains a complicated surgical procedure, with a high mortality rate of 8–10% and neurological complications or spinal injury rate of 7% and 4%, respectively [6]. Its efficacy depends on the stabilization of the aorta’s true lumen and the subsequent thrombosis of the false lumen, a process known as “aortic remodeling” [7]. By the insertion of the stented portion of the hybrid prosthesis into the true lumen, reentries are sealed, and the true lumen (TL) is expanded. False lumen (FL) thrombosis at the level of the stent graft, or even beyond, leads to FL shrinkage by thrombus resorption. This process of TL growth and FL shrinkage is called positive aortic remodeling.
The largest single-center experience with the FET technique using the “E-vita Open” prosthesis (Artivion, Kennesaw, GA, USA) was reported in Essen, Germany, and showed a successful aortic remodeling in 90% of patients with acute dissection, and a correlation of 5-year and 10-year freedom from re-intervention rates of 87% and 74%, respectively [8,9]. The success of the remodeling was influenced by multiple variables, notably the deployment location of the implanted stent and allowing the volumetric increase in the distal hybrid prosthesis.
Historically, the distal anastomosis of the stent graft of the FET Prothesis was performed in aortic Zone 3, before moving proximally to be performed in Zone 2 [1,3,4]. In pursuit of surgical simplicity, and to mitigate technical challenges, many medical institutions have started deploying the FET prosthesis more proximally in Zone 0 [10,11]. In our center, we shifted from deploying the stent in Zone 2 to Zone 0 in 2021. This strategic transition manifested in tangible benefits, notably, a marked decrease in distal ischemia and cerebral perfusion durations, as well as less renal insufficiency requiring temporary dialysis. However, the following question persists: Does the more proximal deployment in Zone 0 ensure long-term aortic remodeling comparable to the traditional FET stenting in aortic Zone 2?
To better understand the changes and their effects on aortic remodeling, we performed an imaging-based retrospective analysis of aortic volumetric changes using 3D segmentation software and compared the results of deploying the FET stent graft in Zone 2 and Zone 0 following acute Type A aortic dissections.
2. Patients and Methods
The research protocol was approved by the local institutional medical ethics committee. The need for informed consent was waived due to the retrospective nature of the analysis and the use of anonymous data.
The inclusion criteria were as follows: (1) operation for acute Type A dissection, (2) survival for at least 6 months, and (3) complete follow-up with high-resolution CT both directly after the surgery and 6 months after. All patients who had proximal anastomosis performed in other aortic zones were excluded.
In our study, 27 patients were included (Zone 0: 12 patients, Zone 2: 15 patients) who were operated on between 2020 and 2022.
Surgical Technique
The operation was performed with the patient in a supine position under endotracheal intubation anesthesia. Skin disinfection was carried out for more than 3 min, according to a standardized hygiene protocol. A puncture of the left femoral artery originating from the true lumen was carried out, and an insertion of a 4 French pigtail catheter via a 5 French sheath, secured by transesophageal echocardiography (TEE), into the true lumen at the level of the left subclavian artery (LSA) was performed. An incision was made on the right infraclavicular area, exposing the right axillary artery, while preserving the plexus, and performing an end-to-side anastomosis with an 8-mm prosthesis. The prosthesis was then flushed with heparin solution.
After that, systemic heparin was intravenously administrated, and then a median sternotomy was performed. The pericardial sac was opened from the diaphragm until the highest point that could be reached superiorly. Retraction stitches were placed on the edges of the pericardial sac and tightly attached to the sternal retractor or to the skin to elevate the heart anteriorly. Cautious preparation of the aortic arch with encircling of the brachiocephalic trunk, left carotid artery, and left subclavian artery, was carried out. Cannulation of the right atrium with a two-stage cannula was performed, connecting the arterial line to the 8-mm prosthesis of the right axillary artery. Initiation of extracorporeal circulation (ECC) and cooling to 26 °C was carried out. An insertion of a left ventricular vent through the superior right pulmonary vein was performed. The aorta was then clamped, and the dissected ascending aorta was incised enough to achieve a good exposure of the coronary ostia. The selective cardioplegia was administrated into both ostia, 1600 mL over >5 min, with ice-water cooling. The ascending aorta was resected up to the sinotubular junction, the hematoma was removed from the dissected right and non-coronary sinus, and the reattachment of the commissures with 4/0 Teflon-coated prolene sutures was performed. In case it was required, the root was glued with Bio-glue.
The sinotubular junction was measured and the appropriate aortic prosthesis was selected. An end-to-end anastomosis with the selected prosthesis reinforced with Teflon-coated prolene 4/0 sutures was performed. After verification of the anastomosis with cardioplegia and the achievement of the target temperature, the ligature of the left subclavian artery was performed. The left common carotid artery (LCA) was clamped, followed by transition to selective cerebral perfusion (18 °C) by clamping the brachiocephalic trunk, the opening of the arch, and resecting the brachiocephalic trunk and left carotid artery.
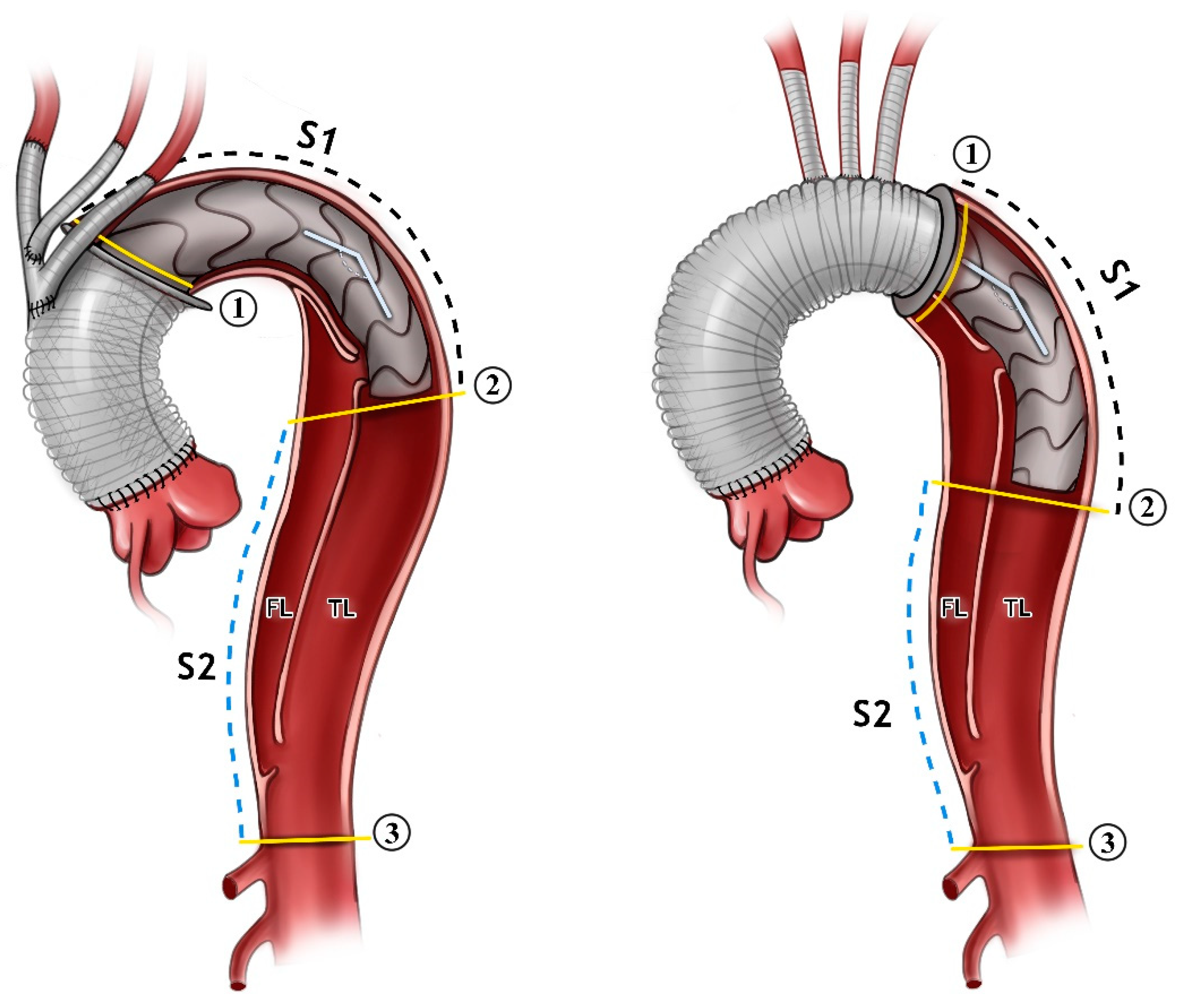
The advancement of the pigtail catheter and Amplatzer guide-wire was performed next. The selection of the appropriate frozen elephant trunk prosthesis and the insertion of the prosthesis into the true lumen guided with the wire was carried out. The release of the prosthesis in Zone 0 or Zone 2 was then performed. Distal anastomosis was then reinforced with Teflon-coated prolene 3/0 in the selected aortic Zone (Figure 1).
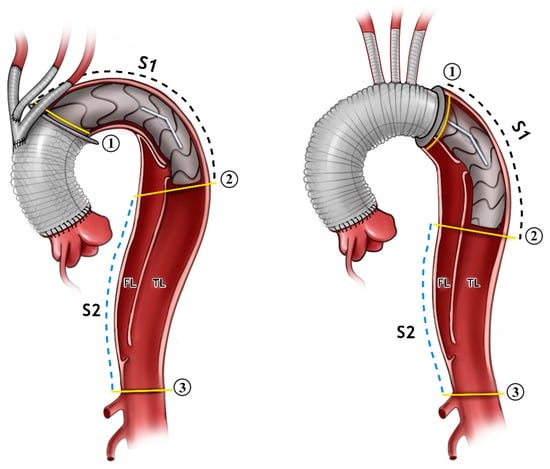
Figure 1.
An illustration showing the FET inserted in Zone 0 to the (left) and Zone 2 to the (right). The angle between the aortic arch and desc. aorta is demonstrated. The measurements were carried out just for the stent part alone (Segment 1, S1), and distally from the stent down to the coeliac trunk (Segment 2, S2). All of the measurements were carried out for the false lumen (FL) and true lumen (TL) in both segments. The aortic cross-sectional area is shown in the yellow lines in position 1, 2, and 3.
The insertion of a Foley catheter into the stent graft segment for perfusion via the adjunct pump to end the distal ischemia was performed.
Then, the anastomosis of the head–neck vessels to the arch prosthesis was performed using mostly a 12-mm prosthesis for the brachiocephalic trunk and a 6-mm prosthesis for the left carotid artery. Afterwards, de-airing in the head-down position and transition to whole-body perfusion was performed. At the end, an end-to-end anastomosis of the ascending aorta prosthesis to the aortic arch prosthesis was performed.
After releasing the aortic clamp, the heart began beating in sinus rhythm.
Then, an incision was made in the left infraclavicular area, exposing the left axillary artery while preserving the plexus, and an end-to-side anastomosis with a 7-mm prosthesis on the undersurface of the vessel was carried out in such a way that the prosthesis could be easily guided extranatomically to the ascending aorta through the 2nd intercostal space.
The 7-mm bypass to the left subclavian artery was guided to the ascending aorta through the second intercostal space, the ascending prosthesis was tangentially clamped, and the bypass was anastomosed end-to-side with prolene 5/0.
After sufficient reperfusion and rewarming, gradual weaning off cardiopulmonary bypass (CPB) was performed. Using stable hemodynamics, the heart–lung machine was decanulated and the protamine was administrated. After hemostasis, and at the end of the procedure, chest drains and temporary pacemaker leads were placed, and the sternotomy was closed using sternal wires.
3. Volumetric Analysis
Semiautomatic segmentation using 3mensio Vascular (Pie Medical Imaging, Netherlands) was performed for all of the CT scans. After manual identification of the true lumen (TL) of the aorta, a centerline was created beginning in the aortic root and extending into the abdominal aorta. The TL extension was automatically identified, and manual corrections were performed on the reconstructed orthogonal CT slices, as required. The resulting segmentation allowed cross-sectional area measurements to be taken at any selected point, as well as length and volume measurements of desired parts.
Areas of the TL were measured at the proximal and distal end of the stent graft and at the level of the celiac trunk (Figure 1).
Volume measurements of the TL and the PFL at the stent graft level (S1), between the stent graft and celiac trunk (S2), and the total thoracic aorta (S1+S2) were taken.
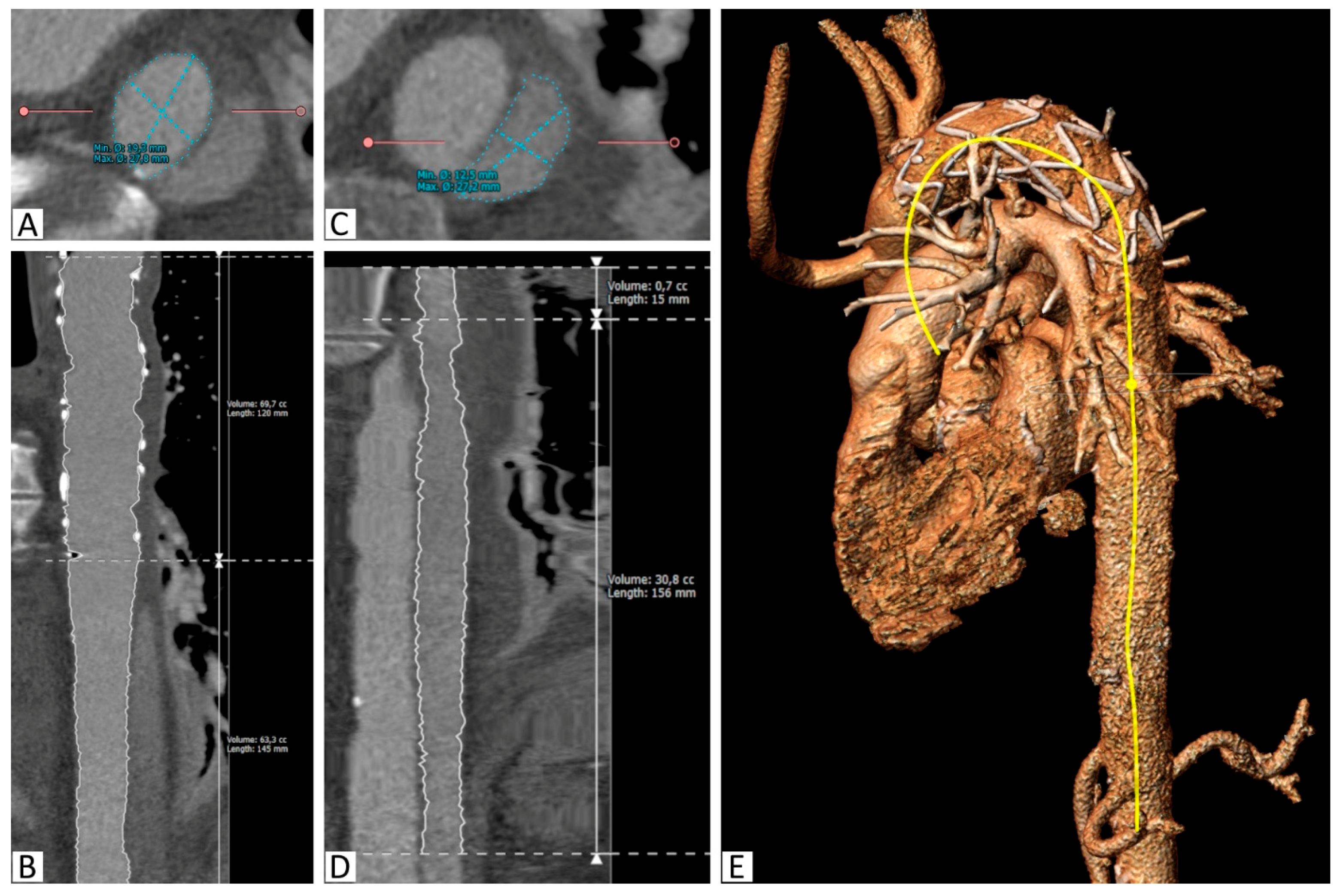
The angle between the aortic arch (measured at the most cranial point of the arch) and the distal end of the stent graft was measured on the created 3D model. All of the measurements were obtained independently by two observers (Figure 1 and Figure 2).
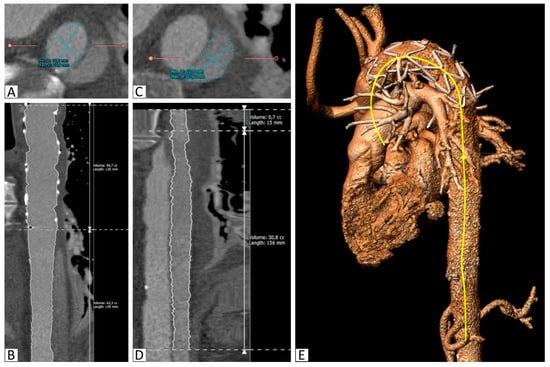
Figure 2.
Segmentation of the aorta with the implanted stent. (A): Segmentation of the true lumen in the descending thoracic aorta distal to the stent graft on a reconstructed slice orthogonal to the vessel, (B): Curved multiplanar reconstruction of the true lumen between the proximal stent graft and the celiac trunk, including length and volume calculations, (C): Segmentation of the false lumen in the descending thoracic aorta distal to the stent graft on a reconstructed slice orthogonal to the vessel, (D): Curved multiplanar reconstruction of the false lumen between its origin in the stent graft and the celiac trunk, including length and volume calculations, (E): 3D reconstruction with the semiautomatically created center-line (yellow).
4. Part Comparison Analysis
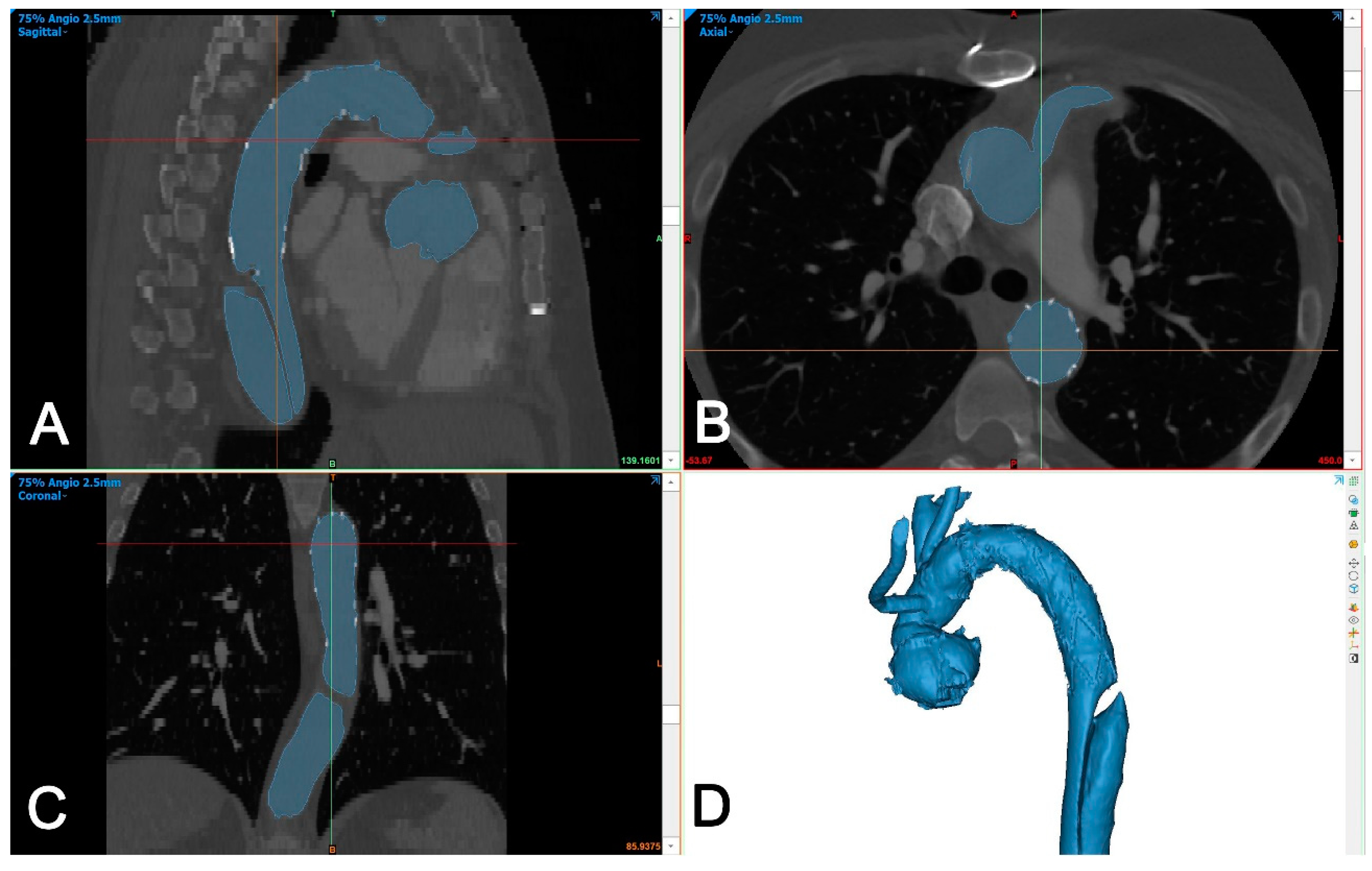
The DICOM dataset was segmented using Mimics Medical version 25 (Mimics Innovation Suite, Materialise, Belgium). The segmentation was performed using a semi-automated (threshold) tool and then the intra-aortic blood volume was separated from the surroundings using the split mask tool (Figure 3).
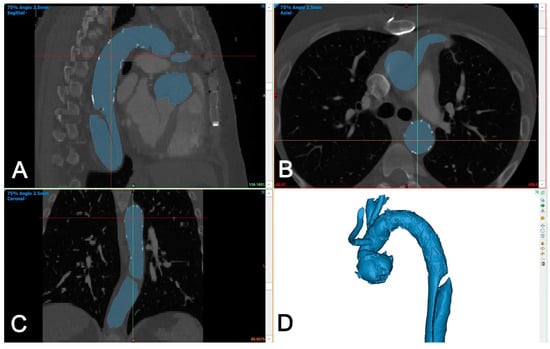
Figure 3.
Segmentation of aortic volume using dedicated 3D-engineering software (Mimis Innovation suite, Materialise, Belgium). (A–C): Aortic volume is semi-automatically segmented (blue color) and manually corrected in three perpendicular planes (sagittal (A), axial (B), and coronal (C)), if needed. (D): A 3D-volume is generated automatically and is used for further processing.
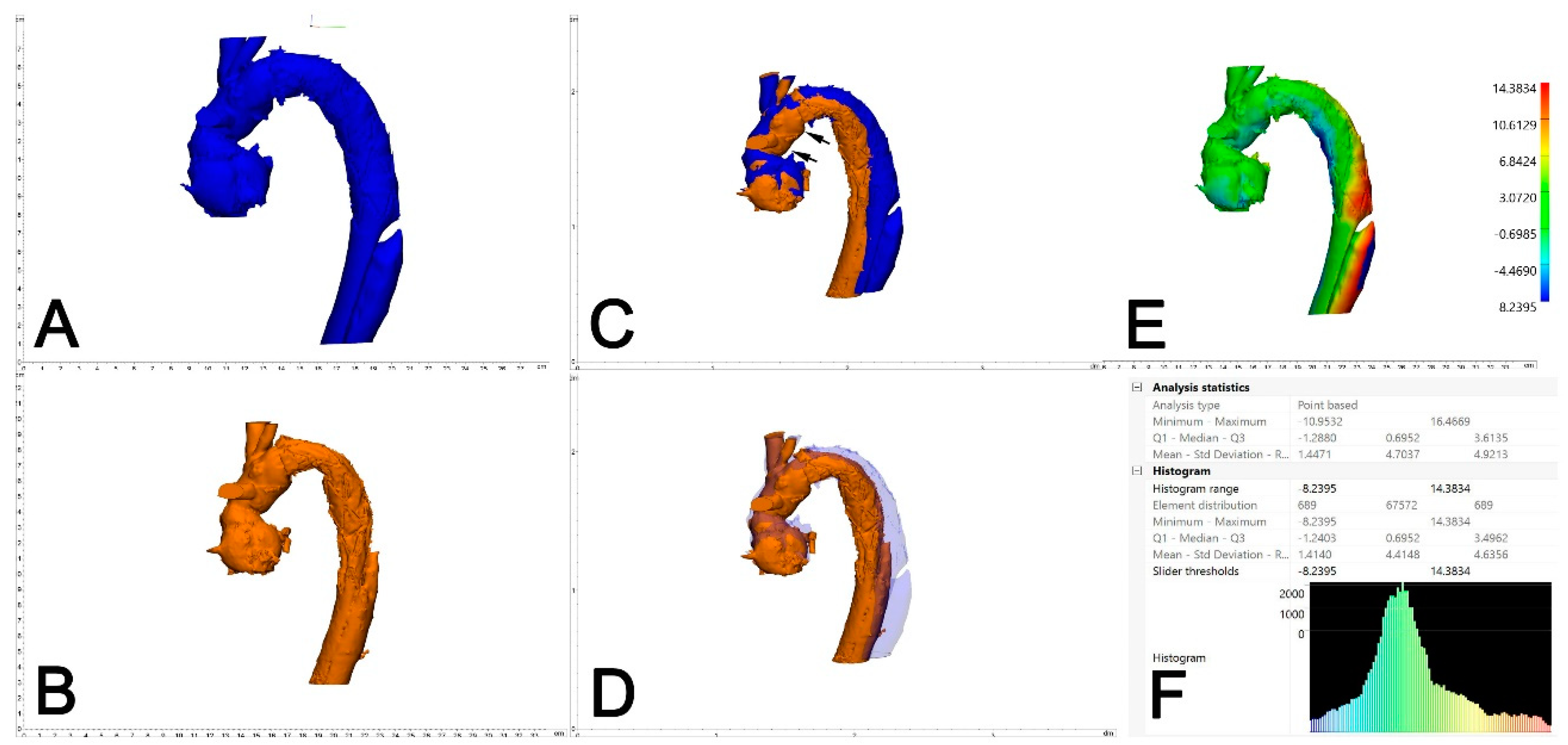
The two generated 3D models of each patient (one generated from postoperative CTA and one generated from the 6-month control CTA) were imported into Materialise 3-matic (Materialise, Belgium). After the alignment of the two chronological models using the interactive translate and global registration tool, a part comparison tool was used. The part comparison tool was used to assess the deviation (distance) of the surfaces of the discharge and 6-month follow-up 3D models. The deviation between the discharge and the 6-month follow-up 3D models was calculated for S1 alone and then combined with the thoracic aorta (S1+S2). The results are shown as a color-coding of the 3D Files (Figure 4). The mean value given in the histogram was used for the statistical analysis.
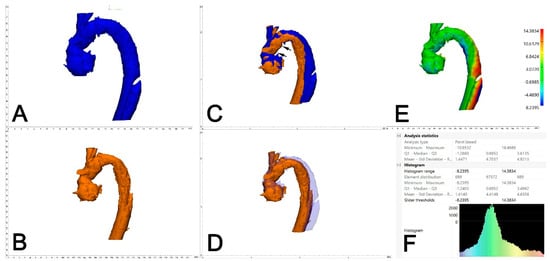
Figure 4.
Assessment of aortic deviation between 0 and 6 months following FET. A dedicated 3D-engineering software-tool (“Part comparison analysis”) was employed. Therefore, the 3D aortic volumes at 6 months (A, blue) and 0 months (B, orange) were aligned/overlayed using the distal and the proximal anastomosis of the Dacron part of the FET (C, black arrows). With reduced transparency of the aortic volume at 6 months (blue), adequate alignment between the two aortic volumes is demonstrated (D). The result of the part comparison tool is depicted as a color-coded “heat-map” of the aortic volume (deviation in mm) (E). The area of maximum conformational change (deviation) is located at aortic Zones 3 and 4. For statistical analysis, the respective values (mean deviation) were retrieved from the PCA analysis statistics (F).
5. Statistical Analysis
The statistical analysis was performed with GraphPad Prism 10 (GraphPad software, Boston, MA, USA). The mean and standard deviation were calculated for continuous variables in case of normality, otherwise the median and quartiles (min. and max.) were reported. The binary variables were compared using the Pearson chi-square or Fisher exact test. Continuous variables were analyzed for normality using the Shapiro–Wilk test. In the case of normal distribution, the paired t-test was used. Otherwise, a Mann–Whitney U test was performed.
6. Results
6.1. Patients’ Demography and Operative Data
Discharge CTA was performed at 8 ± 4 days, and a 6-month CTA was performed 181 ± 47 days postoperatively. No significant difference in patient demographics and comorbidities were noticed between the two groups (Table 1). The durations of surgery, cardiopulmonary bypass (CPB), and aortic cross-clamping were similar. Distal ischemia was significantly reduced in the Zone 0 group (25 ± 6 min for Z0 vs. 31 ± 11 min for Z2, p = 0.041).

Table 1.
Demographic and operative data.
In all of the patients in Zone 2, an E-vita Open prosthesis was utilized. In the Zone 0 group, one patient received a Thoraflex prosthesis (Terumo Aortic, Sunrise, FL, USA), while all of the other patients received E-vita Open neo prostheses. The mean stent length was 14 ± 2.8 cm in the Zone 0 group and 13 ±0.5 cm in the Zone 2 group. Among the Zone 0 group, four patients received an 18-cm stent, three patients received a 13-cm stent, and five patients received a 12-cm stent. However, in the Zone 2 group, all of the patients received a 13-cm stent, except for one patient, who received a 15-cm stent.
6.2. Postoperative Outcome
No mortality was observed within the first 30 days in either group. No significant differences were noticed between the two groups regarding their hospital stay (16 ± 7 in Zone 0 vs. 18 ± 11 days in Zone 2, p = 0.6) or in intensive care unit (ICU) stay (4 ± 2 days in Zone 0 vs. 6 ± 7 days in Zone 2, p = 0.09). Dialysis was only necessary for two patients of the Zone 2 group. The neurological status was impaired preoperatively in two patients of the Zone 0 group, and no progress was documented until discharge. (p = 0.1).
6.3. CTA Analysis
Table 2 presents the volumetric measurements obtained from both groups from the true and false lumen.

Table 2.
Outcome parameters.
6.3.1. Volumetric Analysis
A significant TL increase between discharge and 6-month follow-up was observed in both groups in S1, S2, and the total thoracic aorta (S1+S2). The mean TL volume change in S1 was 30% in the Zone 0 and Zone 2 groups (p = 0.69). In S2, the mean TL volume increase was 50% in the Zone 0 group and 40% in the Zone 2 group (p = 0.78).
Only minimally perfused false lumen (PFL) was found at the stent graft level in Segment 1 (Zone 0: 2.5 +− 8 cc Zone 2: 0.7 +− 1.3 cc) at discharge. This minimal PFL further decreased after 6 months (Zone 0: 0.5 +− 1.7 cc, Zone 2: 0 +− 0.2 cc). In the distal of the stent graft (S2), significant PFL reduction in both groups was observed.
This observation was supported by the measurements of the aortic cross-sectional TL area, which showed a significant increase at level 1 in both groups (p = 0.004 vs. 0.0004). The TL area increase was only significant in the Zone 2 group at level 2.
At the 6-month follow-up endpoint, the total aortic volume in both groups after 6 months was almost identical, averaging 125 cm (p = 0.9).
6.3.2. Angle Measurement Analysis
The postoperative angle measurements and those taken at the 6-month follow-up showed the mean angle in Z-0 FET to be more stable (77° postoperatively, 72° at 6-month follow-up) compared to the Z-2 group (84° postoperatively, 74° at 6-month follow-up).
A significant difference between both groups was observed at discharge (p = 0.042); however, by the 6-month mark, as the curvature of the Zone 2 FET tended to flatten, no significant difference was noted (p = 0.551) (Figure 5).
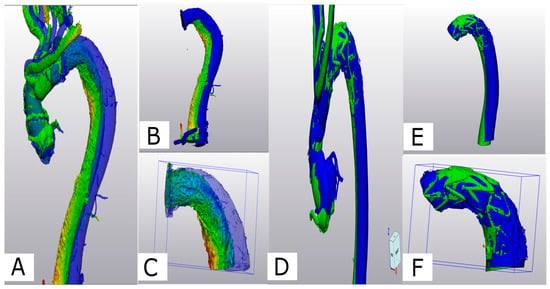
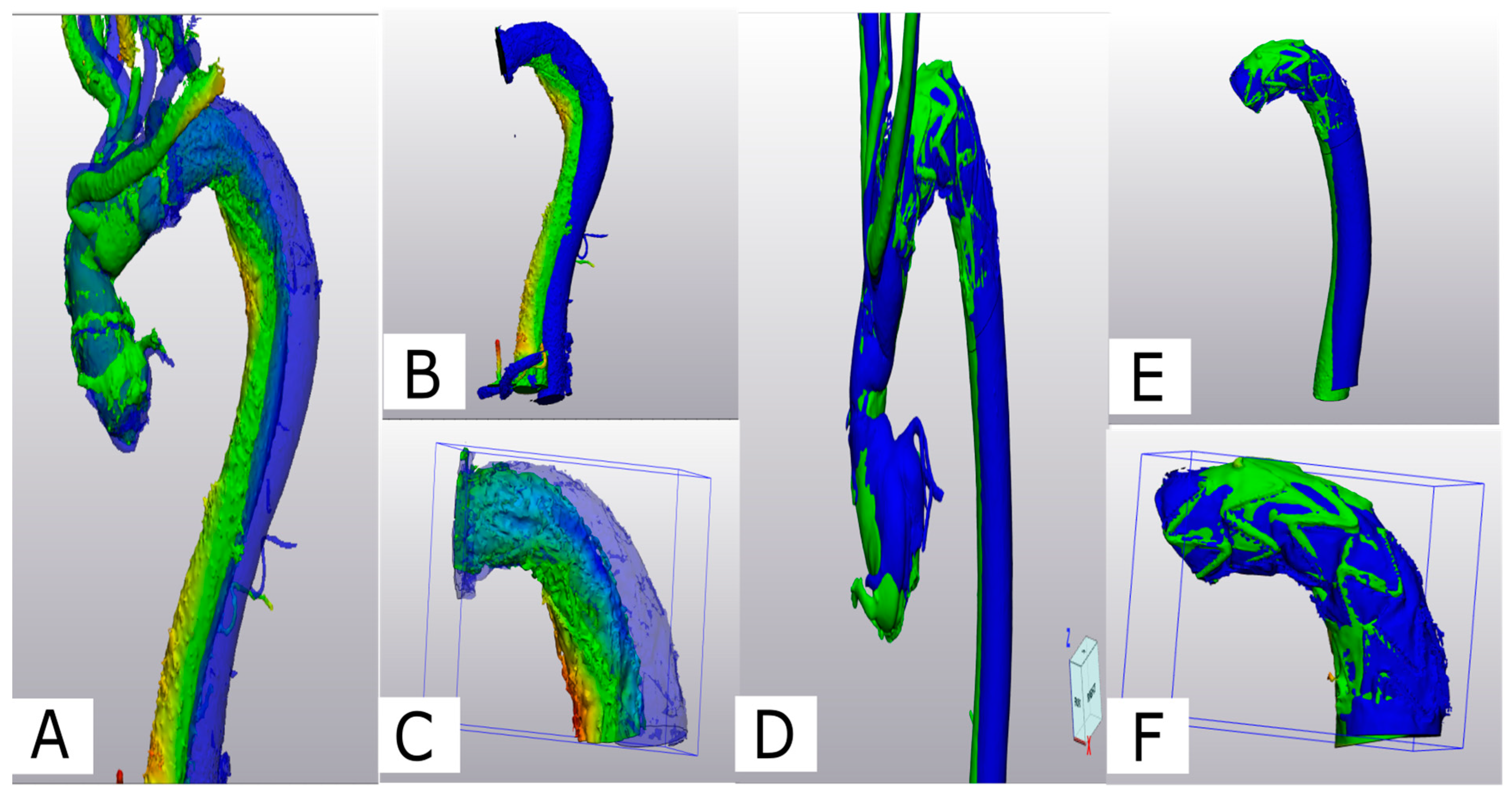
Figure 5.
(A) Comparison of aortic conformational change between Z2- (A–C) and Z0-FET (D–F): Z-2-FET a deviation analysis is shown for the post-operative 3D volume in green and the 6-month 3D volume in blue. (B) Z-2-FET stent with desc. aorta (S1+2) showing the deviation. (C) Z-2-FET stent segment (S1) deviation. (D) Z-0-FET on the middle deviation analysis between the post-operative model in green and the 6-month control model in blue. (E) Z-0-FET stent with desc. aorta (S1+2) showing minimal deviation. (F) Z-0-FET stent segment (S1) with minimal deviation.
6.3.3. Part Comparison Analysis (PCA)
The PCA revealed an average deviation of 4.4 mm across S1 and S2 segments in Z-0 FET, compared to an average deviation of 5.3 mm in Z-2 FET. Specifically, for the S1 segment, a deviation of 1.3 mm was noted in Z-0 FET, while a 2.0-mm deviation was observed in Z-2 FET. The statistical analysis between the two groups did not show a significant difference (p = 0.3).
7. Discussion
In our study, we observed an increase in the true lumen (TL) across both Segment 1 and Segment 2, even within our short (6 months) follow-up. Following the frozen elephant trunk (FET) procedure, the minimally perfused false lumen (FL) at the stent graft level (S1) showed further reduction, and even this minimal partial false lumen (PFL) disappeared after 6 months. The FET’s efficacy in reducing PFL was significant in both groups, regardless of whether it was implanted in Zone 0 (Z0) or Zone 2 (Z2).
On the stent geometry observation, and despite concerns that proximal anastomosis in Zone 0 might lead to a greater deviation of the stent graft, neither our part comparison analysis nor the angle measurements provide any evidence to support those concerns [12].
The use of the FET procedure for the management of acute aortic dissection is increasing, due to its established efficacy [12,13]. Our findings demonstrate that both Zone 0 and Zone 2 FET procedures yield favorable remodeling outcomes [13]. Notably, Zone 0 showed less deviation in outcomes, adding to the advantage of the avoidance of extensive periaortic tissue dissection and aortic arch wall resection, presenting fewer technical challenges and possibly reducing surgery duration [14,15,16,17,18,19].
To our knowledge, this is the first study to demonstrate similar behavior in TL increase and PFL decrease between Zone 0 and Zone 2 FET. It is also important to highlight the significance of our novel 3D-based methodology in assessing postoperative changes in aortic morphology. This approach provides precise and comprehensive evaluations, allowing for detailed comparisons between the different zones of distal anastomosis. By leveraging the analytical capabilities of 3D-engineering software, we were able to detect subtle variations in aortic remodeling patterns, contributing to a deeper understanding of the effects of FET procedures [20,21,22,23].
The results of this study suggest that similar outcomes in terms of aortic remodeling can be expected regardless of the zone of distal anastomosis used. This finding holds significant implications for surgical decision making, as it indicates that Zone 0 FET procedures offer comparable benefits in promoting distal remodeling.
8. Conclusions
The current results reveal no difference between patients operated on in Zone 0 and those operated on in Zone 2 regarding the key markers for aortic remodeling. Taking into account the technical advantage provided by moving the procedure more proximally, this method can continue to be recommended. The final results concerning aortic remodeling, a survival benefit, and long-term outcomes are still pending.
9. Limitations
This study is based on data from a single center with a small sample size and has a retrospective design for a limited follow-up of 6 months. The results of our study might be limited by a survival bias, due to only including patients with an available CT scan at 6 months postoperatively.
Author Contributions
A.G.: Conceptualization, Investigation, Methodology, Software, Validation, Formal analysis, and Writing—original draft. P.P.: Investigation, Methodology, and Writing. R.C.: Formal analysis and Validation. C.P.: Validation, Review, and Editing. D.-S.D.: Validation, Review, and Editing. H.T.: Validation, Review, and Editing. B.D.: Conceptualization, Investigation, Methodology, Validation, Formal analysis, and Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ethical review and approval were waived for this study due to its retrospective nature and the use of anonymous data. The nature of the study is covered by the State Hospital Law, sections 36 and 37, in Rhineland-Palatinate.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the medical illustrator Ashraf Ragab Refaey, a veterinarian, for his help with the medical illustrations. (Figure 1).
Conflicts of Interest
Daniel-S. Dohle was a consultant for Artivion during the time of study. The other authors declare that there are no conflicts of interests.
References
- Di Marco, L.; Pantaleo, A.; Leone, A.; Murana, G.; Di Bartolomeo, R.; Pacini, D. The Frozen Elephant Trunk Technique: European Association for Cardio-Thoracic Surgery Position and Bologna Experience. Korean J. Thorac. Cardiovasc. Surg. 2017, 50, 1–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borst, H.G.; Walterbusch, G.; Schaps, D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac. Cardiovasc. Surg. 1983, 31, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ohnishi, K.; Kaneko, M.; Ueda, T.; Kishi, D.; Mizushima, T.; Matsuda, H. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996, 94 (Suppl. S9), II188–II193. [Google Scholar] [PubMed]
- Karck, M.; Chavan, A.; Hagl, C.; Friedrich, H.; Galanski, M.; Haverich, A. The frozen elephant trunk technique: A new treatment for thoracic aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2003, 125, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Iida, Y.; Fujii, S.; Shimizu, H.; Sawa, S. Patterns of aortic remodelling after total arch replacement with frozen elephant trunk for acute aortic dissection. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Preventza, O.; Liao, J.L.; Olive, J.K.; Simpson, K.; Critsinelis, A.C.; Price, M.D.; Galati, M.; Cornwell, L.D.; Orozco-Sevilla, V.; Omer, S.; et al. Neurologic complications after the frozen elephant trunk procedure: A meta-analysis of more than 3000 patients. J. Thorac. Cardiovasc. Surg. 2020, 160, 20–33.e4. [Google Scholar] [CrossRef] [PubMed]
- Dohle, D.S.; Tsagakis, K.; Janosi, R.A.; Benedik, J.; Kühl, H.; Penkova, L.; Stebner, F.; Wendt, D.; Jakob, H. Aortic remodelling in aortic dissection after frozen elephant trunk. Eur. J. Cardiothorac. Surg. 2016, 49, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.; Sherzad, H.; Bashir, M.; Mariscalco, G. The frozen elephant trunk procedure: Indications, outcomes and future directions. Cardiovasc. Diagn. Ther. 2022, 12, 708–721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jakob, H.; Idhrees, M.; Bashir, M. Frozen elephant trunk with straight vascular prosthesis. Ann. Cardiothorac. Surg. 2020, 9, 164–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wada, T.; Yamamoto, H.; Takagi, D.; Kadohama, T.; Yamaura, G.; Kiryu, K.; Igarashi, I. Aortic remodeling, reintervention, and survival after zone 0 arch repair with frozen elephant trunks for acute type A aortic dissection: Midterm results. JTCVS Tech. 2022, 14, 29–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, K.F.L.; Bhatia, I.; Chan, T.L.D.; Au, W.K.T.; Ho, K.L.C. Proximalization of Frozen Elephant Trunk Procedure: Zone 0 or 1 versus Zone 2 or 3 Arch Repair. Thorac. Cardiovasc. Surg. 2024, 72, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Osswald, A.; Schucht, R.; Schlosser, T.; Jánosi, R.A.; Thielmann, M.; Weymann, A.; Ruhparwar, A.; Tsagakis, K. Changes of stent-graft orientation after frozen elephant trunk treatment in aortic dissection. Eur. J. Cardiothorac. Surg. 2021, 61, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Spinella, G.; Finotello, A.; Conti, M.; Faggiano, E.; Gazzola, V.; Auricchio, F.; Chakfé, N.; Palombo, D.; Pane, B. Assessment of geometrical remodelling of the aortic arch after hybrid treatment. Eur. J. Cardiothorac. Surg. 2019, 55, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Gallingani, A.; Venturini, A.; Scarpanti, M.; Mangino, D.; Formica, F. Frozen Elephant Trunk: Technical Overview and Our Experience with a Patient-Tailored Approach. J. Clin. Med. 2022, 11, 1120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kozlov, B.; Panfilov, D.; Lukinov, V. Frozen Elephant Trunk for Aortic Dissection Using Different Hybrid Grafts: Preliminary Results from a Prospective Study. J. Pers. Med. 2023, 13, 784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, S.Z.C.P.; Lopuszko, A.; Munir, W.; Adams, B.; Bashir, M. Aortic proximalization-Zone 0 versus Zone 2: A concept or true challenge? J. Card. Surg. 2021, 36, 3319–3325. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kadohama, T.; Yamaura, G.; Tanaka, F.; Takagi, D.; Kiryu, K.; Itagaki, Y. Total arch repair with frozen elephant trunk using the "zone 0 arch repair" strategy for type A acute aortic dissection. J. Thorac. Cardiovasc. Surg. 2020, 159, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Jakob, H.; Shehada, S.E.; Dohle, D.; Wendt, D.; El Gabry, M.; Schlosser, T.; Tsagakis, K. New 3-zone hybrid graft: First-in-man experience in acute type I dissection. J. Thorac. Cardiovasc. Surg. 2022, 163, 568–574.e1. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.M.; Ali Hassan, S.M.; Nguyen, G.; Bisleri, G. Zone 0 frozen elephant trunk for type A retrograde acute aortic dissection following endovascular stenting of the arch. J. Card. Surg. 2021, 36, 2124–2126. [Google Scholar] [CrossRef] [PubMed]
- Pichlmaier, M.; Tsilimparis, N.; Hagl, C.; Peterss, S. New anatomical frozen elephant trunk graft for zone 0: Endovascular technology reduces invasiveness of open surgery to the max. Eur. J. Cardiothorac. Surg. 2022, 61, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Okita, Y.; Uchida, N.; Kato, M.; Miyamoto, S.; Matsuda, H.; Nakai, M. J-Open Cardiac Aortic Arch Disease Replacement Surgical Therapy Study Investigators. Comparative study of Japanese frozen elephant trunk device for open aortic arch repairs. J. Thorac. Cardiovasc. Surg. 2022, 164, 1681–1692.e2. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, R.; Pantaleo, A.; Berretta, P.; Murana, G.; Castrovinci, S.; Cefarelli, M.; Folesani, G.; Di Eusanio, M. Frozen elephant trunk surgery in acute aortic dissection. J. Thorac. Cardiovasc. Surg. 2015, 149 (Suppl. S2), S105–S109. [Google Scholar] [CrossRef] [PubMed]
- Dorweiler, B.; Baqué, P.E.; Chaban, R.; Ghazy, A.; Salem, O. Quality Control in 3D Printing: Accuracy Analysis of 3D-Printed Models of Patient-Specific Anatomy. Materials 2021, 14, 1021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).