Efficacy and Safety of Combination Therapy with Low-Dose Rivaroxaban in Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
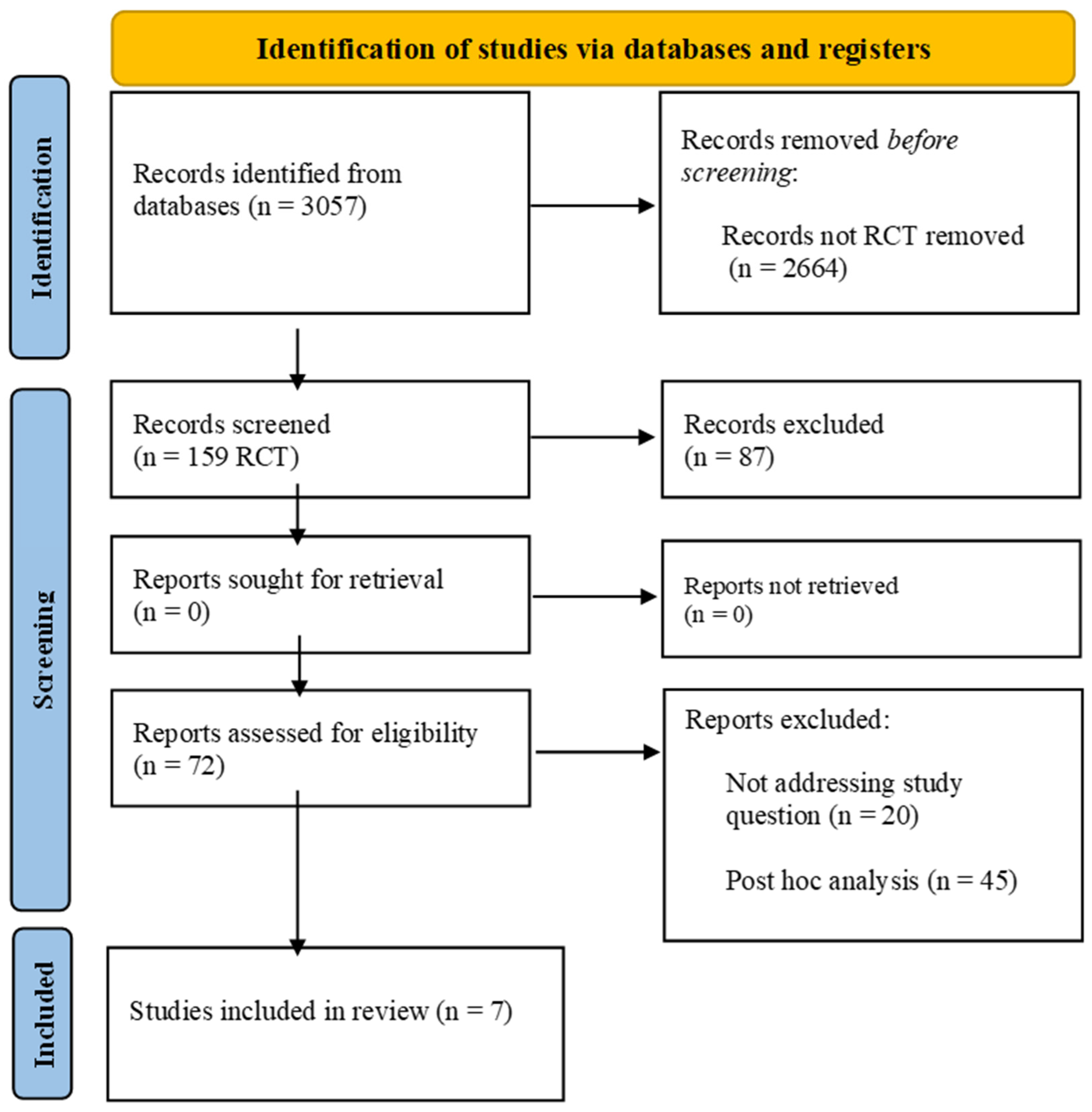
2.1. Searches Strategy and Study Selection
2.2. Data Extraction
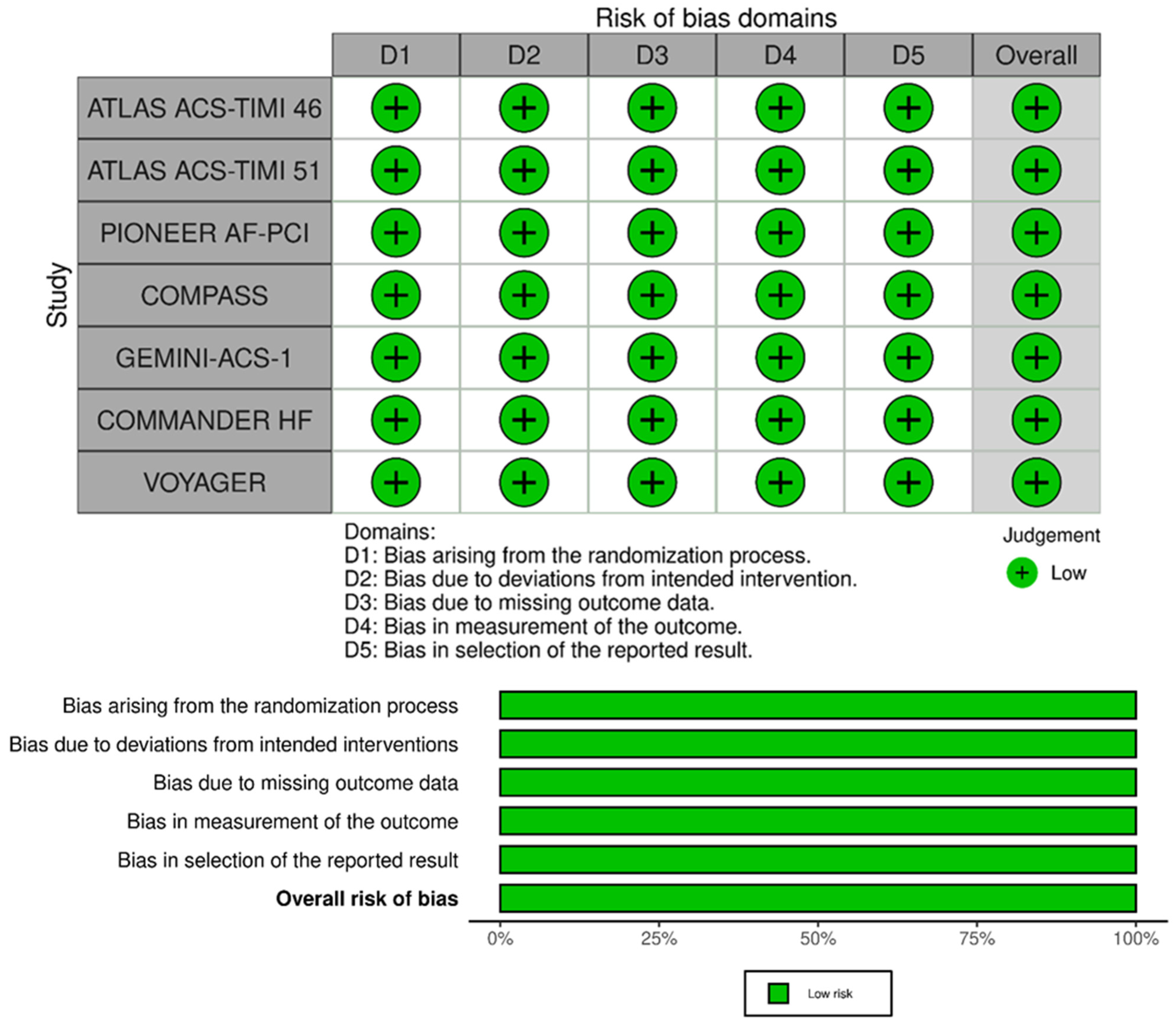
2.3. Risk-of-Bias Assessment
2.4. Treatment Groups
2.5. Study Outcomes
2.6. Statistical Analyses
3. Results
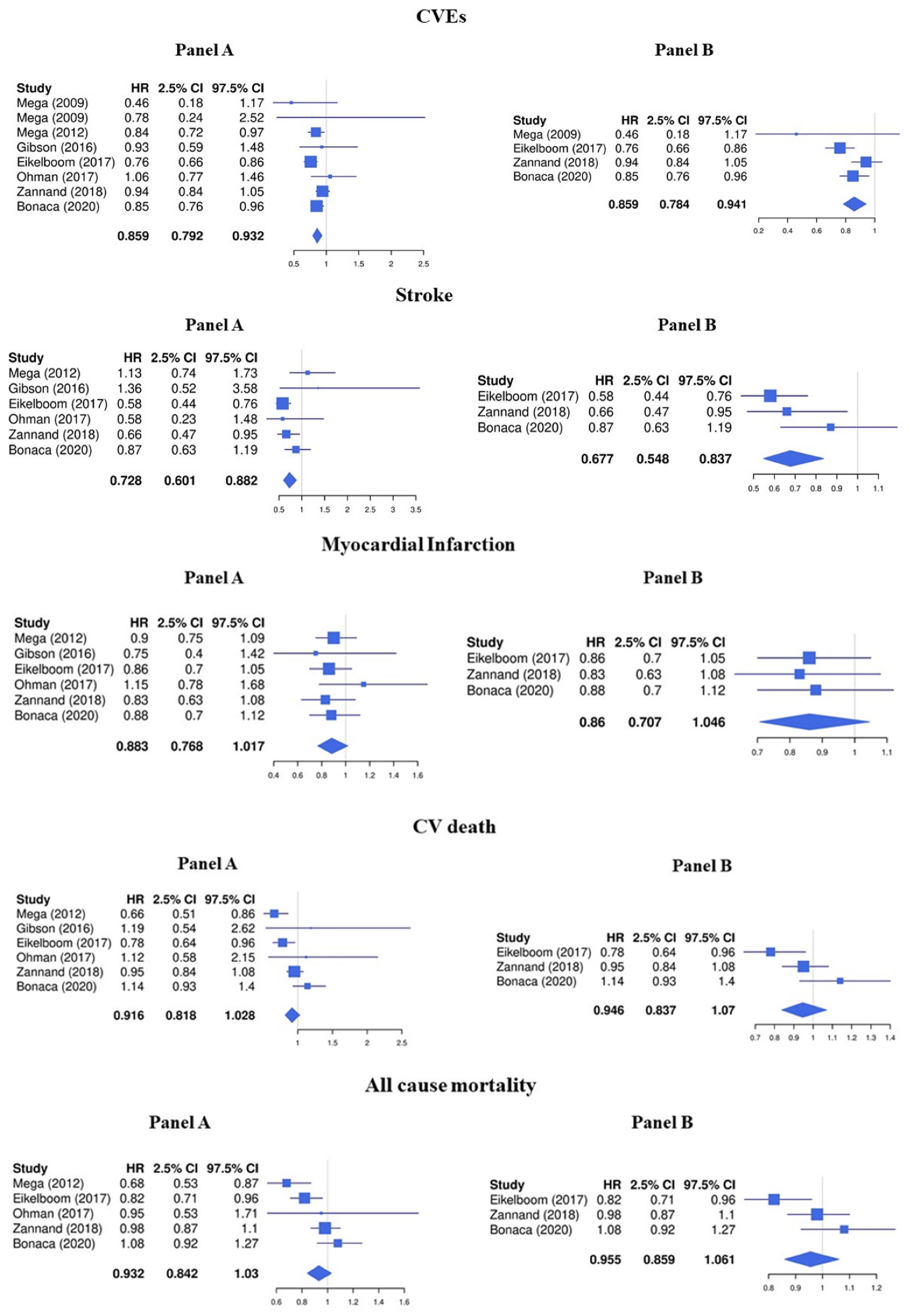
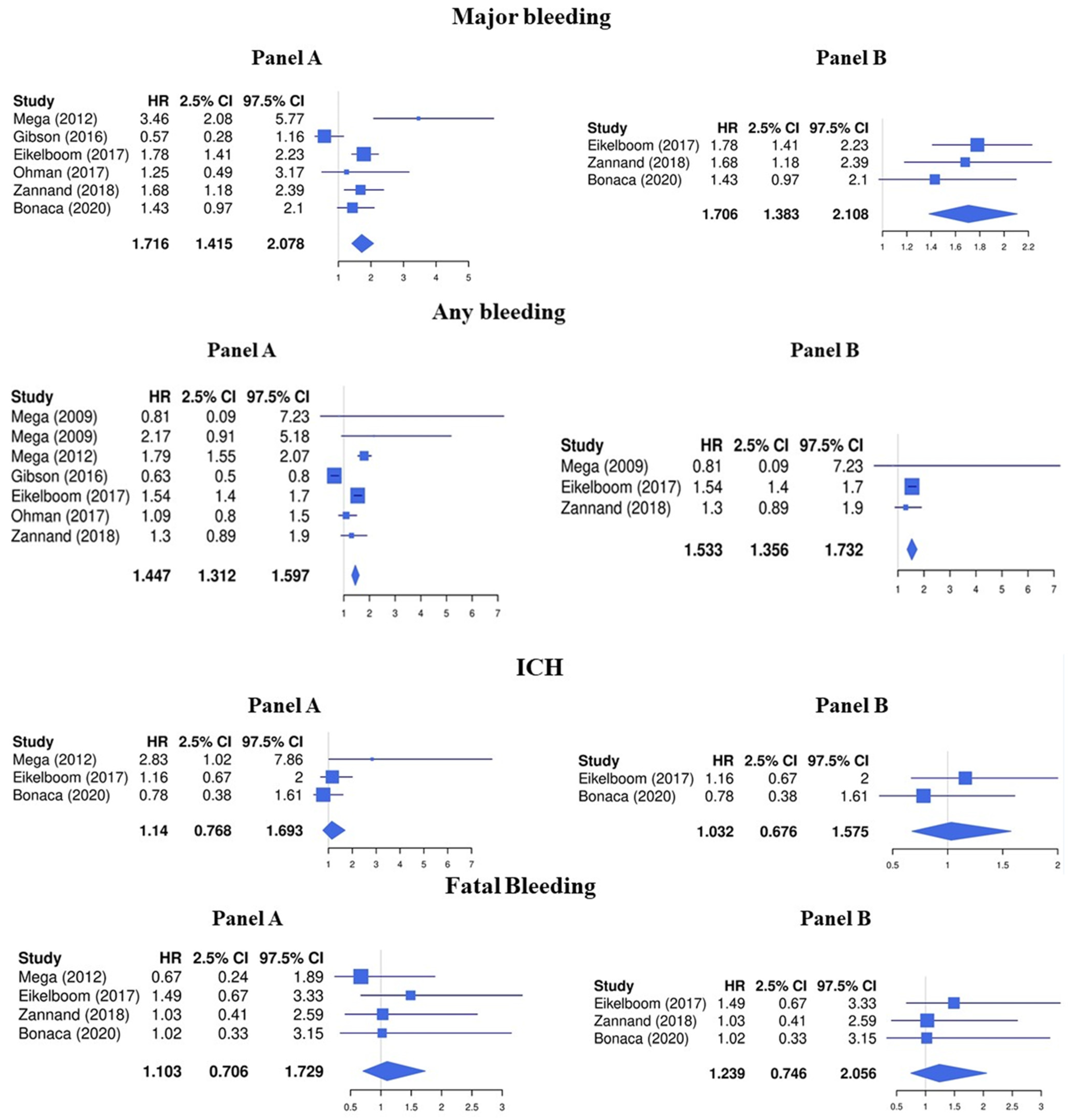
3.1. LDR + Any Antiplatelet vs. Any Antiplatelet
3.2. LDR + ASA vs. ASA Alone
3.3. Sensitivity Analyses
4. Risk of Bias Assessment
5. Discussion
Limitations and Strengths
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poredos, P.; Jug, B. The prevalence of peripheral arterial disease in high risk subjects and coronary or cerebrovascular patients. Angiology 2007, 58, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, S.H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001, 286, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.A.; Mulder, H.; Jones, W.S.; Rockhold, F.W.; Baumgartner, I.; Berger, J.S.; Blomster, J.I.; Fowkes, F.G.R.; Held, P.; Katona, B.G.; et al. Polyvascular Disease and Risk of Major Adverse Cardiovascular Events in Peripheral Artery Disease: A Secondary Analysis of the EUCLID Trial. JAMA Netw. Open 2018, 1, e185239. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Eagle, K.A.; Ohman, E.M.; Hirsch, A.T.; Goto, S.; Mahoney, E.M.; Wilson, P.W.; Alberts, M.J.; D’Agostino, R.; Liau, C.S.; et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010, 304, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Rapsomaniki, E.; Thuresson, M.; Yang, E.; Blin, P.; Hunt, P.; Chung, S.C.; Stogiannis, D.; Pujades-Rodriguez, M.; Timmis, A.; Denaxas, S.C.; et al. Using big data from health records from four countries to evaluate chronic disease outcomes: A study in 114 364 survivors of myocardial infarction. Eur. Heart J. Qual. Care Clin. Outcomes 2016, 2, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Abtan, J.; Bhatt, D.L.; Elbez, Y.; Sorbets, E.; Eagle, K.; Reid, C.M.; Baumgartner, I.; Wu, D.; Hanson, M.E.; Hannachi, H.; et al. Geographic variation and risk factors for systemic and limb ischemic events in patients with symptomatic peripheral artery disease: Insights from the REACH Registry. Clin. Cardiol. 2017, 40, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Farcomeni, A.; Milanese, A.; Del Sole, F.; Menichelli, D.; Hiatt, W.R.; Violi, F. Statins and Major Adverse Limb Events in Patients with Peripheral Artery Disease: A Systematic Review and Meta-Analysis. Thromb. Haemost. 2020, 120, 866–875. [Google Scholar] [CrossRef]
- Mega, J.L.; Braunwald, E.; Mohanavelu, S.; Burton, P.; Poulter, R.; Misselwitz, F.; Hricak, V.; Barnathan, E.S.; Bordes, P.; Witkowski, A.; et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): A randomised, double-blind, phase II trial. Lancet 2009, 374, 29–38. [Google Scholar] [CrossRef]
- Gibson, C.M.; Mehran, R.; Bode, C.; Halperin, J.; Verheugt, F.W.; Wildgoose, P.; Birmingham, M.; Ianus, J.; Burton, P.; van Eickels, M.; et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N. Engl. J. Med. 2016, 375, 2423–2434. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Ohman, E.M.; Roe, M.T.; Steg, P.G.; James, S.K.; Povsic, T.J.; White, J.; Rockhold, F.; Plotnikov, A.; Mundl, H.; Strony, J.; et al. Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEMINI-ACS-1): A double-blind, multicentre, randomised trial. Lancet 2017, 389, 1799–1808. [Google Scholar] [CrossRef]
- Zannad, F.; Anker, S.D.; Byra, W.M.; Cleland, J.G.F.; Fu, M.; Gheorghiade, M.; Lam, C.S.P.; Mehra, M.R.; Neaton, J.D.; Nessel, C.C.; et al. Rivaroxaban in Patients with Heart Failure, Sinus Rhythm, and Coronary Disease. N. Engl. J. Med. 2018, 379, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Fanelli, F.; Capell, W.H.; Diao, L.; Jaeger, N.; et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N. Engl. J. Med. 2020, 382, 1994–2004. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Fox, K.A.A.; Tantry, U.S.; Ten Cate, H.; Weitz, J.I. Combination Antiplatelet and Oral Anticoagulant Therapy in Patients With Coronary and Peripheral Artery Disease. Circulation 2019, 139, 2170–2185. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Pastori, D.; Bartimoccia, S.; Menichelli, D.; Vicario, T.; Nocella, C.; Carnevale, R.; Violi, F. Anti Xa oral anticoagulants inhibit in vivo platelet activation by modulating glycoprotein VI shedding. Pharmacol. Res. 2016, 113, 484–489. [Google Scholar] [CrossRef]
- Cammisotto, V.; Carnevale, R.; Nocella, C.; Stefanini, L.; Bartimoccia, S.; Coluccia, A.; Silvestri, R.; Pignatelli, P.; Pastori, D.; Violi, F. Nox2-mediated platelet activation by glycoprotein (GP) VI: Effect of rivaroxaban alone and in combination with aspirin. Biochem. Pharmacol. 2019, 163, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Petzold, T.; Thienel, M.; Dannenberg, L.; Mourikis, P.; Helten, C.; Ayhan, A.; M’Pembele, R.; Achilles, A.; Trojovky, K.; Konsek, D.; et al. Rivaroxaban Reduces Arterial Thrombosis by Inhibition of FXa-Driven Platelet Activation via Protease Activated Receptor-1. Circ. Res. 2020, 126, 486–500. [Google Scholar] [CrossRef]
- Connolly, S.J.; Eikelboom, J.W.; Bosch, J.; Dagenais, G.; Dyal, L.; Lanas, F.; Metsarinne, K.; O’Donnell, M.; Dans, A.L.; Ha, J.W.; et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 205–218. [Google Scholar] [CrossRef]
- Hess, C.N.; Debus, E.S.; Nehler, M.R.; Anand, S.S.; Patel, M.R.; Szarek, M.; Capell, W.H.; Hsia, J.; Beckman, J.A.; Brodmann, M.; et al. Reduction in Acute Limb Ischemia With Rivaroxaban Versus Placebo in Peripheral Artery Disease After Lower Extremity Revascularization: Insights From VOYAGER PAD. Circulation 2021, 144, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e726–e779. [Google Scholar] [CrossRef] [PubMed]
- Chiarito, M.; Cao, D.; Cannata, F.; Godino, C.; Lodigiani, C.; Ferrante, G.; Lopes, R.D.; Alexander, J.H.; Reimers, B.; Condorelli, G.; et al. Direct Oral Anticoagulants in Addition to Antiplatelet Therapy for Secondary Prevention After Acute Coronary Syndromes: A Systematic Review and Meta-analysis. JAMA Cardiol. 2018, 3, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Khan, M.Z.; Asad, Z.U.A.; Valavoor, S.; Khan, M.U.; Khan, M.S.; Krupica, T.; Alkhouli, M.; Kaluski, E. Efficacy and safety of low dose rivaroxaban in patients with coronary heart disease: A systematic review and meta-analysis. J. Thromb. Thrombolysis 2020, 50, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Capodanno, D.; Benenati, S.; D’Amario, D.; Crea, F.; Andreotti, F.; Angiolillo, D.J. Efficacy and safety of dual pathway inhibition in patients with cardiovascular disease: A systematic review and Meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2021. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; Bassand, J.P.; Bhatt, D.L.; Bode, C.; Burton, P.; Cohen, M.; Cook-Bruns, N.; Fox, K.A.; et al. Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 366, 9–19. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Bhatt, D.L.; Fox, K.A.A.; Bosch, J.; Connolly, S.J.; Anand, S.S.; Avezum, A.; Berkowitz, S.D.; Branch, K.R.H.; Dagenais, G.R.; et al. Mortality Benefit of Rivaroxaban Plus Aspirin in Patients With Chronic Coronary or Peripheral Artery Disease. J. Am. Coll. Cardiol. 2021, 78, 14–23. [Google Scholar] [CrossRef]
- Anand, S.S.; Bosch, J.; Eikelboom, J.W.; Connolly, S.J.; Diaz, R.; Widimsky, P.; Aboyans, V.; Alings, M.; Kakkar, A.K.; Keltai, K.; et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A. Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Biccire, F.G.; Farcomeni, A.; Gaudio, C.; Pignatelli, P.; Tanzilli, G.; Pastori, D. D-dimer for risk stratification and antithrombotic treatment management in acute coronary syndrome patients: Asystematic review and metanalysis. Thromb. J. 2021, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Ardissino, D.; Merlini, P.A.; Bauer, K.A.; Galvani, M.; Ottani, F.; Franchi, F.; Bertocchi, F.; Rosenberg, R.D.; Mannucci, P.M. Coagulation activation and long-term outcome in acute coronary syndromes. Blood 2003, 102, 2731–2735. [Google Scholar] [CrossRef] [PubMed]
- Merlini, P.A.; Bauer, K.A.; Oltrona, L.; Ardissino, D.; Cattaneo, M.; Belli, C.; Mannucci, P.M.; Rosenberg, R.D. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation 1994, 90, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Rho, R.; Tracy, R.P.; Bovill, E.G.; Ball, S.P.; Becker, R.C. Plasma Markers of Procoagulant Activity Among Individuals with Coronary Artery Disease. J. Thromb. Thrombolysis 1995, 2, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Borissoff, J.I.; Joosen, I.A.; Versteylen, M.O.; Spronk, H.M.; ten Cate, H.; Hofstra, L. Accelerated in vivo thrombin formation independently predicts the presence and severity of CT angiographic coronary atherosclerosis. JACC Cardiovasc. Imaging 2012, 5, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke A J. Cereb. Circ. 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.W.; Bassand, J.P.; Agewall, S.; Bax, J.; Boersma, E.; Bueno, H.; Caso, P.; Dudek, D.; Gielen, S.; Huber, K.; et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 2999–3054. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Aday, A.W.; Patel, M.R.; Jones, W.S. Polyvascular Disease: Reappraisal of the Current Clinical Landscape. Circ. Cardiovasc. Interv. 2019, 12, e007385. [Google Scholar] [CrossRef]
| Author (Year) | Study Name | Setting | Age | Men (%) | FU Days | CVEs Definition | Treatment Groups | Total Number of Patients | Total Number of CVEs (%) |
|---|---|---|---|---|---|---|---|---|---|
| Mega (2009) [10] | ATLAS ACS-TIMI 46 | ACS | 57 | 76.5 | 210 | Death, MI, stroke, or severe recurrent ischemia requiring revascularization | LDR + ASA vs. ASA | 329 | 39 (11.9) |
| LDR + DAPT vs. DAPT | 976 | 48 (4.9) | |||||||
| Mega (2012) [29] | ATLAS ACS-TIMI 51 | ACS | 61 | 74.7 | 399 | Death, MI, stroke, or severe recurrent ischemia requiring revascularization | LDR + DAPT vs. DAPT | 10,227 | 689 (6.7) |
| Gibson (2016) [11] | PIONEER AF-PCI | AF with CAD | 70 | 75.5 | 360 | Death from cardiovascular causes, MI, or stroke | LDR + DAPT vs. warfarin + DAPT | 1403 | 72 (5.1) |
| Eikelboom (2017) [30] | COMPASS | PAD with or without CAD | 68 | 74.5 | 690 | Death from cardiovascular causes, MI, or stroke | LDR + ASA vs. ASA | 18,278 | 875 (4.8) |
| Ohman (2017) [13] | GEMINI-ACS-1 | ACS | 62 | 75 | 326 | Cardiovascular death, MI, stroke, or definite stent thrombosis | LDR + P2Y12 vs. DAPT | 3037 | 148 (4.9) |
| Zannand (2018) [14] | COMMANDER HF | HFrEF with CAD | 65 | 77.1 | 633 | Death from any cause, MI, or stroke | LDR + ASA with or without P2Y12 * vs. ASA | 5022 | 1284 (25.6) |
| Bonaca (2020) [15] | VOYAGER | PAD | 67 | 74 | 840 | Acute limb ischemia, major amputation for vascular causes, MI, ischemic stroke, or death from cardiovascular causes | LDR + ASA vs. ASA | 6564 | 1092 (16.3) |
| Study Name + Treatment Arms | Number Patients LDR Group | Number Patients Control Group | Major Bleeding Deficinition | Number of Major Bleedings LDR Group, (%) | Number of Major Bleedings Control Group, (%) | Any Bleeding Definition | Number Any Bleeding LDR Group, (%) | Number Any Bleeding Control Group, (%) | Number ICH LDR Group, (%) | Number ICH Control Group, (%) | Number Fatal Bleedings LDR Group, (%) | Number Fatal Bleeding Control Group, (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATLAS ACS-TIMI 46 LDR + ASA vs. ASA | 77 | 252 | TIMI major bleeding | - | - | Clinically significant bleeding (TIMI major, TIMI minor, or requiring medical attention) | 1 (1.3) | 4 (1.6) | - | - | - | - |
| ATLAS ACS-TIMI 46 LDR + DAPT vs. DAPT | 75 | 901 | 6 (8) | 33 (3.7) | ||||||||
| ATLAS ACS-TIMI 51 LDR + DAPT vs. DAPT | 5114 | 5113 | TIMI major bleeding not associated with CABG | 65 (1.3) | 19 (0.4) | TIMI bleeding requiring medical attention | 492 (9.6) | 282 (5.5) | 14 (0.3) | 5 (0.1) | 6 (0.1) | 6 (0.1) |
| PIONEER AF-PCI LDR + DAPT vs. warfarin + DAPT | 706 | 697 | TIMI major bleeding | 12 (1.7) | 20 (2.9) | TIMI clinically significant bleeding | 117 (16.6) | 167 (24.0) | - | - | - | |
| COMPASS LDR + ASA vs. ASA | 9152 | 9126 | ISTH Major bleeding modified § | 206 (2.3) | 116 (1.3) | Calculated adding minor and major TIMI bleedings | 1044 (11.4) | 673 (7.4) | 28 (0.3) | 24 (0.3) | 15 (0.2) | 10 (0.1) |
| GEMINI-ACS-1 LDR + P2Y12 vs. DAPT | 1519 | 1518 | TIMI major bleeding | 10 (0.7) | 8 (0.5) | TIMI non-CABG clinically significant bleeding | 80 (5.3) | 74 (4.9) | 1 (0) | 0 (0) | 0 (0) | 2 (0) |
| COMMANDER HF LDR + ASA vs. ASA | 2507 | 2515 | ISTH major bleeding | 82 (3.3) | 50 (2.0) | Bleeding requiring hospitalization | 61 (2.4) | 48 (1.9) | - | - | 9 (0.4) | 9 (0.4) |
| VOYAGER LDR + ASA vs. ASA | 3286 | 3278 | TIMI major bleeding | 62 (1.9) | 44 (1.3) | N/A | - | - | 13 (0.4) | 27 (0.8) | 6 (0.2) | 6 (0.2) |
| Study Name + Treatment Arms | Number Patients LDR Group (%) | Number Patients Control Group (%) | Number of CVEs LDR Group (%) | Number of CVEs Control Group (%) | Number MI LDR Group (%) | Number MI Control Group | Number Stroke LDR Group | Number Stroke Control Group | Number All-Cause Mortality LDR Group | Number All-Cause Mortality Control Group | Number CV Death LDR Group | Number CV Death Control Group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATLAS ACS-TIMI 46 LDR + ASA vs. ASA | 77 | 252 | - | - | - | - | - | - | - | - | - | - |
| ATLAS ACS-TIMI 46 LDR + DAPT vs. DAPT | 75 | 901 | - | - | - | - | - | - | - | - | - | - |
| ATLAS ACS-TIMI 51 LDR + DAPT vs. DAPT | 5114 | 5113 | 65 (1.3) | 19 (0.4) | 205 (4.0) | 229 (4.5) | 46 (0.9) | 41 (08) | 103 (2.0) | 153 (3.0) | 94 (1.8) | 143 (2.8) |
| PIONEER AF-PCI LDR+DAPT vs. warfarin + DAPT | 706 | 697 | 12 (1.7) | 20 (0.4) | 17 (2.4) | 21 (3.0) | 10 (1.4) | 7 (1.0) | - | - | 14 (2.0) | 11 (1.6) |
| COMPASS LDR + ASA vs. ASA | 9152 | 9126 | 206 (2.3) | 116 (1.3) | 178 (1.9) | 205 (2.2) | 83 (0.9) | 142 (1.6) | 313 (3.4) | 378 (4.1) | 160 (1.7) | 203 (2.2) |
| GEMINI-ACS-1 LDR + P2Y12 vs. DAPT | 1519 | 1518 | 10 (0.7) | 8 (0.5) | 56 (3.7) | 49 (3.2) | 7 (0.5) | 12 (0.8) | 22 (1.4) | 23 (1.5) | 19 (1.3) | 17 (1.1) |
| COMMANDER HF LDR + ASA vs. ASA | 2507 | 2515 | 82 (3.3) | 50 (2.0) | 98 (3.9) | 118 (4.7) | 51 (2.0) | 76 (3.0) | 546 (21.8) | 556 (22.1) | 453 (18.1) | 476 (18.9) |
| VOYAGER LDR + ASA vs. ASA | 3286 | 3278 | 62 (1.9) | 44 (1.3) | 131 (4.0) | 148 (4.5) | 71 (2.2) | 82 (2.5) | 321 (9.8) | 297 (9.1) | 199 (6.1) | 174 (5.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucci, T.; Del Sole, F.; Menichelli, D.; Galardo, G.; Biccirè, F.G.; Farcomeni, A.; Lip, G.Y.H.; Pignatelli, P.; Pastori, D. Efficacy and Safety of Combination Therapy with Low-Dose Rivaroxaban in Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 2033. https://doi.org/10.3390/jcm13072033
Bucci T, Del Sole F, Menichelli D, Galardo G, Biccirè FG, Farcomeni A, Lip GYH, Pignatelli P, Pastori D. Efficacy and Safety of Combination Therapy with Low-Dose Rivaroxaban in Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2024; 13(7):2033. https://doi.org/10.3390/jcm13072033
Chicago/Turabian StyleBucci, Tommaso, Francesco Del Sole, Danilo Menichelli, Gioacchino Galardo, Flavio Giuseppe Biccirè, Alessio Farcomeni, Gregory Y. H. Lip, Pasquale Pignatelli, and Daniele Pastori. 2024. "Efficacy and Safety of Combination Therapy with Low-Dose Rivaroxaban in Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 13, no. 7: 2033. https://doi.org/10.3390/jcm13072033
APA StyleBucci, T., Del Sole, F., Menichelli, D., Galardo, G., Biccirè, F. G., Farcomeni, A., Lip, G. Y. H., Pignatelli, P., & Pastori, D. (2024). Efficacy and Safety of Combination Therapy with Low-Dose Rivaroxaban in Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 13(7), 2033. https://doi.org/10.3390/jcm13072033







