Correlation between Subjective Nasal Patency and Nasal Capacity in Young Adults: A Pilot Study with a Prototype Device—A Nasoorospirometer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- (1)
- aged between 18–30 years;
- (2)
- gave their written informed consent for the examination with a nasoorospirometer prototype device.
- (1)
- the active upper respiratory tract infection;
- (2)
- history of neoplastic or autoimmune processes;
- (3)
- uncontrolled chronic disease;
- (4)
- current pregnancy.
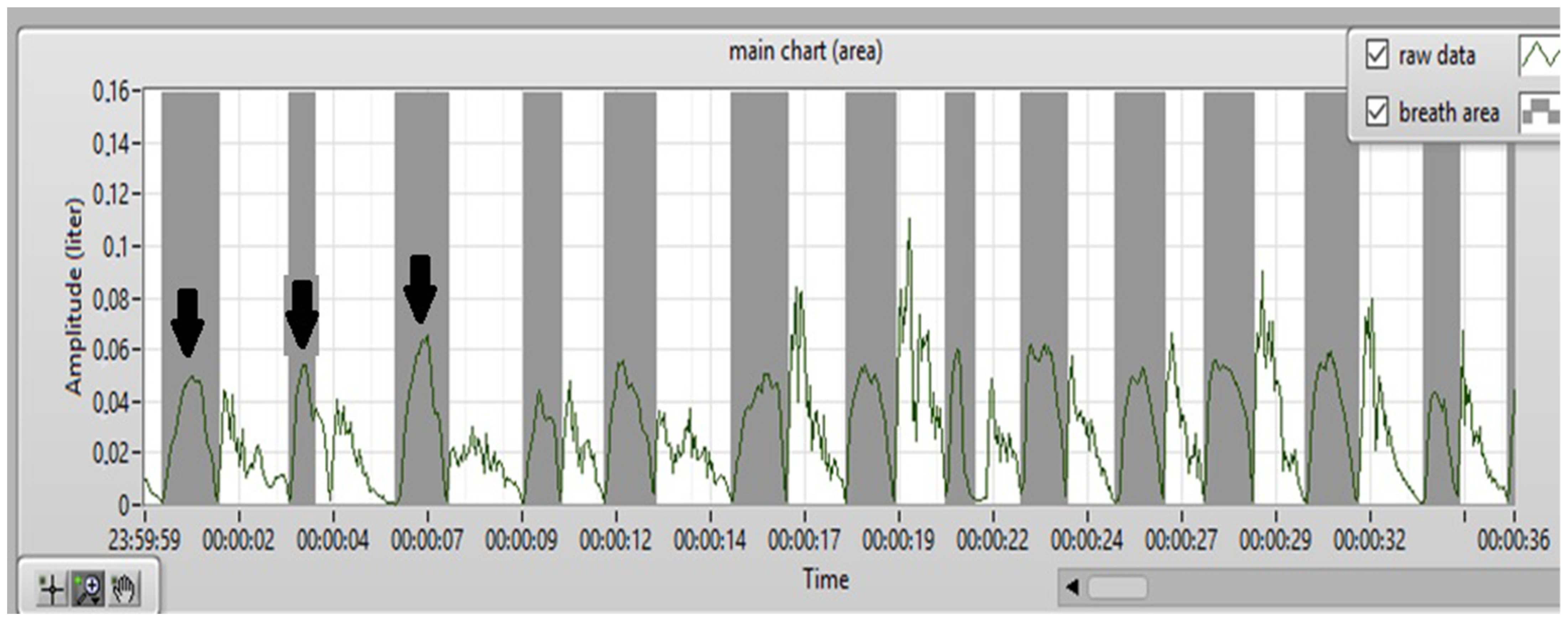
2.2. Nasoorospirometer
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Li, S.; Jin, H.; Song, L.; Li, Y.; Zhong, N.; Zhang, X. High nasal resistance may be a result rather than a cause of obstructive sleep apnea. Eur. Arch. Otorhinolaryngol. 2014, 271, 2999–3003. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Finn, L.; Kim, H.; Palta, M.; Dempsey, J.; Skatrud, J. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J. Allergy Clin. Immunol. 1997, 99, S757–S762. [Google Scholar] [CrossRef] [PubMed]
- Banabilh, S.M.; Suzina, A.H.; Mohamad, H.; Dinsuhaimi, S.; Samsudin, A.R.; Singh, G.D. Assessment of 3-D nasal airway morphology in Southeast Asian adults with obstructive sleep apnea using acoustic rhinometry. Clin. Oral Investig. 2010, 14, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.A.; Su, M.C.; Jiang, R.S. Nasal patency measured by acoustic rhinometry in East Asian patients with sleep-disordered breathing. Am. J. Rhinol. 2006, 20, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Hoel, H.C.; Kvinnesland, K.; Berg, S. Impact of nasal resistance on the distribution of apneas and hypopneas in obstructive sleep apnea. Sleep Med. 2020, 71, 83–88. Available online: https://pubmed.ncbi.nlm.nih.gov/32502854/ (accessed on 31 October 2023). [CrossRef] [PubMed]
- Zeng, B.; Ng, A.T.; Qian, J.; Petocz, P.; Darendeliler, M.A.; Cistulli, P.A. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep 2008, 31, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Zamarrón, C.; Gude, F.; Alvarez, J.M.; Rivera, M.; Gonzalez, F.J.; Rodriguez, J.R. Airway disorders and pulmonary function in snorers. A population-based study. Respir. Med. 2000, 94, 835–840. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, D.-Y.; Cho, J.H.; Jung, Y.G.; Choi, J.H.; Kim, D.-K.; Kim, S.-W.; Kim, H.J.; Kim, H.Y.; Park, S.K.; Park, C.S.; et al. Clinical Practice Guideline: Clinical Efficacy of Nasal Surgery in the Treatment of Obstructive Sleep Apnea. Clin. Exp. Otorhinolaryngol. 2023, 16, 201. [Google Scholar] [CrossRef]
- Maniaci, A.; Lechien, J.R.; La Mantia, I.; Calvo-Henriquez, C.; Iannella, G.; Locatello, L.G.; Saibene, A.M.; Leigh, S.J.; Ingrassia, A.; Nocera, F.; et al. Effectiveness of submucosal turbinoplasty in refractory obstructive rhinitis: A prospective comparative trial. Eur. Arch. Otorhinolaryngol. 2022, 279, 4397–4406. [Google Scholar] [CrossRef]
- Schoustra, E.; van Maanen, P.; den Haan, C.; Ravesloot, M.J.L.; de Vries, N. The Role of Isolated Nasal Surgery in Obstructive Sleep Apnea Therapy—A Systematic Review. Brain Sci. 2022, 12, 1446. [Google Scholar] [CrossRef]
- Agarwal, S.S.; Datana, S.; Sahoo, N.K.; Bhandari, S.K. Correlating Nasal Patency with Obstructive Sleep Apnea in Obese Versus Non-Obese Patients: An Acoustic Rhinometry Study. Indian J. Otolaryngol. Head Neck Surg. 2022, 74 (Suppl. S2), 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, T.; Ruoppi, P.; Seppä, J.; Sahlman, J.; Peltonen, M.; Tukiainen, H.; Gylling, H.; Vanninen, E.; Tuomilehto, H. Effect of weight reduction on rhinometric measurements in overweight patients with obstructive sleep apnea. Am. J. Rhinol. 2008, 22, 410–415. [Google Scholar] [CrossRef]
- Nascimento, J.A.; Genta, P.R.; Fernandes, P.H.; Barroso, L.P.; Carvalho, T.S.; Moriya, H.T.; Madeiro, F.; Lorenzi-Filho, G.; Nakagawa, N.K. Predictors of oronasal breathing among obstructive sleep apnea patients and controls. J. Appl. Physiol. 2019, 127, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Gaberino, C.; Rhee, J.S.; Garcia, G.J.M. Estimates of nasal airflow at the nasal cycle mid-point improve the correlation between objective and subjective measures of nasal patency. Respir. Physiol. Neurobiol. 2017, 238, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Polomis, D.A.; Teodorescu, M.C.; Gangnon, R.E.; Peterson, A.G.; Consens, F.B.; Chervin, R.D.; Jarjour, N.N. Association of obstructive sleep apnea risk with asthma control in adults. Chest 2010, 138, 543–550. [Google Scholar] [CrossRef]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Cheung, Y.; Tai, B.; Loo, G.; Khoo, S.; Cheong, K.Y.; Barbe, F.; Lee, C. Screening for Obstructive Sleep Apnea in the Assessment of Coronary Risk. Am. J. Cardiol. 2017, 119, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Ben-Noun, L.L.; Sohar, E.; Laor, A. Neck Circumference as a Simple Screening Measure for Identifying Overweight and Obese Patients. Obes. Res. 2001, 9, 470–477. [Google Scholar] [CrossRef]
- Nitkiewicz, S.; Barański, R.; Kukwa, A.; Zając, A. Respiratory disorders–measuring method and equipment. Metrol. Meas. Syst. 2018, 25, 187–202. [Google Scholar] [CrossRef]
- Ottaviano, G.; Fokkens, W.J. Measurements of nasal airflow and patency: A critical review with emphasis on the use of peak nasal inspiratory flow in daily practice. Allergy 2016, 71, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Giotakis, A.I.; Tomazic, P.V.; Riechelmann, H.; Vent, J. Objective Assessment of Nasal Patency. Facial Plast. Surg. 2017, 33, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Piromchai, P.; Netnoi, J.; Srirompotong, S.; Thanawirattananit, P. Comparison of nasal patency after nose-blowing between pinch versus no pinch method: A randomized controlled trial. Sci. Rep. 2021, 11, 22084. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.P.; Borojeni, A.A.T.; Koenig, L.J.; Rhee, J.S.; Garcia, G.J.M. Correlation between Subjective Nasal Patency and Intranasal Airflow Distribution. Otolaryngol. Head Neck Surg. 2017, 156, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Primov-Fever, A.; Zaretsky, U.; Elad, D.; Wolf, M. Evaluation of nasal airway patency by analysis of breathing sounds. Acta Otolaryngol. 2016, 136, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, S.; Schmelzer, B. Type and severity of septal deviation are not related with the degree of subjective nasal obstruction. Rhinology 2016, 54, 355–360. [Google Scholar] [CrossRef] [PubMed]
- André, R.F.; Vuyk, H.D.; Ahmed, A.; Graamans, K.; Nolst Trenité, G.J. Correlation between subjective and objective evaluation of the nasal airway. A systematic review of the highest level of evidence. Clin. Otolaryngol. 2009, 34, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Naito, K.; Miyata, S.; Saito, S.; Sakurai, K.; Takeuchi, K. Comparison of perceptional nasal obstruction with rhinomanometric and acoustic rhinometric assessment. Eur. Arch. Otorhinolaryngol. 2001, 258, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Tompos, T.; Garai, T.; Zemplén, B.; Gerlinger, I. Sensation of nasal patency compared to rhinomanometric results after septoplasty. Eur. Arch. Otorhinolaryngol. 2010, 267, 1887–1891. [Google Scholar] [CrossRef]
- Braun, T.; Rich, M.; Kramer, M.F. Correlation of three variables describing nasal patency (HD, MCA, NOSE score) in healthy subjects. Braz. J. Otorhinolaryngol. 2013, 79, 354–358. [Google Scholar] [CrossRef]
- Lam, D.J.; James, K.T.; Weaver, E.M. Comparison of Anatomic, Physiological, and Subjective Measures of the Nasal Airway. Am. J. Rhinol. 2006, 20, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Tjahjono, R.; Singh, N. Correlation between nasal mucosal temperature change and the perception of nasal patency: A literature review. J. Laryngol. Otol. 2021, 135, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Jiang, J.; Blacker, K.; Lyman, B.; Dalton, P.; Cowart, B.J.; Pribitkin, E.A. Regional peak mucosal cooling predicts the perception of nasal patency. Laryngoscope 2014, 124, 589–595. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, I.; Grigaliute, E.; Ragusa, M.; Cocuzza, S.; Radulesco, T.; Saibene, A.M.; Calvo-Henriquez, C.; Fakhry, N.; Michel, J.; Maniaci, A. Effectiveness and rapidity on olfactory fuction recovery in CRS patients treated with Dupilumab: A real life prospective controlled study. Eur. Arch. Otorhinolaryngol. 2024, 281, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Miyazaki, S.; Ohki, M.; Morinaga, M.; Noda, A.; Sugiura, T.; Sugiura, M.; Teranishi, M.; Katayama, N.; Nakashima, T. Reduced nasal resistance after simple tonsillectomy in patients with obstructive sleep apnea. Am. J. Rhinol. 2007, 21, 192–195. [Google Scholar] [CrossRef]
- Blomster, H.; Kemppainen, T.; Numminen, J.; Ruoppi, P.; Sahlman, J.; Peltonen, M.; Seppa, J.; Tuomilehto, H. Impaired nasal breathing may prevent the beneficial effect of weight loss in the treatment of OSA. Rhinology 2011, 49, 587–592. [Google Scholar] [CrossRef]
- Leitzen, K.P.; Brietzke, S.E.; Lindsay, R.W. Correlation between nasal anatomy and objective obstructive sleep apnea severity. Otolaryngol. Head Neck Surg. 2014, 150, 325–331. [Google Scholar] [CrossRef]

| Characteristic | n (%) |
|---|---|
| N of participants | 31 (100%) |
| Age at recruitment (years) | 24.9 (±2.57) |
| Gender: | |
| Male | 18 (58%) |
| Female | 13 (42%) |
| BMI (kg/m2) | 23.72 ± 4.5 |
| NC | 35.32 (±3.94) |
| BQ | |
| High | 5 (16%) |
| low | 26 (84%) |
| NOSE SCORE | Mean: 28.4 |
| Severe | 6 (20%) |
| Moderate | 9 (29%) |
| mild | 14 (45%) |
| No symptoms | 2 (6%) |
| Nasal obstruction | 16 (52%) |
| Earlier septoplasty | 2 (6%) |
| Mean Capacity (R + L) [l] | Mean Capacity (R + L) std [l] | Inhale Number (R + L)/min | Mean Time (R + L) [std(s)] | Mean Time (R + L) [s] | Max Capacity (R + L) | Min Capacity (R + L) |
Sum Capacity (R + L) | |
|---|---|---|---|---|---|---|---|---|
| Mean Capacity (R + L) std [l] | 0.51 p = 0.003 | |||||||
| Inhale number (R + L)/min | −0.35 p = 0.054 | −0.59 p < 0.001 | ||||||
| Mean time (R + L) [std(s)] | 0.20 p = 0.27 | 0.71 p < 0.001 | −0.40 p < 0.026 | |||||
| Mean time (R + L) [s] | 0.64 p < 0.001 | 0.54 p < 0.001 | −0.67 p < 0.001 | 0.45 p < 0.011 | ||||
| Max capacity (R + L) | 0.88 p < 0.001 | 0.73 p < 0.001 | −0.39 p < 0.028 | 0.40 p < 0.024 | 0.65 p < 0.001 | |||
| Min capacity (R + L) | 0.55 p < 0.001 | −0.10 p < 0.59 | −0.20 p < 0.28 | −0.07 p < 0.69 | 0.38 p < 0.034 | 0.42 p < 0.019 | ||
| Sum capacity (R + L) | 0.63 p < 0.001 | 0.07 p < 0.72 | 0.27 p < 0.14 | −0.13 p < 0.5 | 0.16 p < 0.4 | 0.52 p < 0.003 | 0.39 p < 0.03 | |
| NOSE score | 0.18 p < 0.33 | 0.03 p < 0.86 | −0.02 p < 0.93 | −0.14 p < 0.45 | 0.19 p < 0.31 | 0.11 p < 0.56 | −0.02 p < 0.9 | −0.14 p < 0.46 |
| Measure | Body Mass Index | Neck Circumference |
|---|---|---|
| Mean Capacity (R + L) [l] | 0.27 p = 0.14 | 0.45 p= 0.01 |
| Mean Capacity (R + L) std [l] | 0.21 p = 0.27 | 0.21 p = 0.27 |
| Inhale number (R + L)/min | −0.22 p = 0.22 | −0.04 p = 0.83 |
| Mean time (R + L) [std(s)] | 0.07 p = 0.7 | 0.09 p = 0.61 |
| Mean Time (R + L) [s] | 0.40 p= 0.03 | 0.34 p = 0.06 |
| Max capacity (R + L) | 0.23 p = 0.2 | 0.44 p= 0.014 |
| Min capacity (R + L) | 0.19 p = 0.32 | 0.32 p= 0.04 |
| Sum capacity (R + L) | 0.00 p = 1 | 0.38 p= 0.03 |
| Measure | Low Risk, n = 26 | High Risk, n = 5 | p-Value |
|---|---|---|---|
| Inhale number (R + L) | 24 (13, 29) | 30 (24, 37) | 0.3 |
| Mean Capacity (R + L) [l] | 0.47 (0.33, 0.67) | 0.43 (0.31, 0.45) | 0.6 |
| Mean Capacity (R + L) std [l] | 0.15 (0.10, 0.22) | 0.12 (0.08, 0.20) | 0.7 |
| Inhale number (R + L)/min | 12.6 (10.4, 17.6) | 14.3 (11.7, 17.7) | 0.8 |
| Mean time (R + L) [std(s)] | 0.35 (0.27, 0.48) | 0.36 (0.22, 0.47) | 0.7 |
| Mean Time (R + L) [s] | 1.66 (1.35, 2.01) | 1.64 (1.55, 1.86) | >0.9 |
| Max capacity (R + L) | 0.75 (0.58, 1.01) | 0.75 (0.49, 0.75) | 0.7 |
| Min capacity (R + L) | 0.16 (−0.02, 0.39) | 0.17 (0.12, 0.19) | >0.9 |
| Sum capacity (R + L) | 12.4 (7.0, 16.0) | 10.8 (9.2, 16.0) | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zasadzińska-Stempniak, K.; Zajączkiewicz, H.; Kukwa, A. Correlation between Subjective Nasal Patency and Nasal Capacity in Young Adults: A Pilot Study with a Prototype Device—A Nasoorospirometer. J. Clin. Med. 2024, 13, 2506. https://doi.org/10.3390/jcm13092506
Zasadzińska-Stempniak K, Zajączkiewicz H, Kukwa A. Correlation between Subjective Nasal Patency and Nasal Capacity in Young Adults: A Pilot Study with a Prototype Device—A Nasoorospirometer. Journal of Clinical Medicine. 2024; 13(9):2506. https://doi.org/10.3390/jcm13092506
Chicago/Turabian StyleZasadzińska-Stempniak, Katarzyna, Hanna Zajączkiewicz, and Andrzej Kukwa. 2024. "Correlation between Subjective Nasal Patency and Nasal Capacity in Young Adults: A Pilot Study with a Prototype Device—A Nasoorospirometer" Journal of Clinical Medicine 13, no. 9: 2506. https://doi.org/10.3390/jcm13092506
APA StyleZasadzińska-Stempniak, K., Zajączkiewicz, H., & Kukwa, A. (2024). Correlation between Subjective Nasal Patency and Nasal Capacity in Young Adults: A Pilot Study with a Prototype Device—A Nasoorospirometer. Journal of Clinical Medicine, 13(9), 2506. https://doi.org/10.3390/jcm13092506






