Neuroregulatory Effects of Microcone Patch Stimulation on the Auricular Branch of the Vagus Nerve and the Prefrontal Cortex: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
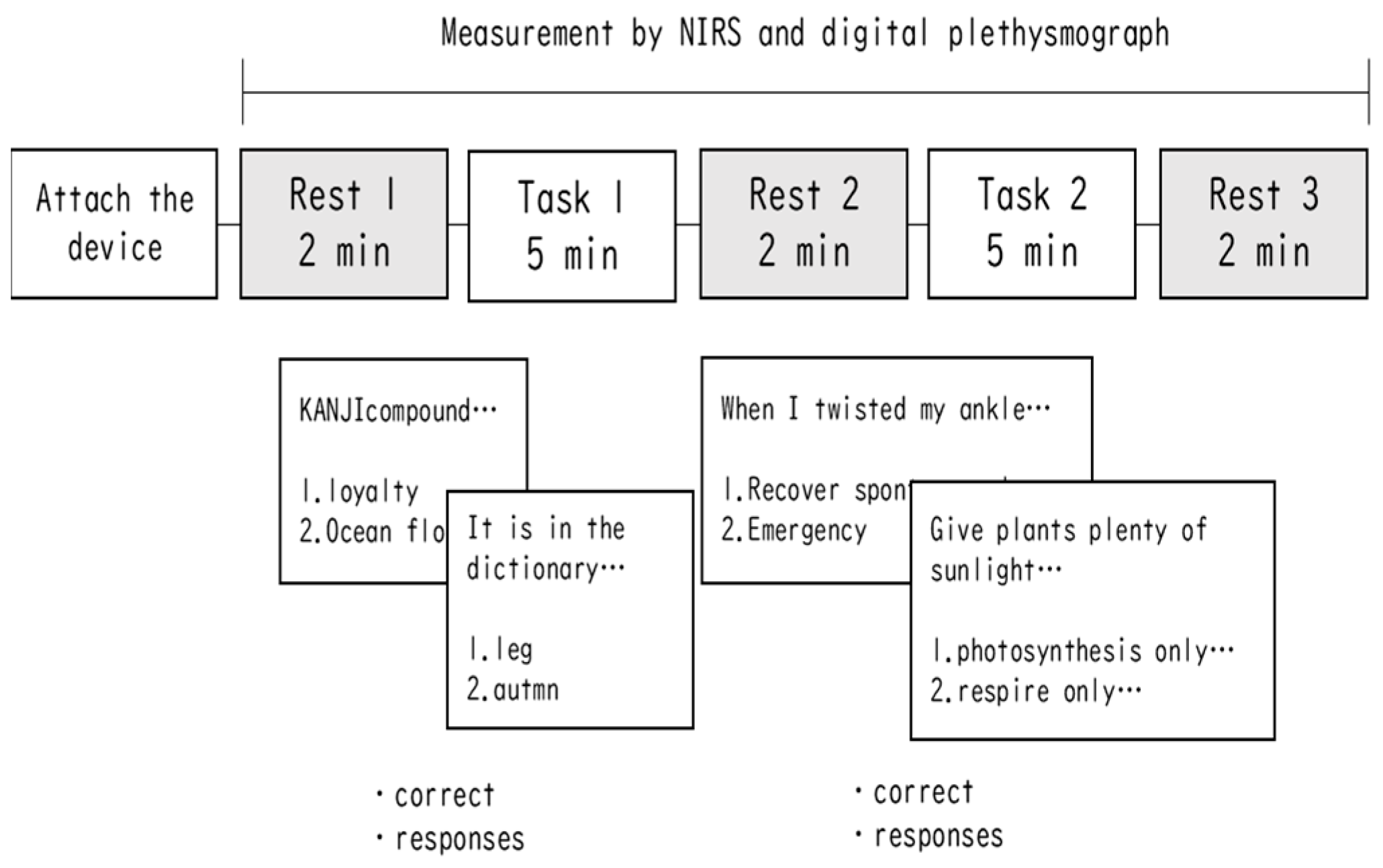
2.2. Task and Procedure
2.3. Characteristics of the Microcone Patch
2.4. Measurement of Autonomic Nervous System Activity
2.5. NIRS Recording and Preprocessing
2.6. Statistical Analysis
3. Result
3.1. Descriptive Statistics and Differences by Condition
3.2. Hierarchical Regression Analysis
3.2.1. Patch-Stimulation Group
3.2.2. Non-Stimulation Group
4. Discussion
4.1. Effects of Simulation on the Cerebral Cortex via Changes in Autonomic Dictation Paths
4.2. Background and Mechanism of Action
4.3. Potential Clinical Applications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guidi, J.; Fava, G.A. Sequential Combination of Pharmacotherapy and Psychotherapy in Major Depressive Disorder: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021, 78, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Santoft, F.; Axelsson, E.; Öst, L.-G.; Hedman-Lagerlöf, M.; Fust, J.; Hedman-Lagerlöf, E. Cognitive behaviour therapy for depression in primary care: Systematic review and meta-analysis. Psychol. Med. 2019, 49, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Simon, N.M. Anxiety Disorders: A Review. JAMA 2022, 328, 2431–2445. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Kasper, S.; Zohar, J.; Souery, D.; Montgomery, S.; Albani, D.; Forloni, G.; Ferentinos, P.; Rujescu, D.; Mendlewicz, J.; et al. Cost-effectiveness of genetic and clinical predictors for choosing combined psychotherapy and pharmacotherapy in major depression. J. Affect. Disord. 2020, 279, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.E.; Fournier, A.-A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). PharmacoEconomics 2021, 39, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux-Lamarche, C.; Berbiche, D.; Vasiliadis, H.-M. Health care system and patient costs associated with receipt of minimally adequate treatment for depression and anxiety disorders in older adults. BMC Psychiatry 2022, 22, 175. [Google Scholar] [CrossRef]
- Khir, S.M.; Wan Mohd Yunus, W.M.A.; Mahmud, N.; Wang, R.; Panatik, S.A.; Sukor, M.S.M.; Nordin, N.A. Efficacy of Progressive Muscle Relaxation in Adults for Stress, Anxiety, and Depression: A Systematic Review. Psychol. Res. Behav. Manag. 2024, 17, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Dolbier, C.L.; Rush, T.E. Efficacy of abbreviated progressive muscle relaxation in a high-stress college sample. Int. J. Stress Manag. 2012, 19, 48–68. [Google Scholar] [CrossRef]
- Kim, A.Y.; Kim, A.Y.; Marduy, A.; Marduy, A.; de Melo, P.S.; de Melo, P.S.; Gianlorenco, A.C.; Gianlorenco, A.C.; Kim, C.K.; Kim, C.K.; et al. Safety of transcutaneous auricular vagus nerve stimulation (taVNS): A systematic review and meta-analysis. Sci. Rep. 2022, 12, 22055. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, Y.; Wang, F.; Zhang, J.; Li, S.; Wu, M.; Li, L.; Rong, P. Transcutaneous auricular vagus nerve stimulators: A review of past, present, and future devices. Expert Rev. Med. Devices 2022, 19, 43–61. [Google Scholar] [CrossRef]
- Watanabe, N.; Piché, M.; Hotta, H. Types of skin afferent fibers and spinal opioid receptors that contribute to touch-induced inhibition of heart rate changes evoked by noxious cutaneous heat stimulation. Mol. Pain 2015, 11, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Nara, M.; Suzuki, S.; Sugie, M.; Yamamoto, T.; Hotta, H. Effects of gentle mechanical skin stimulation on subjective symptoms and joint range of motions in people with chronic neck and shoulder discomfort. J. Physiol. Sci. 2023, 73, 4. [Google Scholar] [CrossRef] [PubMed]
- Briand, M.-M.; Gosseries, O.; Staumont, B.; Laureys, S.; Thibaut, A. Transcutaneous Auricular Vagal Nerve Stimulation and Disorders of Consciousness: A Hypothesis for Mechanisms of Action. Front. Neurol. 2020, 11, 545986. [Google Scholar] [CrossRef]
- Clancy, J.A.; Mary, D.A.; Witte, K.K.; Greenwood, J.P.; Deuchars, S.A.; Deuchars, J. Non-invasive Vagus Nerve Stimulation in Healthy Humans Reduces Sympathetic Nerve Activity. Brain Stimul. 2014, 7, 871–877. [Google Scholar] [CrossRef]
- Yakunina, N.; Kim, S.S.; Nam, E.-C. BOLD fMRI effects of transcutaneous vagus nerve stimulation in patients with chronic tinnitus. PLoS ONE 2018, 13, e0207281. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Rong, P.; Hong, Y.; Fan, Y.; Liu, J.; Wang, H.; Zhang, G.; Chen, X.; Shi, S.; Wang, L.; et al. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol. Psychiatry 2015, 79, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Badran, B.W.; Dowdle, L.T.; Mithoefer, O.J.; LaBate, N.T.; Coatsworth, J.; Brown, J.C.; DeVries, W.H.; Austelle, C.W.; McTeague, L.M.; George, M.S. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul. 2017, 11, 492–500. [Google Scholar] [CrossRef]
- Wu, C.; Liu, P.; Fu, H.; Chen, W.; Cui, S.; Lu, L.; Tang, C. Transcutaneous auricular vagus nerve stimulation in treating major depressive disorder: A systematic review and meta-analysis. Medicine 2018, 97, e13845. [Google Scholar] [CrossRef]
- Hotta, H.; Schmidt, R.F.; Uchida, S.; Watanabe, N. Gentle mechanical skin stimulation inhibits the somatocardiac sympathetic: C-reflex elicited by excitation of unmyelinated C-afferent fibers. Eur. J. Pain 2010, 14, 806–813. [Google Scholar] [CrossRef]
- Bardoni, R.; Tawfik, V.L.; Wang, D.; François, A.; Solorzano, C.; Shuster, S.A.; Choudhury, P.; Betelli, C.; Cassidy, C.; Smith, K.; et al. Delta Opioid Receptors Presynaptically Regulate Cutaneous Mechanosensory Neuron Input to the Spinal Cord Dorsal Horn. Neuron 2014, 81, 1312–1327. [Google Scholar] [CrossRef]
- Watanabe, N.; Ishii, K.; Hotta, H.; Oda, K.; Sakata, M.; Toyohara, J.; Ishiwata, K. Differential human brain activity induced by two perceptually indistinguishable gentle cutaneous stimuli. NeuroReport 2013, 24, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Nakagawa, M. Can Micro-Cone Stimulation of the External Ear Canal Modulate Sympathetic Activity and PFA Function during VOT Task? International Society for Autonomic Neuroscience: Los Angeles, CA, USA, 2022; pp. 4–7. [Google Scholar]
- Wong, S.W.; Massé, N.; Kimmerly, D.S.; Menon, R.S.; Shoemaker, J.K. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. NeuroImage 2007, 35, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Mathias, C.J.; Josephs, O.; O’doherty, J.; Zanini, S.; Dewar, B.; Cipolotti, L.; Shallice, T.; Dolan, R.J. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 2003, 126, 2139–2152. [Google Scholar] [CrossRef] [PubMed]
- Maeoka, H.; Matsuo, A.; Hiyamizu, M.; Morioka, S.; Ando, H. Influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: A study using electroencephalographic power spectrum analysis. Neurosci. Lett. 2012, 512, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.R. Problems with inattention, reading comprehension, and autonomic regulation on the Stroop task. Infant Child Dev. 2020, 29, e2185. [Google Scholar] [CrossRef]
- Becker, D.R.; Carrère, S.; Siler, C.; Jones, S.; Bowie, B.; Cooke, C. Autonomic Regulation on the Stroop Predicts Reading Achievement in School Age Children. Mind Brain Educ. 2012, 6, 10–18. [Google Scholar] [CrossRef]
- Moss, J.; Schunn, C.D. Comprehension through explanation as the interaction of the brain’s coherence and cognitive control networks. Front. Hum. Neurosci. 2015, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gong, D.; Zhang, Y.; Zhang, H.; Liu, G. The bottom-up information transfer process and top-down attention control underlying tonal working memory. Front. Neurosci. 2022, 16, 935120. [Google Scholar] [CrossRef]
- Prat, C.S.; Just, M.A. Exploring the Neural Dynamics Underpinning Individual Differences in Sentence Comprehension. Cereb. Cortex 2010, 21, 1747–1760. [Google Scholar] [CrossRef]
- Takahashi, T.; Mitani, E. Reading and Writing Fluency Task; Chiba Test Center Co., Ltd.: Chiba, Japan, 2022. [Google Scholar]
- Okumura, T.; Kawasaki, A.; Nishioka, Y.; Wakamiya, E.; Miura, T.; Tamai, H. Comprehensive Assessment of Reading Domains (CARD) Guidebook; WEED PLANNIG Co., Ltd.: Otsu, Japan, 2014. [Google Scholar]
- Fedorenko, E.; Behr, M.K.; Kanwisher, N. Functional specificity for high-level linguistic processing in the human brain. Proc. Natl. Acad. Sci. USA 2011, 108, 16428–16433. [Google Scholar] [CrossRef]
- Alaerts, K.; Daniels, N.; Moerkerke, M.; Evenepoel, M.; Tang, T.; Van der Donck, S.; Chubar, V.; Claes, S.; Steyaert, J.; Boets, B.; et al. At the Head and Heart of Oxytocin’s Stress-Regulatory Neural and Cardiac Effects: A Chronic Administration RCT in Children with Autism. Psychother. Psychosom. 2023, 92, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Borges, U.; Knops, L.; Laborde, S.; Klatt, S.; Raab, M. Transcutaneous Vagus Nerve Stimulation May Enhance Only Specific Aspects of the Core Executive Functions. A Randomized Crossover Trial. Front. Neurosci. 2020, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Ridgewell, C.; Heaton, K.J.; Hildebrandt, A.; Couse, J.; Leeder, T.; Neumeier, W.H. The effects of transcutaneous auricular vagal nerve stimulation on cognition in healthy individuals: A meta-analysis. Neuropsychology 2021, 35, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Kiess, O.; Hösl, K.; Terekhin, P.; Kornhuber, J.; Forster, C. CNS BOLD fMRI Effects of Sham-Controlled Transcutaneous Electrical Nerve Stimulation in the Left Outer Auditory Canal—A Pilot Study. Brain Stimul. 2013, 6, 798–804. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Werner, P. Stigma regarding hearing loss and hearing aids: A scoping review. Stigma Health 2016, 1, 59–71. [Google Scholar] [CrossRef]
- Buchweitz, A.; de Azeredo, L.A.; Esper, N.B.; Dalfovo, N.P.; Picoli, F.; da Cunha, F.S.; Viola, T.W.; Grassi-Oliveira, R. Developmental Dyslexia and the Stress of Reading: A Social Stress Study of Neuroendocrine Response in Children. Mind Brain Educ. 2023, 17, 312–323. [Google Scholar] [CrossRef]
- Van Lieshout, P.; Ben-David, B.; Lipski, M.; Namasivayam, A. The impact of threat and cognitive stress on speech motor control in people who stutter. J. Fluen. Disord. 2014, 40, 93–109. [Google Scholar] [CrossRef]


| Index | With Stimulation (M ± 1SD) | Without Stimulation (M ± 1SD) | Statistics |
|---|---|---|---|
| CH1 | 0.022 ± 0.068 | −0.006 ± 0.041 | t(64.377) = 2.25, p = 0.028 |
| CH2 | −0.017 ± 0.104 | 0.013 ± 0.061 | t(63.06) = −1.549, p = 0.127 |
| CH3 | 0.015 ± 0.090 | −0.006 ± 0.047 | t(59.146) = 1.329, p = 0.189 |
| CH4 | −0.018 ± 0.074 | 0.009 ± 0.041 | t(60.99) = −2.017, p = 0.048 |
| LF/HF ratio | 1.450 ± 1.146 | 1.530 ± 0.843 | t(71.665) = −0.355, p = 0.724 |
| pnn50 (%) | 16.486 ± 15.774 | 18.858 ± 17.565 | t(77.115) = −0.635, p = 0.527 |
| Accuracy | 0.920 ± 0.056 | 0.895 ± 0.073 | t(73.293) = 1.673, p = 0.099 |
| CH | Step | Independent Variables | B | 95% CI for B | Se B | β | p of β | R2 | ΔR2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1CH | Step 1 | 0.133 * | ||||||||
| Intercept | 0.022 | 0.002 | 0.043 | 0.010 | ||||||
| LF/HF | 0.022 | 0.004 | 0.040 | 0.009 | 0.365 | 0.021 | ||||
| Step 2 | 0.145 † | 0.012 | ||||||||
| Intercept | 0.022 | 0.002 | 0.043 | 0.010 | ||||||
| LF/HF | 0.024 | 0.005 | 0.044 | 0.010 | 0.412 | 0.017 | ||||
| pnn50 (%) | 0.001 | −0.001 | 0.002 | 0.001 | 0.120 | 0.470 | ||||
| Step 3 | 0.175 † | 0.029 | ||||||||
| Intercept | 0.030 | 0.005 | 0.054 | 0.012 | ||||||
| LF/HF | 0.036 | 0.007 | 0.066 | 0.014 | 0.613 | 0.016 | ||||
| pnn50(%) | 0.001 | −0.001 | 0.003 | 0.001 | 0.319 | 0.193 | ||||
| LF/HF × pnn50 (%) | 0.001 | −0.001 | 0.003 | 0.001 | 0.280 | 0.265 | ||||
| 2CH | Step 1 | 0.112 * | ||||||||
| Intercept | −0.017 | −0.049 | 0.015 | 0.016 | ||||||
| LF/HF | −0.030 | −0.059 | −0.002 | 0.014 | −0.334 | 0.035 | ||||
| Step 2 | 0.114 | 0.002 | ||||||||
| Intercept | −0.017 | −0.049 | 0.015 | 0.016 | ||||||
| LF/HF | −0.032 | 0.015 | −0.001 | 0.015 | −0.354 | 0.042 | ||||
| pnn50 (%) | 0.000 | 0.001 | 0.002 | 0.001 | −0.049 | 0.770 | ||||
| Step 3 | 0.216 * | 0.102 * | ||||||||
| Intercept | 0.004 | −0.033 | 0.040 | 0.018 | ||||||
| LF/HF | 0.002 | −0.042 | 0.046 | 0.022 | 0.022 | 0.928 | ||||
| pnn50 (%) | 0.002 | −0.001 | 0.005 | 0.002 | 0.319 | 0.181 | ||||
| LF/HF × pnn50 (%) | 0.003 | 0.000 | 0.006 | 0.001 | 0.520 | 0.037 | ||||
| 3CH | Step 1 | 0.045 | ||||||||
| Intercept | 0.015 | −0.014 | 0.043 | 0.014 | ||||||
| LF/HF | −0.017 | −0.042 | 0.009 | 0.012 | −0.212 | 0.189 | ||||
| Step 2 | 0.045 | 0.000 | ||||||||
| Intercept | 0.015 | −0.014 | 0.044 | 0.014 | ||||||
| LF/HF | −0.017 | −0.045 | 0.011 | 0.014 | −0.218 | 0.218 | ||||
| pnn50 (%) | 0.000 | −0.002 | 0.002 | 0.001 | −0.016 | 0.927 | ||||
| Step 3 | 0.260 * | 0.215 ** | ||||||||
| Intercept | 0.041 | 0.010 | 0.071 | 0.015 | ||||||
| LF/HF | 0.026 | −0.011 | 0.062 | 0.018 | 0.327 | 0.163 | ||||
| pnn50 (%) | 0.003 | 0.000 | 0.006 | 0.001 | 0.519 | 0.028 | ||||
| LF/HF × pnn50 (%) | 0.004 | 0.001 | 0.006 | 0.001 | 0.756 | 0.003 | ||||
| 4CH | Step 1 | 0.046 | ||||||||
| Intercept | −0.018 | −0.041 | 0.006 | 0.012 | ||||||
| LF/HF | −0.014 | −0.035 | 0.007 | 0.010 | −0.214 | 0.185 | ||||
| Step 2 | 0.054 | 0.009 | ||||||||
| Intercept | −0.018 | −0.041 | 0.006 | 0.012 | ||||||
| LF/HF | −0.016 | −0.039 | 0.006 | 0.011 | −0.253 | 0.153 | ||||
| pnn50 (%) | 0.000 | −0.002 | 0.001 | 0.001 | −0.100 | 0.567 | ||||
| Step 3 | 0.083 | 0.029 | ||||||||
| Intercept | −0.010 | −0.038 | 0.018 | 0.014 | ||||||
| LF/HF | −0.003 | −0.037 | 0.030 | 0.017 | −0.054 | 0.835 | ||||
| pnn50 (%) | 0.000 | −0.002 | 0.003 | 0.001 | 0.095 | 0.708 | ||||
| LF/HF × pnn50 (%) | 0.001 | −0.001 | 0.003 | 0.001 | 0.277 | 0.295 | ||||
| CH | Step | Independent Variables | B | 95% CI for β | Se B | β | p | R2 | ΔR2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1CH | Step 1 | 0.003 | ||||||||
| Intercept | −0.006 | −0.019 | 0.007 | 0.007 | ||||||
| LF/HF | 0.002 | −0.014 | 0.019 | 0.008 | 0.051 | 0.757 | ||||
| Step 2 | 0.005 | 0.003 | ||||||||
| Intercept | −0.006 | −0.020 | 0.008 | 0.007 | ||||||
| LF/HF | 0.001 | −0.017 | 0.019 | 0.009 | 0.027 | 0.884 | ||||
| pnn50(%) | 0.000 | −0.001 | 0.001 | 0.000 | −0.056 | 0.762 | ||||
| Step 3 | 0.066 | 0.061 | ||||||||
| Intercept | −0.001 | −0.016 | 0.014 | 0.007 | ||||||
| LF/HF | 0.005 | −0.013 | 0.024 | 0.009 | 0.103 | 0.582 | ||||
| pnn50(%) | 0.000 | −0.001 | 0.001 | 0.000 | 0.041 | 0.830 | ||||
| LF/HF × pnn50(%) | 0.001 | 0.000 | 0.002 | 0.001 | 0.264 | 0.134 | ||||
| 2CH | Step 1 | 0.009 | ||||||||
| Intercept | 0.013 | −0.007 | 0.033 | 0.010 | ||||||
| LF/HF | −0.007 | −0.031 | 0.017 | 0.012 | −0.094 | 0.562 | ||||
| Step 2 | 0.010 | 0.001 | ||||||||
| Intercept | 0.013 | −0.007 | 0.033 | 0.010 | ||||||
| LF/HF | −0.008 | 0.013 | 0.019 | −0.008 | −0.110 | 0.548 | ||||
| pnn50(%) | 0.000 | 0.001 | 0.001 | 0.000 | −0.036 | 0.845 | ||||
| Step 3 | 0.018 | 0.008 | ||||||||
| Intercept | 0.010 | −0.012 | 0.033 | 0.011 | ||||||
| LF/HF | −0.010 | −0.038 | 0.018 | 0.014 | −0.137 | 0.477 | ||||
| pnn50(%) | 0.000 | −0.002 | 0.001 | 0.001 | −0.070 | 0.722 | ||||
| LF/HF × pnn50(%) | 0.000 | −0.002 | 0.001 | 0.001 | −0.093 | 0.602 | ||||
| 3CH | Step 1 | 0.000 | ||||||||
| Intercept | −0.006 | −0.022 | 0.009 | 0.008 | ||||||
| LF/HF | 0.000 | −0.018 | 0.018 | 0.009 | 0.000 | 1.000 | ||||
| Step 2 | 0.000 | 0.000 | ||||||||
| Intercept | −0.006 | −0.022 | 0.009 | 0.008 | ||||||
| LF/HF | 0.000 | −0.021 | 0.021 | 0.010 | −0.001 | 0.997 | ||||
| pnn50(%) | 0.000 | −0.001 | 0.001 | 0.000 | −0.002 | 0.993 | ||||
| Step 3 | 0.029 | 0.029 | ||||||||
| Intercept | −0.002 | −0.020 | 0.015 | 0.009 | ||||||
| LF/HF | 0.003 | −0.019 | 0.025 | 0.011 | 0.052 | 0.784 | ||||
| pnn50(%) | 0.000 | −0.001 | 0.001 | 0.001 | 0.065 | 0.737 | ||||
| LF/HF × pnn50(%) | 0.001 | −0.001 | 0.002 | 0.001 | 0.183 | 0.305 | ||||
| 4CH | Step 1 | 0.000 | ||||||||
| Intercept | 0.010 | −0.004 | 0.023 | 0.007 | ||||||
| LF/HF | 0.000 | −0.016 | 0.016 | 0.008 | 0.009 | 0.956 | ||||
| Step 2 | 0.000 | 0.000 | ||||||||
| Intercept | 0.010 | −0.004 | 0.023 | 0.007 | ||||||
| LF/HF | 0.000 | 0.009 | 0.018 | 0.009 | 0.004 | 0.982 | ||||
| pnn50(%) | 0.000 | 0.000 | 0.001 | 0.000 | −0.011 | 0.953 | ||||
| Step 3 | 0.022 | 0.022 | ||||||||
| Intercept | 0.013 | −0.003 | 0.028 | 0.007 | ||||||
| LF/HF | 0.002 | −0.016 | 0.021 | 0.009 | 0.050 | 0.792 | ||||
| pnn50(%) | 0.000 | −0.001 | 0.001 | 0.000 | 0.048 | 0.807 | ||||
| LF/HF × pnn50(%) | 0.000 | −0.001 | 0.002 | 0.001 | 0.160 | 0.371 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, A.; Matsuzaki, Y.; Kawada, T. Neuroregulatory Effects of Microcone Patch Stimulation on the Auricular Branch of the Vagus Nerve and the Prefrontal Cortex: A Feasibility Study. J. Clin. Med. 2024, 13, 2399. https://doi.org/10.3390/jcm13082399
Kawasaki A, Matsuzaki Y, Kawada T. Neuroregulatory Effects of Microcone Patch Stimulation on the Auricular Branch of the Vagus Nerve and the Prefrontal Cortex: A Feasibility Study. Journal of Clinical Medicine. 2024; 13(8):2399. https://doi.org/10.3390/jcm13082399
Chicago/Turabian StyleKawasaki, Akihiro, Yutaka Matsuzaki, and Taku Kawada. 2024. "Neuroregulatory Effects of Microcone Patch Stimulation on the Auricular Branch of the Vagus Nerve and the Prefrontal Cortex: A Feasibility Study" Journal of Clinical Medicine 13, no. 8: 2399. https://doi.org/10.3390/jcm13082399
APA StyleKawasaki, A., Matsuzaki, Y., & Kawada, T. (2024). Neuroregulatory Effects of Microcone Patch Stimulation on the Auricular Branch of the Vagus Nerve and the Prefrontal Cortex: A Feasibility Study. Journal of Clinical Medicine, 13(8), 2399. https://doi.org/10.3390/jcm13082399






