PRESERFLO™ Microshunt: 1-Year Results of a 25-Gauge vs. 27-Gauge Needle Tract
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Hussein, M. Reduction of intraocular pressure and glaucoma progression. Evid. Based Eye Care 2003, 4, 137–139. [Google Scholar] [CrossRef]
- Schwartz, G.F. Compliance and persistency in glaucoma follow-up treatment. Curr. Opin. Ophthalmol. 2005, 16, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.F.; Lockwood, A.J.; Shah, P.; Macleod, A.; Broadway, D.C.; King, A.J.; McNaught, A.I.; Agrawal, P. Trabeculectomy in the 21st century: A multicenter analysis. Ophthalmology 2013, 120, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Nichani, P.; Popovic, M.M.; Schlenker, M.B.; Park, J.; Ahmed, I.I.K. Microinvasive glaucoma surgery: A review of 3476 eyes. Surv. Ophthalmol. 2021, 66, 714–742. [Google Scholar] [CrossRef] [PubMed]
- Lavia, C.; Dallorto, L.; Maule, M.; Ceccarelli, M.; Fea, A.M. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PLoS ONE, 2017; 12, e0183142. [Google Scholar]
- Pinchuk, L.; Riss, I.; Batlle, J.F.; Kato, Y.P.; Martin, J.B.; Arrieta, E.; Palmberg, P.; Parrish, R.K.; Weber, B.A.; Kwon, Y.; et al. The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2017, 105, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Scheres, L.M.J.; Kujovic-Aleksov, S.; Ramdas, W.D.; de Crom, R.M.; Roelofs, L.C.; Berendschot, T.T.; Webers, C.A. XEN® Gel Stent compared to PRESERFLOTM MicroShunt implantation for primary open-angle glaucoma: Two-year results. Acta Ophthalmol. 2021, 99, e433–e440. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, M.B.; Durr, G.M.; Michaelov, E.; Ahmed, I.I.K. Intermediate Outcomes of a Novel Standalone Ab Externo SIBS Microshunt With Mitomycin C. Am. J. Ophthalmol. 2020, 215, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Batlle, J.F.; Fantes, F.; Riss, I.; Pinchuk, L.; Alburquerque, R.; Kato, Y.P.; Arrieta, E.; Peralta, A.C.; Palmberg, P.; Parrish, R.K.; et al. Three-year follow-up of a novel aqueous humor microshunt. J. Glaucoma 2016, 25, e58–e65. [Google Scholar] [CrossRef] [PubMed]
- Batlle, J.F.; Corona, A.; Albuquerque, R. Long-term Results of the PRESERFLO® MicroShunt in Patients with Primary Open-angle Glaucoma from a Single-center Non-randomized Study. J. Glaucoma 2021, 30, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Pillunat, K.R.; Herber, R.; Haase, M.A.; Jamke, M.; Jasper, C.S.; Pillunat, L.E. PRESERFLOTM MicroShunt versus trabeculectomy: First results on efficacy and safety. Acta Ophthalmol. 2021, 100, E779–E790. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.M.; Schuster, A.K.; Munder, A.; Muehl, M.; Chronopoulos, P.; Pfeiffer, N.; Hoffmann, E.M. Comparison of subconjunctival microinvasive glaucoma surgery and trabeculectomy. Acta Ophthalmol. 2021, 100, E1120–E1126. [Google Scholar] [CrossRef]
- Lewis, R.A. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J. Cataract. Refract. Surg. 2014, 40, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephoerster, R.T.; Parel, J.-M.; Kennedy, J.P. Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials 2008, 29, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Resch, H.; Kiss, B.; Buda, D.; Vass, C. Needling and open filtering bleb revision after XEN-45 implantation—A retrospective outcome comparison. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Beckers, H.J.M.; Aptel, F.; Webers, C.A.B.; Bluwol, E.; Martínez-De-La-Casa, J.M.; García-Feijoó, J.; Lachkar, Y.; Méndez-Hernández, C.D.; Riss, I.; Shao, H.; et al. Safety and Effectiveness of the PRESERFLO® MicroShunt in Primary Open-Angle Glaucoma. Ophthalmol. Glaucoma 2022, 5, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Baker, n.D.; Barnebey, H.S.; Moster, M.R.; Stiles, M.C.; Vold, S.D.; Khatana, A.K.; Flowers, B.E.; Grover, D.S.; Strouthidis, n.G.; Panarelli, J.F.; et al. Ab-Externo MicroShunt versus Trabeculectomy in Primary Open-Angle Glaucoma: One-Year Results from a 2-Year Randomized, Multicenter Study. Ophthalmology 2021, 128, 1710–1721. [Google Scholar] [CrossRef]
- Fea, A.M.; Laffi, G.L.; Martini, E.; Economou, M.A.; Caselgrandi, P.; Sacchi, M.; Au, L. Effectiveness of MicroShunt in Patients with Primary Open-Angle and Pseudoexfoliative Glaucoma. Ophthalmol. Glaucoma 2021, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Durr, G.M.; Schlenker, M.B.; Samet, S.; Ahmed, I.I.K. One-year outcomes of stand-alone ab externo SIBS microshunt implantation in refractory glaucoma. Br. J. Ophthalmol. 2020, 106, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ibarz Barberá, M.; Martínez-Galdón, F.; Caballero-Magro, E.; Rodríguez-Piñero, M.; Tañá-Rivero, P. Efficacy and Safety of the Preserflo Microshunt With Mitomycin C for the Treatment of Open Angle Glaucoma. J. Glaucoma 2022, 31, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.; Haddad, F.; Fajardo-Sanchez, J.; Nguyen, E.; Thong, K.X.; Ah-Moye, S.; Perl, N.; Abu-Bakra, M.; Kulkarni, A.; Trikha, S.; et al. One-year surgical outcomes of the PreserFlo MicroShunt in glaucoma: A multicentre analysis. Br. J. Ophthalmol. 2022, 107, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Vastardis, I.; Fili, S.; Perdikakis, G.; Kontopoulou, K.; Balidis, M.; Gatzioufas, Z.; Kohlhaas, M. Preliminary results of Preserflo Microshunt versus Preserflo Microshunt and Ologen implantation. Eye Vis. 2021, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Nobl, M.; Freissinger, S.; Kassumeh, S.; Priglinger, S.; Mackert, M.J. One-year outcomes of microshunt implantation in pseudoexfoliation glaucoma. PLoS ONE 2021, 16, e0256670. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Gillmann, K.; Rao, H.L.; Guidotti, J.; Mermoud, A. Prospective evaluation of XEN gel implant in eyes with pseudoexfoliative glaucoma. J. Glaucoma 2018, 27, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Gillmann, K.; Bravetti, G.E.; Mermoud, A.; Rao, H.L.; Mansouri, K. XEN gel stent in pseudoexfoliative glaucoma: 2-year results of a prospective evaluation. J. Glaucoma 2019, 28, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Heidinger, A.; Schwab, C.; Lindner, E.; Riedl, R.; Mossböck, G. A Retrospective Study of 199 Xen45 Stent Implantations From 2014 to 2016. J. Glaucoma. 2019, 28, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, I.E.; Allen, F.; Morley, C.; Pearsall, T.; Bowes, O.M.; Ruben, S. Efficacy and safety data for the XEN45 implant at 2 years: A retrospective analysis. Br. J. Ophthalmol. 2019, 104, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Widder, R.A.; Dietlein, T.S.; Dinslage, S.; Kühnrich, P.; Rennings, C.; Rössler, G. The XEN45 Gel Stent as a minimally invasive procedure in glaucoma surgery: Success rates, risk profile, and rates of re-surgery after 261 surgeries. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Charles, R.; Abdel-Hay, A.; Shah, B.; Byles, D.; Lim, L.-A.; Rossiter, J.; Kuo, C.-H.; Chapman, P.; Robertson, S. 1-year outcomes of the Xen45 glaucoma implant. Eye 2019, 33, 761–766. [Google Scholar] [CrossRef]
- Reitsamer, H.; Sng, C.; Vera, V.; Lenzhofer, M.; Barton, K.; Stalmans, I. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 983–996. [Google Scholar] [CrossRef] [PubMed]

| All | 25-Gauge | 27-Gauge | p-Value | |
|---|---|---|---|---|
| n = 60 | n = 19 | n = 41 | ||
| Age (years). median (IQR) | 72 (58–79) | 74 (57–81) | 71 (58–79) | 0.251 |
| Sex. n (%) | 0.511 | |||
| Female | 29 (48) | 8 (42) | 21 (51) | |
| Eye. n (%) | 0.896 | |||
| Left | 34 (57) | 11 (58) | 23 (56) | |
| Glaucoma type. n (%) | 0.635 | |||
| Primary open-angle glaucoma | 29 (48) | 10 (53) | 19 (46) | |
| Pseudoexfoliative glaucoma | 15 (25) | 5 (26) | 10 (24) | |
| Pigment dispersion glaucoma | 4 (7) | 0 (0) | 4 (10) | |
| Primary angle-closure glaucoma | 1 (2) | 0 (0) | 1 (2) | |
| Other * | 11 (18) | 4 (21) | 7 (17) | |
| History of glaucoma surgery. n (%) | 13 (22) | 2 (11) | 11 (27) | 0.154 |
| Trabeculectomy | 8 (13) | 1 (5) | 7 (17) | |
| Xen | 5 (8) | 1 (5) | 4 (10) | |
| Lens status. n (%) | 0.967 | |||
| Pseudophakic | 16 (27) | 5 (26) | 11 (27) |
| All n = 60 | 25-Gauge n = 19 | 27-Gauge n = 41 | p-Value | ||
|---|---|---|---|---|---|
| Pre OP | IOP (mmHg) | 23.4 ± 8.6 | 24.2 ± 7.3 | 23.1 ± 9.2 | 0.639 |
| GDS | 3.1 ± 0.9 | 3.0 ± 0.8 | 3.1 ± 1.0 | 0.701 | |
| n | 60 | 19 | 41 | ||
| Day 1–2 | IOP (mmHg) | 10.6 ± 7.0 | 10.1 ± 4.3 | 10.9 ± 8.2 | 0.717 |
| GDS | 0.1 ± 0.5 | 0.0 ± 0.0 | 0.2 ± 0.6 | 0.247 | |
| n | 49 | 18 | 31 | ||
| Week 1 | IOP (mmHg) | 11.6 ± 5.7 | 10.1 ± 3.5 | 12.2 ± 6.3 | 0.238 |
| GDS | 0.1 ± 0.6 | 0.0 ± 0.0 | 0.2 ± 0.7 | 0.283 | |
| n | 49 | 15 | 34 | ||
| Week 2 | IOP (mmHg) | 11.3 ± 6.7 | 10.2 ± 4.8 | 11.7 ± 7.4 | 0.499 |
| GDS | 0.2 ± 0.7 | 0.0 ± 0.0 | 0.3 ± 0.8 | 0.264 | |
| n | 42 | 13 | 29 | ||
| Month 1 | IOP (mmHg) | 12.1 ± 4.4 | 10.6 ± 3.3 | 12.8 ± 4.7 | 0.072 |
| GDS | 0.1 ± 0.4 | 0.1 ± 0.2 | 0.2 ± 0.5 | 0.315 | |
| n | 58 | 18 | 30 | ||
| Month 3 | IOP (mmHg) | 12.4 ± 3.2 | 11.2 ± 3.0 | 13.0 ± 3.2 | 0.061 |
| GDS | 0.2 ± 0.6 | 0.0 ± 0.1 | 0.3 ± 0.7 | 0.192 | |
| n | 52 | 16 | 36 | ||
| Month 6 | IOP (mmHg) | 14.3 ± 4.0 | 13.5 ± 4.2 | 14.8 ± 3.8 | 0.267 |
| GDS | 0.6 ± 1.0 | 0.3 ± 0.7 | 0.8 ± 1.1 * | 0.046 | |
| n | 48 | 17 | 31 | ||
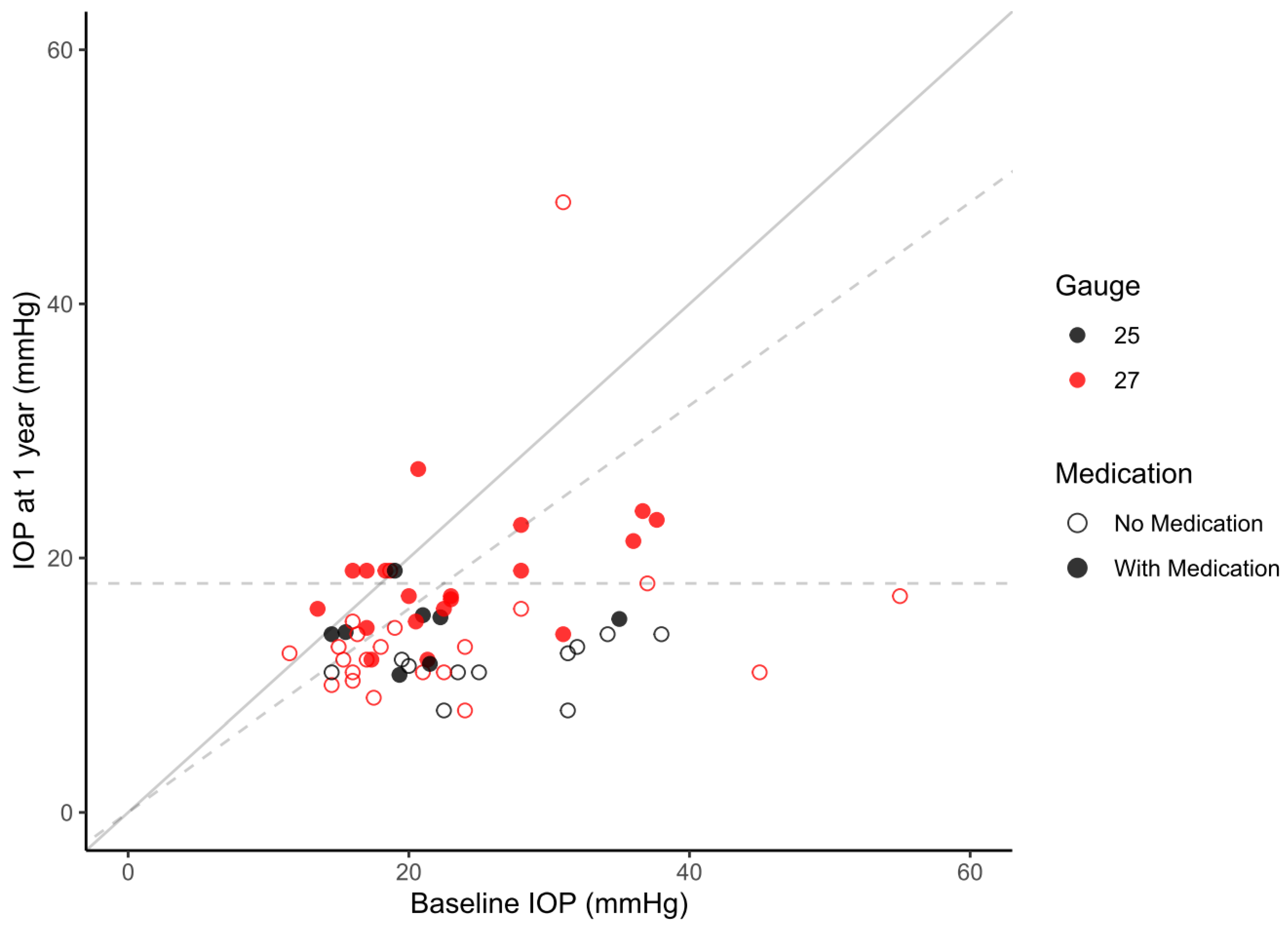
| Year 1 | IOP (mmHg) | 15.1 ± 5.9 | 12.7 ± 2.7 | 16.2 ± 6.7 * | 0.035 |
| GDS | 0.8 ± 1.1 | 0.5 ± 0.8 | 0.9 ± 1.2 | 0.137 | |
| n | 60 | 19 | 41 |
| Overall | 25-Gauge | 27-Gauge | p-Value | Stand-Alone Surgery | Combined Surgery | p-Value | |
|---|---|---|---|---|---|---|---|
| Mean Survival | Mean Survival | Mean Survival | Mean Survival | Mean Survival | |||
| n = 60 | n = 19 | n = 41 | n = 51 | n = 9 | |||
| QS21 | 359 (313–404) | 448 (385–511) | 308 (254–361) * | 0.005 | 344 (295–393) | 435 (339–532) | 0.192 |
| QS18 | 352 (308–397) | 448 (385–511) | 300 (250–352) * | 0.002 | 340 (292–389) | 421 (327–515) | 0.296 |
| FS21 | 315 (268–361) | 398 (330–467) | 270 (215–325) * | 0.010 | 299 (251–347) | 377 (259–496) | 0.274 |
| FS18 | 311 (265–357) | 396 (327–466) | 266 (212–319) * | 0.007 | 298 (249–346) | 361 (248–475) | 0.455 |
| Covariates | QS21 | FS18 | |||
|---|---|---|---|---|---|
| n | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age | 60 | 1.02 (0.98–1.07) | 0.308 | 1.02 (0.98–1.06) | 0.274 |
| Sex: Male | 31 | 1.00 (0.43–2.30) | 0.994 | 0.82 (0.39–1.73) | 0.596 |
| Baseline IOP | 60 | 1.03 (0.96–1.11) | 0.452 | 1.04 (0.97–1.12) | 0.244 |
| Baseline number of glaucoma medication | 60 | 0.85 (0.50–1.45) | 0.560 | 0.95 (0.58–1.55) | 0.828 |
| Diagnosis: Primary open angle | 28 | Reference | Reference | ||
| Diagnosis: PEX | 15 | 0.72 (0.23–2.28) | 0.579 | 0.60 (0.20–1.79) | 0.356 |
| Diagnosis: Pigmentary glaucoma | 4 | 3.48 (0.63–19.17) | 0.152 | 2.92 (0.60–14.12) | 0.183 |
| Diagnosis: Other | 12 | 0.37 (0.13–1.03) | 0.058 | 0.34 (0.13–0.92) | 0.033 |
| Glaucoma surgery prior to PMS | 13 | 2.42 (0.97–6.08) | 0.059 | 2.65 (1.12–6.23) | 0.026 |
| Combined PMS with cataract extraction | 9 | 0.47 (0.13–1.70) | 0.248 | 0.81 (0.28–2.32) | 0.692 |
| Conjunctival opening: Limbus based | 23 | 1.23 (0.46–3.33) | 0.678 | 1.11 (0.45–2.76) | 0.815 |
| Needle diameter: 25G | 19 | 0.47 (0.24–0.90) | 0.022 | 0.48 (0.27–0.86) | 0.013 |
| Number of created tunnels | 60 | 1.28 (0.76–2.14) | 0.360 | 1.12 (0.67–1.87) | 0.658 |
| All | 25-Gauge | 27-Gauge | p-Value | |
|---|---|---|---|---|
| n = 60 | n = 19 | n = 41 | ||
| Transient ocular hypotension | 13 (21) | 7 (37) | 6 (15) | 0.089 |
| Clinically relevant ocular hypotension | 4 (7) | 4 (21) | 0 (0) * | 0.008 |
| Shallowing of the anterior chamber | 1 (2) | 1 (5) | 0 (0) | 0.317 |
| Choroidal effusion | 5 (8) | 4 (21) | 1 (2) * | 0.031 |
| Wound dehiscence | 1 (2) | 0 (0) | 1 (2) | 0.683 |
| Hyphema | 1 (2) | 0 (0) | 1 (2) | 0.683 |
| Device touch to the iris | 1 (2) | 1 (5) | 0 (0) | 0.317 |
| Device dislocation | 1 (2) | 1 (5) | 0 (0) | 0.317 |
| One of the recorded complications | 18 (30) | 10 (53) | 8 (20) * | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steiner, S.; Resch, H.; Kiss, B.; Vass, C. PRESERFLO™ Microshunt: 1-Year Results of a 25-Gauge vs. 27-Gauge Needle Tract. J. Clin. Med. 2024, 13, 1979. https://doi.org/10.3390/jcm13071979
Steiner S, Resch H, Kiss B, Vass C. PRESERFLO™ Microshunt: 1-Year Results of a 25-Gauge vs. 27-Gauge Needle Tract. Journal of Clinical Medicine. 2024; 13(7):1979. https://doi.org/10.3390/jcm13071979
Chicago/Turabian StyleSteiner, Stefan, Hemma Resch, Barbara Kiss, and Clemens Vass. 2024. "PRESERFLO™ Microshunt: 1-Year Results of a 25-Gauge vs. 27-Gauge Needle Tract" Journal of Clinical Medicine 13, no. 7: 1979. https://doi.org/10.3390/jcm13071979
APA StyleSteiner, S., Resch, H., Kiss, B., & Vass, C. (2024). PRESERFLO™ Microshunt: 1-Year Results of a 25-Gauge vs. 27-Gauge Needle Tract. Journal of Clinical Medicine, 13(7), 1979. https://doi.org/10.3390/jcm13071979






