Exploring Chronic Hypocalcemia: Insights into Autoimmune Polyglandular Syndrome Type 1—A Case Study and Literature Review
Abstract
1. Introduction
2. Case Report
2.1. Patient’s Medical History
2.2. Symptoms and Clinical Findings
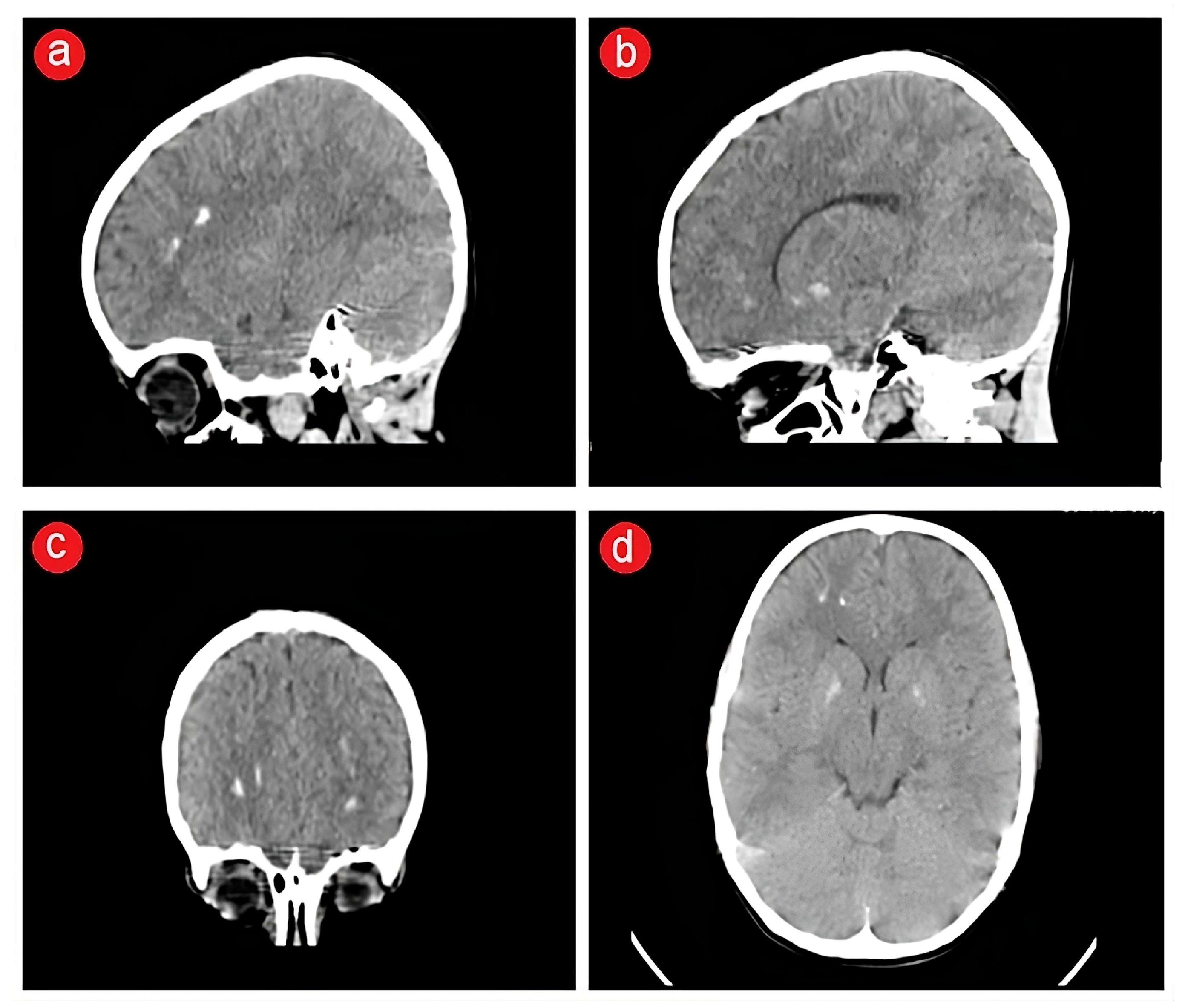
2.3. Biological and Paraclinical Assessment
2.4. Therapeutic Intervention
2.5. Follow-Up and Outcome
3. Discussion
3.1. Literature Review
3.1.1. Definition of APS-1
3.1.2. Epidemiology
3.1.3. Pathogenesis and Autoimmunity of APS-1
3.1.4. Correlation between Genotype and Phenotype in APS-1
3.1.5. Clinical Features of APS-1
3.1.6. Therapeutic Approach in APS-1
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcucci, G.; Cianferotti, L.; Brandi, M.L. Clinical presentation and management of hypoparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, F.; Pepe, J.; Danese, V.C.; Fassino, V.; Cecchetti, V.; De Lucia, F.; Biamonte, F.; Colangelo, L.; Ferrazza, G.; Panzini, E.; et al. The relative influence of serum ionized calcium and 25- hydroxyvitamin D in regulating PTH secretion in healthy subjects. Bone 2019, 125, 200–206. [Google Scholar] [CrossRef]
- Bharill, S.; Wu, M. Hypocalcemia and Hypercalcemia in Children. Pediatr. Rev. 2023, 44, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Cusano, N.E.; Bilezikian, J.P. Signs and symptoms of hypoparathyroidism. Endocrinol. Metab. Clin. N. Am. 2018, 47, 759–770. [Google Scholar] [CrossRef]
- Underbjerg, L.; Sikjaer, T.; Mosekilde, L.; Rejnmark, L. Cardiovascular and renal complications to postsurgical hypoparathyroidism: A Danish nationwide controlled historic follow-up study. J. Bone Miner. Res. 2013, 28, 2277–2285. [Google Scholar] [CrossRef]
- Dittmar, M.; Kahaly, G.J. Polyglandular autoimmune syndromes: Immunogenetics and long-term follow-up. J. Clin. Endocrinol. Metab. 2003, 88, 2983–2992. [Google Scholar] [CrossRef]
- Neufeld, M.; Maclaren, N.; Blizzard, R. Autoimmune polyglandular syndromes. Pediatr. Ann. 1980, 9, 154–162. [Google Scholar] [CrossRef]
- Michels, A.W.; Gottlieb, P.A. Autoimmune polyglandular syndromes. Nat. Rev. Endocrinol. 2010, 6, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Anaya, J.M.; Rojas-Villarraga, A.; Garcıa-Carrasco, M. The Autoimmune Tautology: From Polyautoimmunity and Familial Autoimmunity to the Autoimmune Genes. Autoimmune Dis. 2012, 2012, 297193. [Google Scholar] [CrossRef]
- Bousfiha, A.; Moundir, A.; Tangye, S.G.; Picard, C.; Jeddane, L.; Al-Herz, W.; Rundles, C.C.; Franco, J.L.; Holland, S.M.; Klein, C.; et al. The 2022 Update of IUIS Phenotypical Classification for Human Inborn Errors of Immunity. J. Clin. Immunol. 2022, 42, 1508–1520. [Google Scholar] [CrossRef]
- Guo, C.J.; Leung, P.S.C.; Zhang, W.; Ma, X.; Gershwin, M.E. The immunobiology and clinical features of type 1 autoimmune polyglandular syndrome (APS-1). Autoimmun. Rev. 2018, 17, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Husebye, E.S.; Perheentupa, J.; Rautemaa, R.; Kämpe, O. Clinical Manifestations and Management of Patients with Autoimmune Polyendocrine Syndrome Type I. J. Intern. Med. 2009, 265, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Nuralieva, N.; Yukina, M.; Sozaeva, L.; Donnikov, M.; Kovalenko, L.; Troshina, E.; Orlova, E.; Gryadunov, D.; Savvateeva, E.; Dedov, I. Diagnostic Accuracy of Methods for Detection of Antibodies against Type I Interferons in Patients with Endocrine Disorders. J. Pers. Med. 2022, 12, 1948. [Google Scholar] [CrossRef] [PubMed]
- Husebye, E.S.; Anderson, M.S.; Kämpe, O. Autoimmune polyendocrine syndromes. N. Engl. J. Med. 2018, 378, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Zlotogora, J.; Shapiro, M.S. Polyglandular autoimmune syndrome type I among Iranian Jews. J. Med. Genet. 1992, 29, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, P.; Myllarniemi, S.; Sipila, I.; Perheentupa, J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N. Engl. J. Med. 1990, 322, 1829–1836. [Google Scholar] [CrossRef]
- Betterle, C.; Presotto, F. Autoimmune Polyendocrine Syndrome (APS) or multiple autoimmune syndrome (MAS). In Handbook of Systemic Autoimmune Diseases, Endocrine Manifestations of Systemic Autoimmune Diseases; Walker, S., Jara, L.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 135–148. [Google Scholar]
- Proust-Lemoine, E.; Saugier-Veber, P.; Wémeau, J.L. Polyglandular autoimmune syndrome type I. Presse Med. 2012, 41, e651–e662. [Google Scholar] [CrossRef] [PubMed]
- Sato, U.; Horikawa, R.; Katsumata, N.; Asakura, Y.; Kitanaka, S.; Tanaka, T. Novel compound heterozygous AIRE mutations in a Japanese patient with APECED. J. Pediatr. Endocrinol. Metab. 2004, 17, 917–921. [Google Scholar] [CrossRef]
- Capalbo, D.; Improda, N.; Esposito, A.; De Martino, L.; Barbieri, F.; Betterle, C.; Pignata, C.; Salerno, M. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy from the pediatric perspective. J. Endocrinol. Investig. 2013, 36, 903–912. [Google Scholar]
- Ferre, E.M.; Rose, S.R.; Rosenzweig, S.D.; Burbelo, P.D.; Romito, K.R.; Niemela, J.E.; Rosen, L.B.; Break, T.J.; Gu, W.; Hunsberger, S.; et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight 2016, 1, e88782. [Google Scholar] [CrossRef]
- Pellegrino, M.; Bellacchio, E.; Dhamo, R.; Frasca, F.; Betterle, C.; Fierabracci, A. A novel homozygous mutation of the AIRE gene in an APECED patient from Pakistan: Case report and review of the literature. Front. Immunol. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Venanzi, E.S.; Klein, L.; Chen, Z.; Berzins, S.P.; Turley, S.J.; von Boehmer, H.; Bronson, R.; Dierich, A.; Benoist, C.; et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 2002, 298, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Strassburg, C.P.; Obermayer-Straub, P.; Brabant, G.; Manns, M.P. The genetic background of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy and its autoimmune disease components. J. Mol. Med. 2002, 80, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Perniola, R.; Musco, G. The biophysical and biochemical properties of the autoimmune regulator (AIRE) protein. Biochim. Biophys. Acta. 2014, 1842, 326–337. [Google Scholar] [CrossRef]
- Liiv, I.; Haljasorg, U.; Kisand, K.; Maslovskaja, J.; Laan, M.; Peterson, P. AIRE-induced apoptosis is associated with nuclear translocation of stress sensor protein GAPDH. Biochem. Biophys. Res. Commun. 2012, 423, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Rosatelli, M.C.; Meloni, A.; Meloni, A.; Devoto, M.; Cao, A.; Scott, H.S.; Peterson, P.; Heino, M.; Krohn, D.-M.N.; Nagamine, K.; et al. A common mutation in Sardinian autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. Hum. Genet. 1998, 103, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Fierabracci, A. Type 1 diabetes in autoimmune polyendocrinopathycandidiasis-ectodermal dystrophy syndrome (APECED): A “rare” manifestation in a “rare” disease. Int. J. Mol. Sci. 2016, 17, 1106. [Google Scholar] [CrossRef] [PubMed]
- Malchow, S.; Leventhal, D.S.; Nishi, S.; Fischer, B.I.; Shen, L.; Paner, G.P.; Amit, A.S.; Kang, C.; Geddes, J.E.; Allison, J.P.; et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 2013, 339, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.D.; Gilmore, D.C.; Dileepan, T.; Nawrocka, W.I.; Chao, J.L.; Schoenbach, M.H.; Jenkins, M.K.; Adams, E.J.; Savage, P.A. Identification of Natural Regulatory T Cell Epitopes Reveals Convergence on a Dominant Autoantigen. Immunity 2017, 47, 107–117.e8. [Google Scholar] [CrossRef]
- Deleeuw, R.J.; Kost, S.E.; Kakal, J.A.; Nelson, B.H. The Prognostic Value of FoxP3+ Tumor-Infiltrating Lymphocytes in Cancer: A Critical Review of the Literature. Clin. Cancer Res. 2012, 18, 3022. [Google Scholar] [CrossRef]
- Zhao, B.; Chang, L.; Fu, H.; Sun, G.; Yang, W. The Role of Autoimmune Regulator (AIRE) in Peripheral Tolerance. J. Immunol. Res. 2018, 2018, 3930750. [Google Scholar] [CrossRef] [PubMed]
- Philippot, Q.; Casanova, J.L.; Puel, A. Candidiasis in patients with APS-1: Low IL-17, high IFN-γ, or both? Curr. Opin. Immunol. 2021, 72, 318–323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kashem, S.W.; Binstadt, B.A. Pathogenic and Protective Autoantibodies in Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy (APECED). Antibodies 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, B.E.; Hellesen, A.; Erichsen, M.M.; Bratland, E.; Vardi, A.; Perheentupa, J.; Kemp, E.H.; Fiskerstrand, T.; Viken, M.K.; Weetman, A.P.; et al. Dominant mutations in the autoimmune regulator AIRE are associated with common organ-specific autoimmune diseases. Immunity 2015, 42, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Cetani, F.; Barbesino, G.; Borsari, S.; Pardi, E.; Cianferotti, L.; Pinchera, A.; Marcocci, C. A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J. Clin. Endocrinol. Metab. 2001, 86, 4747–4752. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.K.; Huoh, Y.S.; Reynolds, P.R.; Yu, L.; Rewers, M.; Reddy, M.; Anderson, M.S.; Hur, S.; Gelfand, E.W. Dominant-negative loss of function arises from a second, more frequent variant within the SAND domain of autoimmune regulator (AIRE). J. Autoimmun. 2018, 88, 114–120. [Google Scholar] [CrossRef]
- Constantine, G.M.; Lionakis, M.S. Lessons from primary immunodeficiencies: Autoimmune regulator and autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Immunol. Rev. 2019, 287, 103–112. [Google Scholar] [CrossRef]
- Oftedal, B.E.; Assing, K.; Baris, S.; Safgren, S.L.; Johansen, I.S.; Jakobsen, M.A.; Babovic-Vuksanovic, D.; Agre, K.; Klee, E.W.; Majcic, E.; et al. Dominant-negative heterozygous mutations in AIRE confer diverse autoimmune phenotypes. iScience 2023, 26, 106818. [Google Scholar] [CrossRef]
- Human Gene Mutation Database. Available online: https://www.hgmd.cf.ac.uk/ac/gene.php?gene=AIRE (accessed on 30 December 2023).
- Björses, P.; Halonen, M.; Palvimo, J.J.; Kolmer, M.; Aaltonen, J.; Ellonen, P.; Perheentupa, J.; Ulmanen, I.; Peltonen, L. Mutations in the AIRE gene: Effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am. J. Hum. Genet. 2000, 66, 378–392. [Google Scholar] [CrossRef]
- Cervato, S.; Mariniello, B.; Lazzarotto, F.; Morlin, L.; Zanchetta, R.; Radetti, G.; De Luca, F.; Valenzise, M.; Giordano, R.; Rizzo, D.; et al. Evaluation of the autoimmune regulator (AIRE) gene mutations in a cohort of Italian patients with autoimmune-polyendocrinopathy-candidiasis-ectodermal-dystrophy (APECED) and in their relatives. Clin. Endocrinol. 2009, 70, 421–428. [Google Scholar] [CrossRef]
- De Martino, L.; Capalbo, D.; Improda, N.; D’Elia, F.; Di Mase, R.; D’Assante, R.; D’Acunzo, I.; Pignata, C.; Salerno, M. APECED: A Paradigm of Complex Interactions between Genetic Background and Susceptibility Factors. Front. Immunol. 2013, 4, 331. [Google Scholar] [CrossRef] [PubMed]
- Perheentupa, J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J. Clin. Endocrinol. Metab. 2006, 912, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.H.; Cheetham, T.; Imrie, H.; Vaidya, B.; Barnes, N.D.; Bilous, R.W.; Carr, D.; Meeran, K.; Shaw, N.J.; Smith, C.S.; et al. A common and recurrent 13-bp deletion in the autoimmune regulator gene in British kindreds with autoimmune polyendocrinopathy type 1. Am. J. Hum. Genet. 1998, 63, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Willcox, N.; Meager, A.; Atzeni, M.; Wolff, A.S.; Husebye, E.S.; Furcas, M.; Rosatelli, M.C.; Cao, A.; Congia, M. Autoimmune polyendocrine syndrome type 1: An extensive longitudinal study in Sardinian patients. J. Clin. Endocrinol. Metab. 2012, 97, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Fierabracci, A.; Lanzillotta, M.; Vorgučin, I.; Palma, A.; Katanić, D.; Betterle, C. Report of two siblings with APECED in Serbia: Is there a founder effect of c.769C>T AIRE genotype? Ital. J. Pediatr. 2021, 47, 126. [Google Scholar] [CrossRef] [PubMed]
- Kisand, K.; Bøe Wolff, A.S.; Podkrajsek, K.T.; Tserel, L.; Link, M.; Kisand, K.V.; Ersvaer, E.; Perheentupa, J.; Erichsen, M.M.; Bratanic, N.; et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 2010, 207, 299–308. [Google Scholar] [CrossRef]
- Ferré, E.M.N.; Break, T.J.; Burbelo, P.D.; Allgäuer, M.; Kleiner, D.E.; Jin, D.; Xu, Z.; Folio, L.R.; Mollura, D.J.; Swamydas, M.; et al. Lymphocyte-driven regional immunopathology in pneumonitis caused by impaired central immune tolerance. Sci. Transl. Med. 2019, 11, eaav5597. [Google Scholar] [CrossRef] [PubMed]
- Chascsa, D.M.; Ferré, E.M.N.; Hadjiyannis, Y.; Alao, H.; Natarajan, M.; Quinones, M.; Kleiner, D.E.; Simcox, T.L.; Chitsaz, E.; Rose, S.R.; et al. APECED-Associated Hepatitis: Clinical, Biochemical, Histological and Treatment Data from a Large, Predominantly American Cohort. Hepatology 2021, 73, 1088–1104. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, D.; Røyrvik, E.C.; Aranda-Guillén, M.; Berger, A.H.; Landegren, N.; Artaza, H.; Hallgren, Å.; Grytaas, M.A.; Ström, S.; Bratland, E.; et al. GWAS for autoimmune Addison’s disease identifies multiple risk loci and highlights AIRE in disease susceptibility. Nat. Commun. 2021, 12, 959. [Google Scholar] [CrossRef]
- Laisk, T.; Lepamets, M.; Koel, M.; Abner, E.; Estonian Biobank Research, T.; Magi, R. Genome-wide association study identifies five risk loci for pernicious anemia. Nat. Commun. 2021, 12, 3761. [Google Scholar] [CrossRef]
- Kliegman, R.; Behrman, R.E.; Nelson, W.E. (Eds.) Nelson Textbook of Pediatrics, 20th ed.; Elsevier: Philadelphia, PA, USA, 2016; ISBN 978-1-4557-7566-8. [Google Scholar]
- Nambam, B.; Winter, W.E.; Schatz, D.A. IgG4 antibodies in autoimmune polyglandular disease and IgG4-related endocrinopathies: Pathophysiology and clinical characteristics. Curr. Opin. Pediatr. 2014, 26, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Naletto, L.; Frigo, A.C.; Ceccato, F.; Sabbadin, C.; Scarpa, R.; Presotto, F.; Dalla Costa, M.; Faggian, D.; Plebani, M.; Censi, S.; et al. The natural history of autoimmune Addison’s disease from the detection of autoantibodies to development of the disease: A long-term follow-up study on 143 patients. Eur. J. Endocrinol. 2019, 180, 223–234. [Google Scholar] [CrossRef]
- Garelli, S.; Dalla Costa, M.; Sabbadin, C.; Barollo, S.; Rubin, B.; Scarpa, R.; Masiero, S.; Fierabracci, A.; Bizzarri, C.; Crinò, A.; et al. Autoimmune polyendocrine syndrome type 1: An Italian survey on 158 patients. J. Endocrinol. Investig. 2021, 44, 2493–2510. [Google Scholar] [CrossRef] [PubMed]
- Proust-Lemoine, E.; Guyot, S. Polyendocrinopathies auto-immunes de type 1 et pathologies buccales. Presse Méd. 2017, 46, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Skrabic, V.; Skrabic, I.; Skrabic, R.; Roje, B.; Simunovic, M. Clinical Characteristics in the Longitudinal Follow-Up of APECED Syndrome in Southern Croatia-Case Series. Genes 2022, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Myhre, A.G.; Halonen, M.; Eskelin, P.; Ekwall, O.; Hedstrand, H.; Rorsman, F.; Kämpe, O.; Husebye, E.S. Autoimmune Polyendocrine Syndrome Type 1 (APS I) in Norway. Clin. Endocrinol. 2001, 54, 211–217. [Google Scholar] [CrossRef]
- Kang, M.S.; Sandhu, C.S.; Singh, N.; Evans, T. Initiation of levothyroxine in a patient with hypothyroidism inducing adrenal crisis requiring VA ECMO: A tale of preventable disaster. BMJ Case. Rep. 2019, 12, e230601. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, D.; Bossini, B.; Benvenuto, S.; Pellegrin, M.C.; Tornese, G. Pediatric Adrenal Insufficiency: Challenges and Solutions. Ther. Clin. Risk Manag. 2022, 18, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Abate, E.G.; Clarke, B.L. Review of Hypoparathyroidism. Front. Endocrinol. 2017, 7, 172. [Google Scholar] [CrossRef]
- Sanda, S.; Sclingmann, K.P.; Newfield, R.S. Autosomal dominant hypoparathyroidism with severe hypomagnesemia and hypocalcemia successfully treated with recombinant PTH and continuous subcutaneous magnesium infusion. J. Pediatr. Endocrinol. Metab. 2008, 21, 385–391. [Google Scholar] [CrossRef]
- Matarazzo, P.; Tuli, G.; Fiore, L.; Mussa, A.; Feyles, F.; Peiretti, V.; Lala, R. Teriparatide (rhPTH) treatment in children with syndromic hypoparathyroidism. J. Pediatr. Endocrinol. Metab. 2014, 27, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tuli, G.; Buganza, R.; Tessaris, D.; Einaudi, S.; Matarazzo, P.; de Sanctis, L. Teriparatide (rhPTH 1-34) treatment in the pediatric age: Long-term efficacy and safety data in a cohort with genetic hypoparathyroidism. Endocrine 2020, 67, 457–465. [Google Scholar] [CrossRef]
- Winer, K.K.; Kelly, A.; Johns, B.S.A.; Zhang, B.; Dowdy, R.N.K.; Kim, L.; Reynolds, J.C.; Albert, P.S.; Cutler Jr, G.B. Long-Term Parathyroid Hormone 1-34 Replacement Therapy in Children with Hypoparathyroidism. J. Pediatr. 2018, 203, 391–399. [Google Scholar] [CrossRef]
- Winer, K.K.; Sinaii, N.; Peterson, D.; Sainz, B., Jr.; Cutler, G.B., Jr. Effects of once versus twice-daily parathyroid hormone 1–34 therapy in children with hypoparathyroidism. J. Clin. Endocrinol. Metab. 2008, 93, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Vahle, J.L.; Sato, M.; Long, G.G.; Young, J.K.; Francis, P.C.; Engelhardt, J.A.; Westmore, M.S.; Linda, Y.; Nold, J.B. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol. Pathol. 2002, 30, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.B.; Gilsenan, A.W.; Midkiff, K.; Sherrill, B.; Wu, Y.; Mann, B.H.; Masica, D. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: Study design and findings from the first 7 years. J. Bone Miner. Res. 2012, 27, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Gilsenan, A.; Harding, A.; Kellier-Steele, N.; Harris, D.; Midkiff, K.; Andrews, E. The Forteo Patient Registry linkage to multiple state cancer registries: Study design and results from the first 8 years. Osteoporos. Int. 2018, 29, 2335–2343. [Google Scholar] [CrossRef]
- Humbert, L.; Cornu, M.; Proust-Lemoine, E.; Bayry, J.; Wemeau, J.L.; Vantyghem, M.C.; Sendid, B. Chronic Mucocutaneous Candidiasis in Autoimmune Polyendocrine Syndrome Type 1. Front. Immunol. 2018, 9, 2570. [Google Scholar] [CrossRef]
- Grace, M.L. Preventing infections in children and adults with asplenia. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 328–335. [Google Scholar]



| Biological Investigation * | Result | Reference |
|---|---|---|
| TSH | 2.9 μIU/mL | 0.66–4.14 μIU/mL |
| FT4 | 18.91 pmol/L | 11.6–21.5 pmol/L |
| FT3 | 7.06 pmol/L | 4.1–7.9 pmol/L |
| Anti-thyroid peroxidase abs | <10 UI/mL | <35.0 UI/mL |
| Anti-thyroglobulin abs | <1.3 UI/mL | < 4.5 UI/mL |
| ACTH am | 29.50 pg/ml | ≤46.00 pg/ml |
| Cortisol am | 505.1 nmol/L | 171–536 nmol/L |
| Steroid 17-hydroxylase abs | 9.55 | <10, GZ < 15 |
| Steroid 21-hydroxylase abs | 6.43 | <10, GZ 10–15 |
| IgA tTg abs | 2.45 UI/mL | <20 UI/mL |
| IgG tTg abs | 1.64 UI/mL | <20 UI/mL |
| fecal calprotectin | 48.40 µg/g | <50.00 µg/g |
| IgG anti-Sm abs | 1.4 UI/mL | <15.0 UI/mL |
| IgG anti-LKM1 abs | negative | negative |
| ANA | negative | negative |
| pANCA | 3.5 U/mL | <7 U/mL |
| ASCA | 1.3 U/mL | <7 U/mL |
| IgG ab to H(+)/K(+) ATPase | 3.2 U/mL | <10.0 U/mL |
| IgG intrinsic factor abs | 0.7 U/mL | <6.0 U/mL |
| folate | 10.83 ng/mL | >5.38 ng/mL |
| ICA | 0.3 | <0.90 |
| anti-GAD 2 | 2.3 UI/mL | <10.0 UI/mL |
| IA2 | 3.5 UI/mL | <10.0 UI/mL |
| B12 vitamin | 763 pg/mL | 211–911 pg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brad, G.-F.; Nicoară, D.-M.; Scutca, A.-C.; Bugi, M.-A.; Asproniu, R.; Olariu, L.-G.; Jugănaru, I.; Cristun, L.-I.; Mărginean, O. Exploring Chronic Hypocalcemia: Insights into Autoimmune Polyglandular Syndrome Type 1—A Case Study and Literature Review. J. Clin. Med. 2024, 13, 2368. https://doi.org/10.3390/jcm13082368
Brad G-F, Nicoară D-M, Scutca A-C, Bugi M-A, Asproniu R, Olariu L-G, Jugănaru I, Cristun L-I, Mărginean O. Exploring Chronic Hypocalcemia: Insights into Autoimmune Polyglandular Syndrome Type 1—A Case Study and Literature Review. Journal of Clinical Medicine. 2024; 13(8):2368. https://doi.org/10.3390/jcm13082368
Chicago/Turabian StyleBrad, Giorgiana-Flavia, Delia-Maria Nicoară, Alexandra-Cristina Scutca, Meda-Ada Bugi, Raluca Asproniu, Laura-Gratiela Olariu, Iulius Jugănaru, Lucian-Ioan Cristun, and Otilia Mărginean. 2024. "Exploring Chronic Hypocalcemia: Insights into Autoimmune Polyglandular Syndrome Type 1—A Case Study and Literature Review" Journal of Clinical Medicine 13, no. 8: 2368. https://doi.org/10.3390/jcm13082368
APA StyleBrad, G.-F., Nicoară, D.-M., Scutca, A.-C., Bugi, M.-A., Asproniu, R., Olariu, L.-G., Jugănaru, I., Cristun, L.-I., & Mărginean, O. (2024). Exploring Chronic Hypocalcemia: Insights into Autoimmune Polyglandular Syndrome Type 1—A Case Study and Literature Review. Journal of Clinical Medicine, 13(8), 2368. https://doi.org/10.3390/jcm13082368







