Long-Term Efficacy and Safety of Direct Oral Anticoagulants at Reduced Doses in the Secondary Prevention of Venous Thromboembolism and Post-Thrombotic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Procedures, and Follow-Up
2.2. Study Endpoints
2.3. Statistical Analysis
3. Results
4. Discussion
- (I)
- Long-term extended therapy for VTE with reduced DOACs was effective and as safe as with a full dose, as none of the patients experienced recurrences, no MB occurred, and CRNMB events were equal. Notably, the average time of treatment with DOACs at full dose before dosage reduction was 24 months, and in about one-third of cases the reason for dosage reduction was due to haemorrhagic phenomena. Additionally, patients in the RD group were significantly older. Therefore, the safety results are reassuring when considering the haemorrhagic risk of the RD population.
- (II)
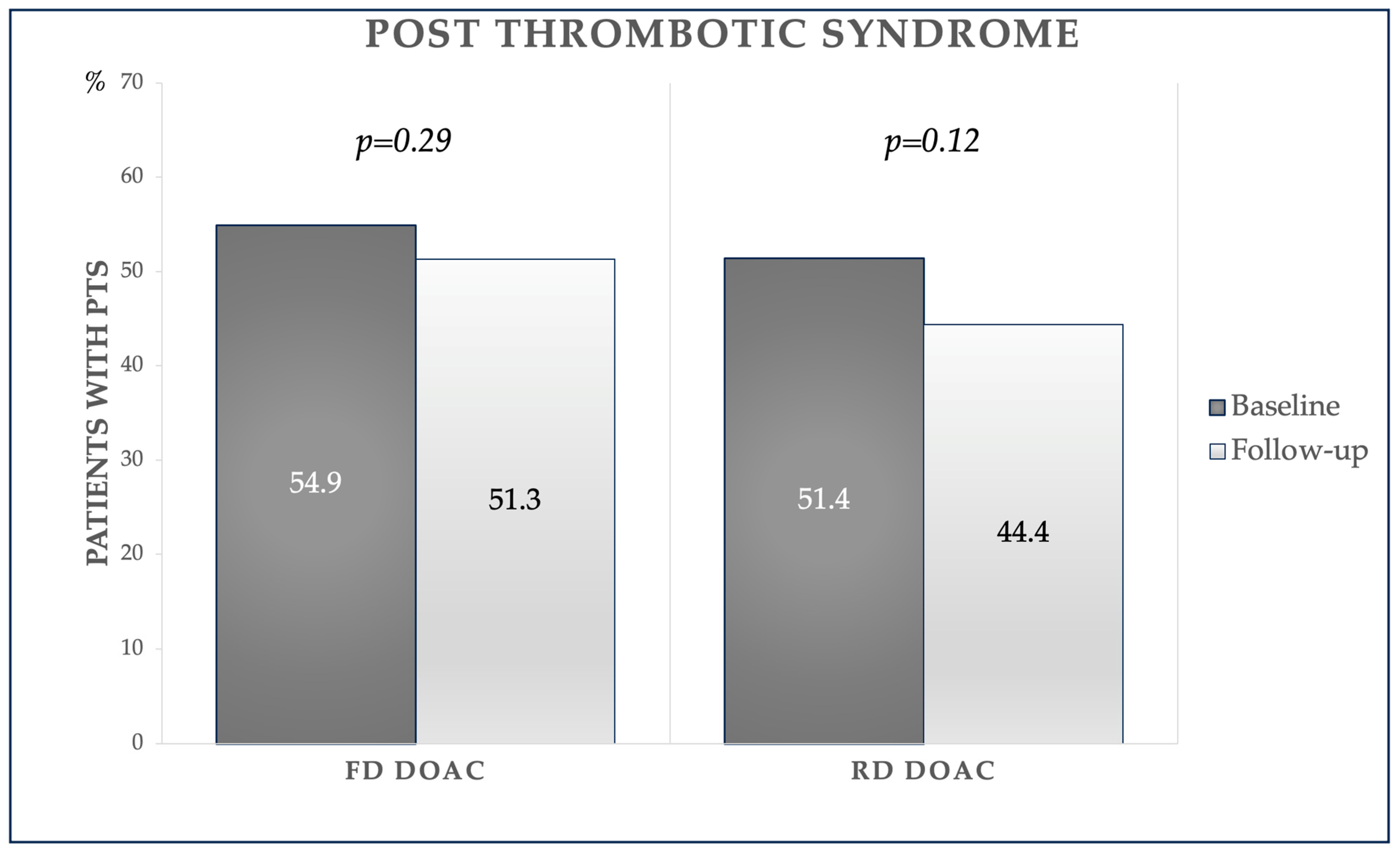
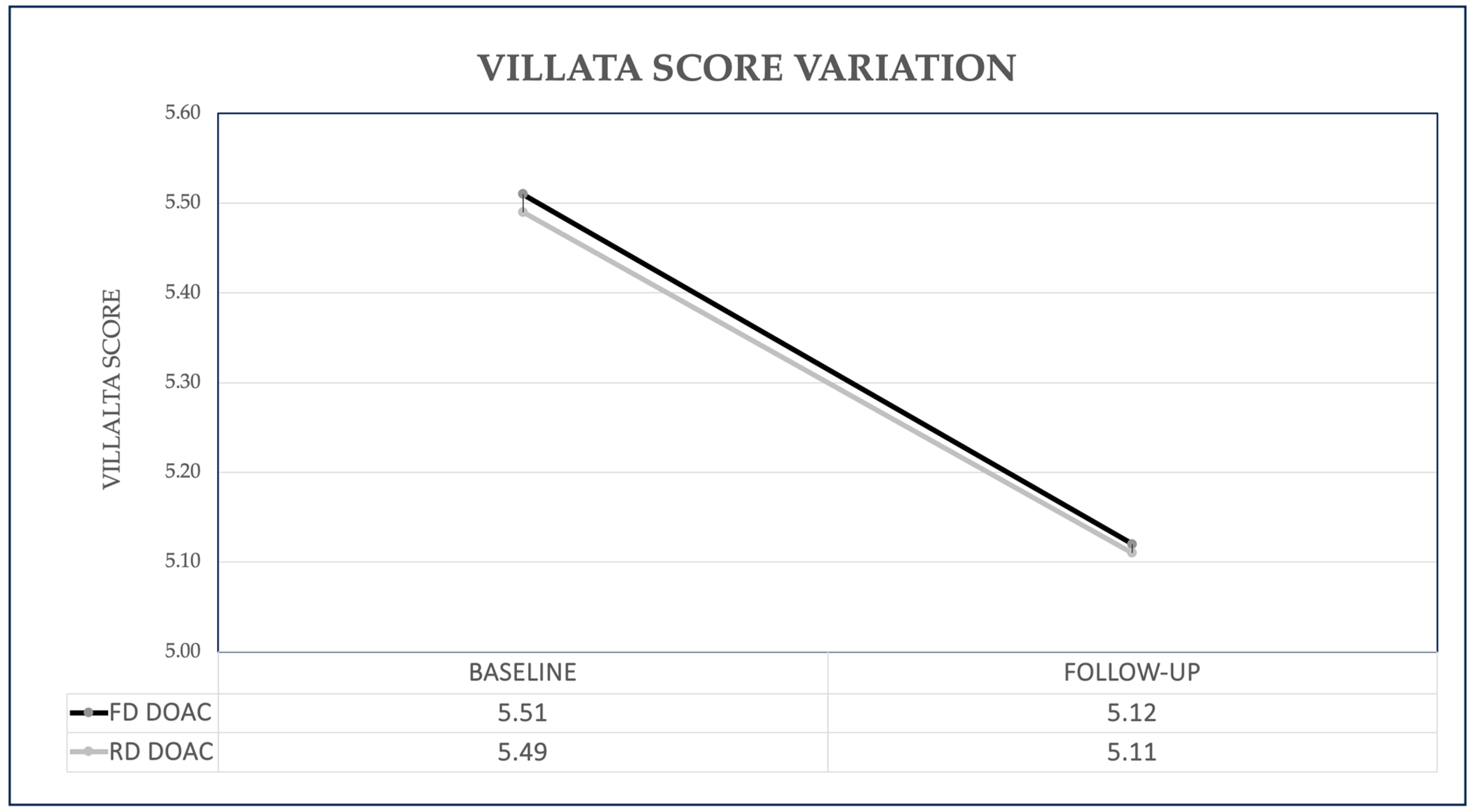
- The progression of PTS was not negatively impacted by r-DOACs; however, there was even a significant improvement in the Villalta score.
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Andresen, M.S.; Sandven, I.; Brunborg, C.; Njaastad, A.M.; Strekerud, F.; Abdelnoor, M.; Smith, P.; Abildgaard, U. Mortality and recurrence after treatment of VTE: Long term follow-up of patients with good life-expectancy. Thromb. Res. 2011, 127, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Predictors of survival after deep vein thrombosis and pulmonary embolism: A population-based, cohort study. Arch. Intern. Med. 1999, 159, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Nelson, R.E.; Nyarko, K.A.; Richardson, L.C.; Raskob, G.E. The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs. Thromb. Res. 2016, 137, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Spencer, F.A.; Emery, C.; Joffe, S.W.; Pacifico, L.; Lessard, D.; Reed, G.; Gore, J.M.; Goldberg, R.J. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J. Thromb. Thrombolysis 2009, 28, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Spencer, F.A.; White, R.H. The epidemiology of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Rahman, A.; Carrier, M.; Kearon, C.; Schulman, S.; Couturaud, F.; Prandoni, P.; Eichinger, S.; Becattini, C.; Agnelli, G.; et al. Long-term risk of recurrence after discontinuing anticoagulants for a first unprovoked venous thromboembolism: Protocol for a systematic review and meta-analysis. BMJ Open 2017, 7, 16950. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Zakai, N.A. Epidemiology and prevention of venous thromboembolism. Nat. Reviews. Cardiol. 2023, 20, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Ageno, W.; Cannegieter, S.C.; Cosmi, B.; Geersing, G.J.; Kyrle, P.A.; Subcommittees on Control of Anticoagulation, and Predictive and Diagnostic Variables in Thrombotic Disease. Categorization of patients as having provoked or unprovoked venous thromboembolism: Guidance from the SSC of ISTH. J. Thromb. Haemost. 2016, 14, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Makedonov, I.; Kahn, S.R.; Galanaud, J.-P. Prevention and Management of the Post-Thrombotic Syndrome. J. Clin. Med. 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Ashrani, A.A.; Heit, J.A. Incidence and cost burden of post-thrombotic syndrome. J. Thromb. Thrombolysis 2009, 28, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Ageno, W.; Weitz, J.I.; Goldhaber, S.Z.; Turpie, A.G.G.; Goto, S.; Angchaisuksiri, P.; Dalsgaard Nielsen, J.; Kayani, G.; Zaghdoun, A.; et al. Anticoagulation therapy patterns for acute treatment of venous thromboembolism in GARFIELD-VTE patients. J. Thromb. Haemost. 2019, 17, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, S.K.; Gohel, M.; Baekgaard, N.; Bauersachs, R.; Bellmunt-Montoya, S.; Black, S.A.; Ten Cate-Hoek, A.J.; Elalamy, I.; Enzmann, F.K.; Geroulakos, G.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) Clinical Practice Guidelines on the Management of Venous Thrombosis. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 9–82. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M.; Woller, S.C.; Kreuziger, L.B.; Bounameaux, H.; Doerschug, K.; Geersing, G.J.; Huisman, M.V.; Kearon, C.; King, C.S.; Knighton, A.J.; et al. Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest 2021, 160, e545–e608. [Google Scholar] [CrossRef] [PubMed]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimeénez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Porcari, A.; Raskob, G.E.; Weitz, J.I.; AMPLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. New Engl. J. Med. 2013, 368, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Lensing, A.W.A.; Prins, M.H.; Bauersachs, R.; Beyer-Westendorf, J.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; Davidson, B.L.; Decousus, H.; et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. New Engl. J. Med. 2017, 376, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J. Thromb. Haemost. 2009, 7, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M.; Woller, S.C.; Baumann Kreuziger, L.; Doerschug, K.; Geersing, G.J.; Klok, F.A.; King, C.S.; Murin, S.; Vintch, J.R.E.; Wells, P.S.; et al. Antithrombotic Therapy for VTE Disease: Compendium and Review of CHEST Guidelines 2012–2021. Chest 2024. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Le Gal, G.; Wells, P.S.; Rodger, M.A. Systematic review: Case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann. Intern. Med. 2010, 152, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, L.A.; Cameron, C.; Le Gal, G.; Rodger, M.A.; Coyle, D.; Wells, P.S.; Clifford, T.; Gandara, E.; Wells, G.; Carrier, M. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: Systematic review and network meta-analysis. BMJ 2013, 347, f5133. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Iorio, A.; Palareti, G.; Subcommittee on Control of Anticoagulation of the SSC of the ISTH. Risk of recurrent venous thromboembolism after stopping treatment in cohort studies: Recommendation for acceptable rates and standardized reporting. J. Thromb. Haemost. 2010, 8, 2313–2315. [Google Scholar] [CrossRef] [PubMed]
- Verhovsek, M.; Douketis, J.D.; Yi, Q.; Shrivastava, S.; Tait, R.C.; Baglin, T.; Poli, D.; Lim, W. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann. Intern. Med. 2008, 149, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Rodger, M.A.; Le Gal, G.; Anderson, D.R.; Schmidt, J.; Pernod, G.; Kahn, S.R.; Righini, M.; Mismetti, P.; Kearon, C.; Meyer, G.; et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: Multinational prospective cohort management study. BMJ 2017, 356, j1065. [Google Scholar] [CrossRef] [PubMed]
- Palareti, G.; Poli, D.; Ageno, W.; Legnani, C.; Antonucci, E.; Bucherini, E.; Testa, S.; Paoletti, O.; Chistolini, A.; Serrao, A.; et al. D-dimer and reduced-dose apixaban for extended treatment after unprovoked venous thromboembolism: The Apidulcis study. Blood Adv. 2022, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- de Winter, M.A.; Büller, H.R.; Carrier, M.; Cohen, A.T.; Hansen, J.B.; Kaasjager, K.A.H.; Kakkar, A.K.; Middeldorp, S.; Raskob, G.E.; Sørensen, H.T.; et al. Recurrent venous thromboembolism and bleeding with extended anticoagulation: The VTE-PREDICT risk score. Eur. Heart J. 2023, 44, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tritschler, T.; Kimpton, M.; Wells, P.S.; Kearon, C.; Weitz, J.I.; Büller, H.R.; Raskob, G.E.; Ageno, W.; Couturaud, F.; et al. Long-Term Risk for Major Bleeding During Extended Oral Anticoagulant Therapy for First Unprovoked Venous Thromboembolism: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2021, 174, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Ageno, W.; Donadini, M. Breadth of complications of long-term oral anticoagulant care. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, R.; Mantovani, L.G.; Haas, S.; Monje, D.; Schneider, J.; Bugge, J.P.; Gebel, M.; Tamm, M.; Ageno, W.; Turpie, A.G.G. XALIA-LEA: An observational study of venous thromboembolism treatment with rivaroxaban and standard anticoagulation in the Asia-Pacific, Eastern Europe, the Middle East, Africa and Latin America. Thromb. Res. 2019, 176, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Beyer-Westendorf, J.; Förster, K.; Pannach, S.; Ebertz, F.; Gelbricht, V.; Thieme, C.; Michalski, F.; Köhler, C.; Werth, S.; Sahin, K.; et al. Rates, management, and outcome of rivaroxaban bleeding in daily care: Results from the Dresden NOAC registry. Blood 2014, 124, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Bova, C.; Bianco, A.; Mascaro, V.; Nobile, C.G. Extended anticoagulation and mortality in venous thromboembolism. A meta-analysis of six randomized trials. Thromb. Res. 2016, 139, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tan, J.; Deng, Y.; Hua, L.; Guo, T. Clinical and Safety Outcomes Associated with Extended Treatment of Venous Thromboembolism: A Network Meta-Analysis. J. Cardiovasc. Dev. Dis. 2022, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Ebraheem, M.; Alzahrani, I.; Crowther, M.; Rochwerg, B.; Almakadi, M. Extended DOAC therapy in patients with VTE and potential risk of recurrence: A systematic review and meta-analysis. J. Thromb. Haemost. 2020, 18, 2308–2317. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Bertoletti, L.; Cucherat, M.; Jardel, S.; Grange, C.; Provencher, S.; Lega, J.C. Extended anticoagulation for the secondary prevention of venous thromboembolic events: An updated network meta-analysis. PLoS ONE 2019, 14, e0214134. [Google Scholar] [CrossRef]
- Vasanthamohan, L.; Boonyawat, K.; Chai-Adisaksopha, C.; Crowther, M. Reduced-dose direct oral anticoagulants in the extended treatment of venous thromboembolism: A systematic review and meta-analysis. J. Thromb. Haemost. 2018, 16, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Comerota, A.J.; Cushman, M.; Evans, N.S.; Ginsberg, J.S.; Goldenberg, N.A.; Gupta, D.K.; Prandoni, P.; Vedantham, S.; Walsh, M.E.; et al. The postthrombotic syndrome: Evidence-based prevention, diagnosis, and treatment strategies: A scientific statement from the American Heart Association. Circulation 2014, 130, 1636–1661. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, L.; Francaviglia, B.; Frazzetto, M.; Valenti, N.; Capranzano, P. Impact of direct oral anticoagulants on evolution of post-thrombotic syndrome. Thromb Res. 2021, 207, 10–15. [Google Scholar] [CrossRef] [PubMed]
| AMPLIFY-EX [16] | EINSTEIN CHOICE [17] | |
|---|---|---|
| Number of patients | 2486 | 3365 |
| Drug and dose | Placebo vs. ApiX 2.5 mg BID vs. ApiX 5 mg BID | ASA 100 mg OD vs. RivaX 10 mg OD vs. RivaX 20 mg OD |
| Recurrent VTE and VTE-related Death | Placebo: 8.8% | ASA 100 mg: 4.4% |
| ApiX 2.5 mg: 1.7% | RivaX 10 mg: 1.2% | |
| ApiX 5 mg: 1.7% | RivaX 20 mg: 1.5% | |
| Major bleeding | Placebo: 0.5% | ASA 100 mg: 0.3% |
| ApiX 2.5 mg: 0.2% | RivaX 10 mg: 0.4% | |
| ApiX 5 mg: 0.1% | RivaX 20 mg: 0.5% | |
| Clinically Relevant non-major bleeding | Placebo: 2.3% | ASA 100 mg: 1.8% |
| ApiX 2.5 mg: 3% | RivaX 10 mg: 2.0% | |
| ApiX 5 mg: 4.2% | RivaX 20 mg: 2.7% | |
| Recurrent VTE or Mortality for all causes | Placebo: 11.6% | ASA 100 mg: 4.9% |
| ApiX 2.5 mg: 3.8% | RivaX 10 mg:1.3% | |
| ApiX 5 mg: 4.2% | RivaX 20 mg: 2.1% |
| Demographic and Clinical Characteristics of the Population (n = 185) | |||
|---|---|---|---|
| Therapeutic DOACs (n = 113) | Reduced DOACs (n = 72) | p | |
| Age (years), mean ± SD | 57.00 ± 15.74 | 64.11 ± 14.47 | <0.001 |
| Male sex, n (%) | 70 (61.9) | 40 (55.6) | 0.44 |
| BMI, mean ± SD | 26.28 ± 4.80 | 26.86 ± 4.10 | 0.23 |
| Recurrent VTE, n (%) | 44 (38.9) | 30 (41.7) | 0.71 |
| PE, n. (%) | 28 (24.8) | 25 (34.7) | 0.18 |
| Proximal VTE, n (%) | 94 (83.2) | 64 (88.9) | 0.29 |
| Provoked thrombosis, n (%) | 23 (20.4) | 15 (20.8) | 1 |
| Smokers, n (%) | 37 (32.7) | 20 (27.8) | 0.52 |
| Arterial hypertension, n (%) | 53 (46.9) | 42 (58.3) | 0.13 |
| eGFR (ml/min), mean ± SD | 91.12 ± 21.29 | 88.99 ± 22.46 | 0.44 |
| Active cancer, n (%) | 10 (8.8) | 8 (11.1%) | 0.61 |
| Heterozygosity Factor V Leiden, n (%) | 15 (13.3) | 9 (12.5) | 1 |
| Heterozygosity Factor II, n (%) | 8 (7.1) | 7 (9.7) | 0.58 |
| Protein C deficiency, n (%) | 1 (0.9) | 1 (1.4) | 1 |
| Protein S deficiency, n (%) | 24 (21.2) | 12 (16.7) | 0.46 |
| Antithrombin III deficiency n (%) | 2 (1.8) | 4 (5.6) | 0.21 |
| Results of the Study | |||
|---|---|---|---|
| Full-Dose DOACs (n = 113) | Reduced-Dose DOACs (n = 72) | p | |
| VTE occurrence/recurrence, n (%) | 0 (0%) | 0 (0%) | - |
| MB, n (%) | 0 (0%) | 0 (0) | - |
| CRNMB, n (%) | 3 (2.7%) | 3 (4.2%) | 0.57 |
| Villalta Score, mean ± SD | 5.24 ± 4.51 | 5.11 ± 3.73 | 0.72 |
| PTS, n (%) | 59 (52.2%) | 32 (44.4%) | 0.30 |
| Elastic stockings adherence, n (%) | 101 (89.4%) | 66 (91.7%) | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanzo, L.; Di Paola, F.; Pedi, A.M.; Failla, G.; Mangiafico, M. Long-Term Efficacy and Safety of Direct Oral Anticoagulants at Reduced Doses in the Secondary Prevention of Venous Thromboembolism and Post-Thrombotic Syndrome. J. Clin. Med. 2024, 13, 2394. https://doi.org/10.3390/jcm13082394
Costanzo L, Di Paola F, Pedi AM, Failla G, Mangiafico M. Long-Term Efficacy and Safety of Direct Oral Anticoagulants at Reduced Doses in the Secondary Prevention of Venous Thromboembolism and Post-Thrombotic Syndrome. Journal of Clinical Medicine. 2024; 13(8):2394. https://doi.org/10.3390/jcm13082394
Chicago/Turabian StyleCostanzo, Luca, Federico Di Paola, Anastasia Maria Pedi, Giacomo Failla, and Marco Mangiafico. 2024. "Long-Term Efficacy and Safety of Direct Oral Anticoagulants at Reduced Doses in the Secondary Prevention of Venous Thromboembolism and Post-Thrombotic Syndrome" Journal of Clinical Medicine 13, no. 8: 2394. https://doi.org/10.3390/jcm13082394
APA StyleCostanzo, L., Di Paola, F., Pedi, A. M., Failla, G., & Mangiafico, M. (2024). Long-Term Efficacy and Safety of Direct Oral Anticoagulants at Reduced Doses in the Secondary Prevention of Venous Thromboembolism and Post-Thrombotic Syndrome. Journal of Clinical Medicine, 13(8), 2394. https://doi.org/10.3390/jcm13082394





