Treatment-Free Remission in Chronic Myeloid Leukemia
Abstract
1. Introduction
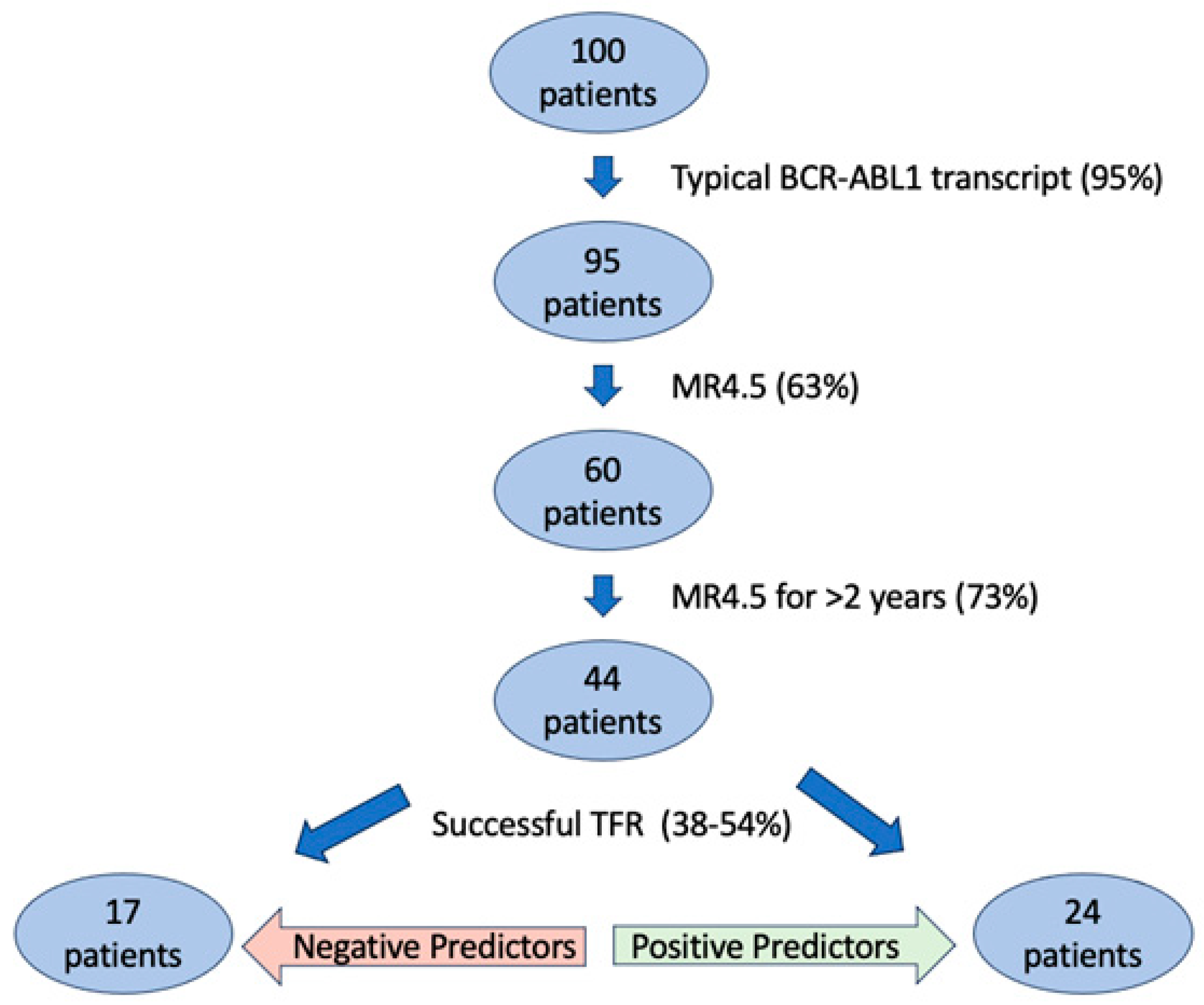
2. TFR Eligibility
3. Strategies to Increase TFR Eligibility
4. TFR Efficacy
5. TFR Failure
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henderson, C.A. Imatinib: The promise of a “magic bullet” for cancer fulfilled. J. Med. Assoc. Ga. 2003, 92, 12–14, 22. [Google Scholar]
- Definition of Philadelphia Chromosome—NCI Dictionary of Cancer Terms—NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/philadelphia-chromosome (accessed on 4 February 2024).
- Kantarjian, H.; O’Brien, S.; Jabbour, E.; Garcia-Manero, G.; Quintas-Cardama, A.; Shan, J.; Rios, M.B.; Ravandi, F.; Faderl, S.; Kadia, T.; et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: A single-institution historical experience. Blood 2012, 119, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; Johnson, J.R.; Pazdur, R.U.S. Food and Drug Administration Drug Approval Summary: Conversion of imatinib mesylate (STI571; Gleevec) tablets from accelerated approval to full approval. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 12–19. [Google Scholar] [CrossRef]
- Survival Rates for Chronic Myeloid Leukemia. Available online: https://www.cancer.org/cancer/types/chronic-myeloid-leukemia/detection-diagnosis-staging/survival-rates.html (accessed on 4 February 2024).
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef]
- Guidelines Detail. NCCN. Available online: https://www.nccn.org/guidelines/guidelines-detail (accessed on 3 September 2021).
- Vener, C.; Banzi, R.; Ambrogi, F.; Ferrero, A.; Saglio, G.; Pravettoni, G.; Sant, M. First-line imatinib vs second- and third-generation TKIs for chronic-phase CML: A systematic review and meta-analysis. Blood Adv. 2020, 4, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Abboud, C.; Berman, E.; Cohen, A.; Cortes, J.; DeAngelo, D.; Deininger, M.; Devine, S.; Druker, B.; Fathi, A.; Jabbour, E.; et al. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: From the perspective of a large group of CML experts. Blood 2013, 121, 4439–4442. [Google Scholar] [CrossRef]
- Shih, Y.-C.T.; Cortes, J.E.; Kantarjian, H.M. Treatment Value of Second-generation Bcr-Abl1 TKIs Compared With Imatinib to Achieve Treatment-free Remission in Patients With Chronic Myeloid Leukemia: A Modelling Study. Lancet Haematol. 2019, 6, e398–e408. [Google Scholar] [CrossRef]
- Narra, R.K.; Flynn, K.E.; Atallah, E. Chronic Myeloid Leukemia- The Promise of Tyrosine Kinase Inhibitor Discontinuation. Curr. Hematol. Malig. Rep. 2017, 12, 415–423. [Google Scholar] [CrossRef]
- Saifullah, H.H.; Lucas, C.M. Treatment-Free Remission in Chronic Myeloid Leukemia: Can We Identify Prognostic Factors? Cancers 2021, 13, 4175. [Google Scholar] [CrossRef]
- Sharf, G.; Marin, C.; Bradley, J.A.; Pemberton-Whiteley, Z.; Bombaci, F.; Christensen, R.I.O.; Gouimi, B.; Deekes, N.B.; Daban, M.; Geissler, J. Treatment-free remission in chronic myeloid leukemia: The patient perspective and areas of unmet needs. Leukemia 2020, 34, 2102–2112. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network—Home. Available online: https://www.nccn.org/ (accessed on 9 March 2024).
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Mahon, F.-X.; Réa, D.; Guilhot, J.; Guilhot, F.; Huguet, F.; Nicolini, F.; Legros, L.; Charbonnier, A.; Guerci, A.; Varet, B.; et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010, 11, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E.; Choi, S.Y.; Song, H.-Y.; Kim, S.-H.; Choi, M.-Y.; Park, J.S.; Kim, H.-J.; Kim, S.-H.; Zang, D.Y.; Oh, S.; et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: The KID study. Haematologica 2016, 101, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Branford, S.; Seymour, J.F.; Schwarer, A.P.; Arthur, C.; Yeung, D.T.; Dang, P.; Goyne, J.M.; Slader, C.; Filshie, R.J.; et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: Results from the TWISTER study. Blood 2013, 122, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Rousselot, P.; Charbonnier, A.; Cony-Makhoul, P.; Agape, P.; Nicolini, F.E.; Varet, B.; Gardembas, M.; Etienne, G.; Réa, D.; Roy, L.; et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Polydoros, F.; Apperley, J.F.; Milojkovic, D.; Pocock, C.; Smith, G.; Byrne, J.L.; de Lavallade, H.; O’Brien, S.G.; Coffey, T.; et al. De-escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): An interim analysis of a non-randomised, phase 2 trial. Lancet Haematol. 2017, 4, e310–e316. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Imagawa, J.; Murai, K.; Hino, M.; Kitawaki, T.; Okada, M.; Tanaka, H.; Shindo, M.; Kumagai, T.; Ikezoe, T.; et al. Treatment-free remission after first-line dasatinib discontinuation in patients with chronic myeloid leukaemia (first-line DADI trial): A single-arm, multicentre, phase 2 trial. Lancet Haematol. 2020, 7, e218–e225. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Imagawa, J.; Tanaka, H.; Nakamae, H.; Hino, M.; Murai, K.; Ishida, Y.; Kumagai, T.; Sato, S.; Ohashi, K.; et al. Final 3-year Results of the Dasatinib Discontinuation Trial in Patients with Chronic Myeloid Leukemia Who Received Dasatinib as a Second-line Treatment. Clin. Lymphoma Myeloma Leuk. 2018, 18, 353–360.e1. [Google Scholar] [CrossRef]
- Fujisawa, S.; Ueda, Y.; Usuki, K.; Kobayashi, H.; Kondo, E.; Doki, N.; Nakao, T.; Kanda, Y.; Kosugi, N.; Kosugi, H.; et al. Feasibility of the imatinib stop study in the Japanese clinical setting: Delightedly overcome CML expert stop TKI trial (DOMEST Trial). Int. J. Clin. Oncol. 2019, 24, 445–453. [Google Scholar] [CrossRef]
- Mori, S.; Vagge, E.; le Coutre, P.; Abruzzese, E.; Martino, B.; Pungolino, E.; Elena, C.; Pierri, I.; Assouline, S.; D’Emilio, A.; et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: The ISAV study. Am. J. Hematol. 2015, 90, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Tauchi, T.; Kitamura, K.; Miyamura, K.; Saburi, Y.; Hatta, Y.; Miyata, Y.; Kobayashi, S.; Usuki, K.; Matsumura, I.; et al. Deeper molecular response is a predictive factor for treatment-free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: The JALSG-STIM213 study. Int. J. Hematol. 2018, 107, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Polydoros, F.; Apperley, J.F.; Milojkovic, D.; Rothwell, K.; Pocock, C.; Byrne, J.; de Lavallade, H.; Osborne, W.; Robinson, L.; et al. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): A non-randomised, phase 2 trial. Lancet Haematol. 2019, 6, e375–e383. [Google Scholar] [CrossRef]
- Imagawa, J.; Tanaka, H.; Okada, M.; Nakamae, H.; Hino, M.; Murai, K.; Ishida, Y.; Kumagai, T.; Sato, S.; Ohashi, K.; et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): A multicentre phase 2 trial. Lancet Haematol. 2015, 2, e528–e535. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Nakaseko, C.; Nishiwaki, K.; Yoshida, C.; Ohashi, K.; Takezako, N.; Takano, H.; Kouzai, Y.; Murase, T.; Matsue, K.; et al. Dasatinib cessation after deep molecular response exceeding 2 years and natural killer cell transition during dasatinib consolidation. Cancer Sci. 2018, 109, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P.; García-Gutiérrez, V.; Jiménez-Velasco, A.; Larson, S.; Saussele, S.; Rea, D.; Mahon, F.-X.; Levy, M.Y.; Gómez-Casares, M.T.; Pane, F.; et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: The DASFREE study. Leuk. Lymphoma 2020, 61, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Nicolini, F.E.; Tulliez, M.; Guilhot, F.; Guilhot, J.; Guerci-Bresler, A.; Gardembas, M.; Coiteux, V.; Guillerm, G.; Legros, L.; et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: Interim analysis of the STOP 2G-TKI study. Blood 2017, 129, 846–854. [Google Scholar] [CrossRef]
- Takahashi, N.; Nishiwaki, K.; Nakaseko, C.; Aotsuka, N.; Sano, K.; Ohwada, C.; Kuroki, J.; Kimura, H.; Tokuhira, M.; Mitani, K.; et al. Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan. Haematologica 2018, 103, 1835–1842. [Google Scholar] [CrossRef]
- Mahon, F.-X.; Boquimpani, C.; Kim, D.-W.; Benyamini, N.; Clementino, N.C.D.; Shuvaev, V.; Ailawadhi, S.; Lipton, J.H.; Turkina, A.G.; De Paz, R.; et al. Treatment-Free Remission After Second-Line Nilotinib Treatment in Patients With Chronic Myeloid Leukemia in Chronic Phase: Results From a Single-Group, Phase 2, Open-Label Study. Ann. Intern. Med. 2018, 168, 461–470. [Google Scholar] [CrossRef]
- Hochhaus, A.; Masszi, T.; Giles, F.J.; Radich, J.P.; Ross, D.M.; Gómez Casares, M.T.; Hellmann, A.; Stentoft, J.; Conneally, E.; García-Gutiérrez, V.; et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: Results from the ENESTfreedom study. Leukemia 2017, 31, 1525–1531. [Google Scholar] [CrossRef]
- Ross, D.M.; Masszi, T.; Gómez Casares, M.T.; Hellmann, A.; Stentoft, J.; Conneally, E.; Garcia-Gutierrez, V.; Gattermann, N.; le Coutre, P.D.; Martino, B.; et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J. Cancer Res. Clin. Oncol. 2018, 144, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, F.E.; Dulucq, S.; Boureau, L.; Cony-Makhoul, P.; Charbonnier, A.; Escoffre-Barbe, M.; Rigal-Huguet, F.; Coiteux, V.; Varet, B.; Dubruille, V.; et al. Evaluation of Residual Disease and TKI Duration Are Critical Predictive Factors for Molecular Recurrence after Stopping Imatinib First-line in Chronic Phase CML Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 6606–6613. [Google Scholar] [CrossRef]
- Etienne, G.; Guilhot, J.; Rea, D.; Rigal-Huguet, F.; Nicolini, F.; Charbonnier, A.; Guerci-Bresler, A.; Legros, L.; Varet, B.; Gardembas, M.; et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Atallah, E.; Schiffer, C.A.; Radich, J.P.; Weinfurt, K.P.; Zhang, M.-J.; Pinilla-Ibarz, J.; Kota, V.; Larson, R.A.; Moore, J.O.; Mauro, M.J.; et al. Assessment of Outcomes After Stopping Tyrosine Kinase Inhibitors Among Patients With Chronic Myeloid Leukemia: A Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, 42–50. [Google Scholar] [CrossRef]
- Alam, A.; Lal, A.; Osman, H.Y.; Hussain, S.; Lee, D.; Kristensen, J. Deep Molecular Response (DMR) in Chronic Myeloid Leukemia (CML) the Tawam Experience. Blood 2015, 126, 5152. [Google Scholar] [CrossRef]
- Costa, A.; Breccia, M. How to improve treatment-free remission eligibility in chronic myeloid leukaemia? Br. J. Haematol. 2024, 204, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Burchert, A.; Saußele, S.; Baerlocher, G.M.; Mayer, J.; Brümmendorf, T.H.; La Rosee, P.; Heim, D.; Krause, S.W.; Le Coutre, P.; et al. S157: Nilotinib vs. nilotinib + peg-interferon αlpha induction and nilotinib or peg-interferon αlpha maintenance therapy for newly diagnosed chronic myeloid leukemia patients. the tiger trial. HemaSphere 2023, 7, e4695659. [Google Scholar] [CrossRef]
- Nicolini, F.E.; Etienne, G.; Huguet, F.; Guerci-Bresler, A.; Charbonnier, A.; Escoffre-Barbe, M.; Dubruille, V.; Johnson-Ansah, H.; Legros, L.; Coiteux, V.; et al. Treatment-Free Remissions in Newly Diagnosed CP CML Patients Treated with the Combination of Nilotinib + Pegylated Interferon Alpha 2a Versus Nilotinib Alone in the National Phase III Petals Trial. Blood 2021, 138, 2553. [Google Scholar] [CrossRef]
- Ernst, T.; Le Coutre, P.; Crysandt, M.; Brümmendorf, T.H.; Franke, G.-N.; Illmer, T.; Burchert, A.; Lang, F.; Saussele, S.; Lars Teichmann, L.; et al. S156: Frontline asciminib combination in chronic phase chronic myeloid leukemia patients. the fascination trial. HemaSphere 2023, 7, e34543a6. [Google Scholar] [CrossRef]
- Cortes, J.E.; Jiang, Q.; Wang, J.; Weng, J.; Zhu, H.; Liu, X.; Hochhaus, A.; Kim, D.-W.; Radich, J.; Savona, M.; et al. Dasatinib vs. imatinib in patients with chronic myeloid leukemia in chronic phase (CML-CP) who have not achieved an optimal response to 3 months of imatinib therapy: The DASCERN randomized study. Leukemia 2020, 34, 2064–2073. [Google Scholar] [CrossRef]
- Pane, F.; Castagnetti, F.; Luciano, L.; Russo Rossi, A.; Abruzzese, E.; Bassan, R.; Binotto, G.; Caocci, G.; Cimino, G.; Fazi, P.; et al. S156: International, prospective study comparing nilotinib versus imatinib with early switch to nilotinib to obtain sustained treatment-free remission in patients with with chronic myeloid leukemia. HemaSphere 2022, 6, 57. [Google Scholar] [CrossRef]
- Cheung, Y.M.; Ip, H.W.; Kwong, Y.L. Induction–maintenance approach for the chronic phase of chronic myeloid leukaemia (IMPACT-I): A prospective, single-arm, phase 2 study. Lancet Oncol. 2022, 23, S7. [Google Scholar] [CrossRef]
- Breccia, M.; Abruzzese, E.; Stagno, F.; Iurlo, A.; Pene, F.; Attolica, I.; Sportoletti, P.; Pregno, P.; Galimberti, S.; Scappini, B.; et al. First Interim Analysis of the Italian Dante Study: De-Escalation before Treatment-Free Remission in Patients with Chronic Myeloid Leukemia Treated with First-Line Nilotinib. Blood 2021, 138, 1474. [Google Scholar] [CrossRef]
- Gener-Ricos, G.; Haddad, F.G.; Sasaki, K.; Issa, G.C.; Skinner, J.; Masarova, L.; Borthakur, G.; Alvarado, Y.; Garcia-Manero, G.; Jabbour, E.; et al. Low-Dose Dasatinib (50 mg Daily) Frontline Therapy in Newly Diagnosed Chronic Phase Chronic Myeloid Leukemia: 5-Year Follow-Up Results. Clin. Lymphoma Myeloma Leuk. 2023, 23, 742–748. [Google Scholar] [CrossRef]
- Abraham, A.; Jain, H.; Bhattacharyya, J.; Biswajit, D.; PK, J.; Bhurani, D.; Bala, S.C.; Pramanik, S.; Devadas, S.; Kulkarni, U.P.; et al. Low Dose Dasatinib Is Not As Active in a CML CP Cohort Enriched with Intermediate/High-Risk CML Chronic Phase: A Phase IIb Multi-Center Trial. Blood 2022, 140, 6789–6790. [Google Scholar] [CrossRef]
- Kim, Y.; Go, T.-H.; Jang, J.; Lee, J.B.; Lim, S.T.; Shim, K.Y.; Lee, J.I.; Kong, J.H. Survival impact of adherence to tyrosine kinase inhibitor in chronic myeloid leukemia. Korean J. Intern. Med. 2021, 36, 1450–1458. [Google Scholar] [CrossRef]
- Campiotti, L.; Suter, M.B.; Guasti, L.; Piazza, R.; Gambacorti-Passerini, C.; Grandi, A.M.; Squizzato, A. Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR-ABL transcript level: A systematic review and a meta-analysis. Eur. J. Cancer 2017, 77, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Du, T.; Xiong, P.; Fan, G.; Yang, W. Discontinuation of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia With Losing Major Molecular Response as a Definition for Molecular Relapse: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 372. [Google Scholar] [CrossRef]
- Molica, M.; Abruzzese, E.; Breccia, M. Prognostic Significance of Transcript-Type BCR—ABL1 in Chronic Myeloid Leukemia. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020062. [Google Scholar] [CrossRef]
- Etienne, G.; Dulucq, S.; Bauduer, F.; Adiko, D.; Lifermann, F.; Dagada, C.; Lenoir, C.; Schmitt, A.; Klein, E.; Madene, S.; et al. Incidences of Deep Molecular Responses and Treatment-Free Remission in de Novo CP-CML Patients. Cancers 2020, 12, 2521. [Google Scholar] [CrossRef]
- Dulucq, S.; Astrugue, C.; Etienne, G.; Mahon, F.-X.; Benard, A. Risk of molecular recurrence after tyrosine kinase inhibitor discontinuation in chronic myeloid leukaemia patients: A systematic review of literature with a meta-analysis of studies over the last ten years. Br. J. Haematol. 2020, 189, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.D.H.; Busque, L.; Forrest, D.L.; Savoie, L.; Bence-Bruckler, I.; Couban, S.; Delage, R.; Xenocostas, A.; Liew, E.; Laneuville, P.; et al. Second Attempt of TKI Discontinuation with Dasatinib for Treatment-Free Remission after Failing First Attempt with Imatinib: Treatment-Free Remission Accomplished By Dasatinib (TRAD) Trial. Blood 2018, 132, 787. [Google Scholar] [CrossRef]
- Kim, E.; Hwang, E.-J.; Lee, J.; Kim, D.-Y.; Kim, J.-Y.; Kim, D.-W. Patient-specific molecular response dynamics can predict the possibility of relapse during the second treatment-free remission attempt in chronic myelogenous leukemia. Neoplasia 2022, 32, 100817. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, E.; Sakurai, M.; Karigane, D.; Kasahara, H.; Kikuchi, T.; Shimizu, T.; Yokoyama, K.; Mori, T.; Okamoto, S. Second Attempt to Discontinue TKI in CML Patients Who Have Sustained CMR for over 2 Years Is Rarely Successful Even with the Use of Second Generation TKIs. Blood 2016, 128, 1887. [Google Scholar] [CrossRef]
- Legros, L.; Nicolini, F.E.; Etienne, G.; Rousselot, P.; Rea, D.; Giraudier, S.; Guerci, A.; Huguet, F.; Gardembas, M.; Ianotto, J.-C.; et al. The TKI-Free Duration after a First Discontinuation Attempt That Failed in CP CML Patients Is a Predictive Factor of TKI-Free Remission after a Second Attempt. Blood 2019, 134, 28. [Google Scholar] [CrossRef]
- Legros, L.; Nicolini, F.E.; Etienne, G.; Rousselot, P.; Rea, D.; Giraudier, S.; Guerci-Bresler, A.; Huguet, F.; Gardembas, M.; Escoffre, M.; et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer 2017, 123, 4403–4410. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Pagani, I.S.; Shanmuganathan, N.; Kok, C.H.; Seymour, J.F.; Mills, A.K.; Filshie, R.J.; Arthur, C.K.; Dang, P.; Saunders, V.A.; et al. Long-term treatment-free remission of chronic myeloid leukemia with falling levels of residual leukemic cells. Leukemia 2018, 32, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Ureshino, H.; Kamachi, K.; Nishioka, A.; Okamoto, S.; Katsuya, H.; Yoshimura, M.; Kubota, Y.; Ando, T.; Kimura, S. Subsequent attempt tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia; a single institute experience. Hematol. Oncol. 2021, 39, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Kota, V.; Atallah, E. Musculoskeletal Pain in Patients With Chronic Myeloid Leukemia After Tyrosine Kinase Inhibitor Therapy Cessation. Clin. Lymphoma Myeloma Leuk. 2019, 19, 480–487. [Google Scholar] [CrossRef]
- Article—Billing and Coding: MolDx: BCR-ABL (A55233). Available online: https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleid=55233&ver=19&keywordtype=starts&keyword=bcr-abl&bc=0 (accessed on 10 February 2024).
- Nguyen, J.T.; Cole, A.L.; Leech, A.A.; Wood, W.A.; Dusetzina, S.B. Cost-Effectiveness of First-Line Tyrosine Kinase Inhibitor Therapy Initiation Strategies for Chronic Myeloid Leukemia. Value Health 2020, 23, 1292–1299. [Google Scholar] [CrossRef]
| Drug Name | Notable AEs | Dose | Average Wholesale Price (USD, 2022) |
|---|---|---|---|
| Imatinib | Myalgias, arthralgias, skin rash, N/V/D, edema, renal insufficiency, pancreatitis | 400 mg qD | USD 564–142,000 |
| Dasatinib | Pleural/pericardial effusion, PHTN, QT prolongation, bleeding | 100 mg qD | USD 228,000 |
| 50 mg qD | USD 127,000 | ||
| 20 mg qD | USD 63,000 | ||
| Nilotinib | MI, peripheral arterial occlusive disease, diabetes, QT prolongation, pancreatitis, rash | 300 mg BID | USD 240,000 |
| 150–200 mg BID | USD 120,000 | ||
| Ponatinib | HTN, HF, MI, stroke, peripheral arterial occlusive disease, arrhythmias, liver failure | 15 or 30 or 45 mg qD | USD 271,000 |
| Bosutinib | N/V/D, pancreatitis, increased ALT, renal insufficiency, rash | 400 mg qD | USD 250,000 |
| Asciminib | Arthralgias, dyspnea, dizziness, pancreatitis | 40 mg BID | USD 258,000 |
| 200 mg BID | USD 1,289,000 |
| Trial | Treatment | Inclusion Criteria | Molecular Relapse Definition |
|---|---|---|---|
| STIM1 (n = 100) | Imatinib de novo (n = 50) or after IFN (n = 50) | UMRD for ≥2 years | Loss of UMRD |
| KID (n = 90) | Imatinib de novo (n = 82) or after IFN (n = 8) | UMRD for ≥2 years | Loss of MMR |
| TWISTER (n = 40) | Imatinib de novo (n = 19) or after IFN (n = 21) | UMRD for ≥2 years | Loss of UMRD |
| ASTIM (n = 80) | Imatinib de novo (n = 37) or after IFN (n = 42) or cytarabine (n = 1); combined therapy (n = 15) | UMRD for ≥2 years | Loss of UMRD |
| Loss of MMR | |||
| ISAV (n = 108) | Imatinib de novo (n = 71) or after IFN (n = 36); 1 missing | UMRD for ≥1.5 years | Loss of MMR |
| EURO-SKI (n = 755) | Frontline: Imatinib (n = 710), dasatinib (n = 14), nilotinib (n = 33), unknown (n = 1); second line: imatinib (n = 7), nilotinib (n = 47), dasatinib (n = 62) | MR4.0 for ≥1 year | Loss of MMR |
| STOP 2G-TKI (n = 60) | Dasatinib (n = 30) or nilotinib (n = 30) | UMRD for ≥2 years | Loss of MMR |
| ENESTFreedom (n = 190) | Nilotinib | MR4.5 for ≥1 year | Loss of MMR |
| ENESTop (n = 126) | Nilotinib second line after imatinib intolerance (n = 51), imatinib resistance (n = 30) or preference for a physician (n = 45) | MR4.5 for ≥1 year | Loss of MR4 |
| DADI (n = 63) | Dasatinib therapy after imatinib for resistance (n = 13), intolerance (n = 36) or a patient’s request (n = 14) | MR4.0 for ≥1 year | Loss of MR4 |
| DASfree (n = 84) | Dasatinib frontline or subsequent therapy, no IFN | MR4.5 for ≥1 year | Loss of MMR |
| DESTINY (n = 174) | Imatinib (n = 148), nilotinib (n = 16) or dasatinib (n = 10) | MR4.0 for ≥1 year | Loss of MMR |
| MMR for ≥1 year | Loss of MMR | ||
| LAST (n = 172) | Imatinib (n = 102), dasatinib (n = 27) nilotinib (n = 39) or bosutinib (n = 4) | MR4.0 for ≥2 years | Loss of MMR |
| DOMEST (n = 99) | Imatinib de novo (n = 83) or after IFN (n = 16) | MR4.0 for ≥2 years | Loss of MR4 |
| D-STOP (n = 54) | Dasatinib frontline (n =19) or second line: imatinib (n = 34) or unknown (n = 1) | MR4.0 for ≥2 years | Loss of MR4 |
| STAT2 (n = 78) | Nilotinib second line after imatinib (n = 73) or other treatment (n = 5); after IFN (n = 12) | MR4.5 for ≥2 years | Loss of MR4.5 |
| STIM2 (n = 218) | Imatinib | UMRD for ≥2 years | Loss of UMRD |
| JALSG-STIM213 (n= 68) | Imatinib de novo (n = 55) or after IFN (n = 13) | MR4.0 for ≥2 years | Loss of MMR |
| First-line DADI (n= 58) | Dasatinib | MR4.0 for ≥1 year | Loss of MR4 |
| Trial | Predictors of Response | Patients in TFR (%) | CML Related Death Rates (%) | Progression Rates (%) | MMR Recovery Rates (%) |
|---|---|---|---|---|---|
| STIM1 (n = 100) | Low-risk Sokal, longer treatment duration | 38 at 60 months | 0% | 0% | 96% |
| KID (n = 90) | Withdrawal syndrome, negative dPCR, longer treatment duration | 69 at 24 months | NR | NR | 100% |
| TWISTER (n = 40) | Low-risk Sokal, longer duration of IFN treatment prior to TKI | 45 at 60 months | 0% | 0% | 100% |
| ASTIM (n = 80) | Previous IFN treatment | 44 at 36 months (loss of UMRD) | 0% | 1% | 100% |
| 61 at 36 months (loss of MMR) | |||||
| ISAV (n = 108) | Older age, undetectable dPCR at time of TKI discontinuation | 48 at 36 months | NR | 0% | 100% |
| EURO-SKI (n = 755) | Longer treatment duration, longer MR4 prior to TKI discontinuation | 49 at 24 months | 0% | 0% | 86% |
| STOP 2G-TKI (n = 60) | Prior intolerance or resistance to TKIs | 54 at 48 months | 0% | 0% | 100% |
| ENESTFreedom (n = 190) | Low-risk Sokal, longer nilotinib exposure, longer MR4.5 | 49 at 96 weeks | 0% | 0% | 99% |
| ENESTop (n = 126) | Longer MR4.5 duration prior to TKI discontinuation | 53 at 96 weeks | 0% | 0% | 98% |
| DADI (n = 63) | Imatinib resistance, NK-cell counts, γδ+ T-cell count, CD4+ regulatory T-cell count | 44 at 36 months | NR | 0% | 100% |
| DASfree (n = 84) | Older age, first-line therapy, longer treatment duration | 46 at 24 months | 0% | 0% | 96% |
| DESTINY (n = 174) | Longer treatment duration, MR4 group | 72 at 36 months (MR 4.0 > 1 year) | 0% | 0% | 100% |
| 36 at 36 months (MMR > 1 year) | |||||
| LAST (n = 172) | Undetectable RQ-PCR 3 months after TKI discontinuation, undetectable digital PCR at time of TKI discontinuation | 61 at 42 months | 0% | NR | 96% |
| DOMEST (n = 99) | Longer duration of imatinib therapy, longer time from diagnosis to imatinib discontinuation, low-risk Sokal score | 64 at 24 months | NR | NR | 96% |
| D-STOP (n = 54) | None | 63 at 12 months | NR | 0% | 100% |
| STAT2 (n = 78) | MRD negative prior to TFR | 63 at 36 months | 0% | 0% | 100% |
| STIM2 (n = 218) | NR | 50 at 24 months | NR | NR | 100% |
| JALSG-STIM213 (n = 68) | UMRD before TFR | 65 at 36 months | NR | 0% | 100% |
| First-line DADI (n = 58) | Lower CD4-cell count prior to dasatinib discontinuation | 55 at 12 months | NR | NR | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourne, G.; Bhatia, R.; Jamy, O. Treatment-Free Remission in Chronic Myeloid Leukemia. J. Clin. Med. 2024, 13, 2567. https://doi.org/10.3390/jcm13092567
Bourne G, Bhatia R, Jamy O. Treatment-Free Remission in Chronic Myeloid Leukemia. Journal of Clinical Medicine. 2024; 13(9):2567. https://doi.org/10.3390/jcm13092567
Chicago/Turabian StyleBourne, Garrett, Ravi Bhatia, and Omer Jamy. 2024. "Treatment-Free Remission in Chronic Myeloid Leukemia" Journal of Clinical Medicine 13, no. 9: 2567. https://doi.org/10.3390/jcm13092567
APA StyleBourne, G., Bhatia, R., & Jamy, O. (2024). Treatment-Free Remission in Chronic Myeloid Leukemia. Journal of Clinical Medicine, 13(9), 2567. https://doi.org/10.3390/jcm13092567





