Coronary Chronic Total Occlusion Revascularization: When, Who and How?
Abstract
1. Introduction
2. Methodology
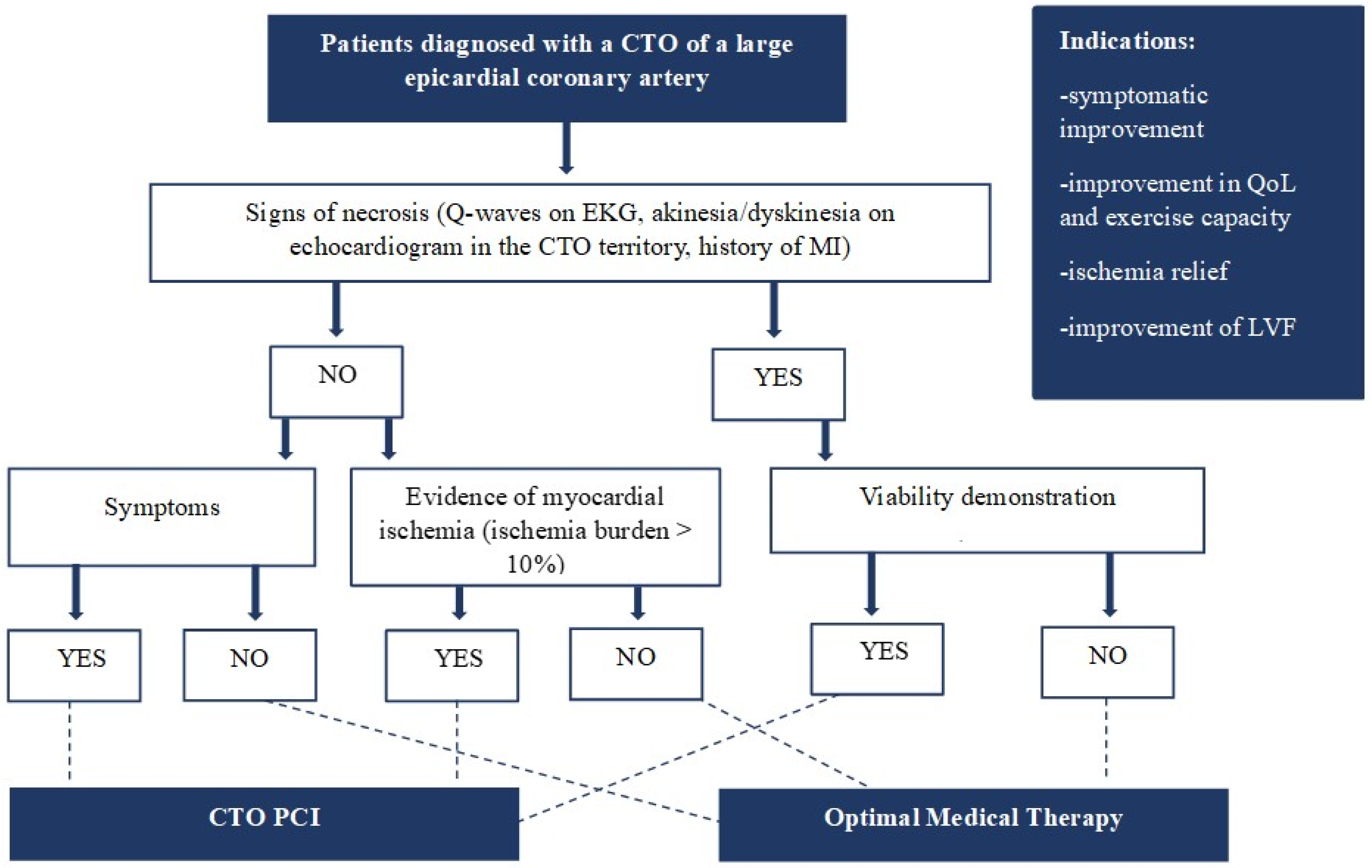
3. CTO Revascularization: When? Who?
3.1. Current Evidence and Future Directions
3.2. Patients with Symptoms and Myocardial Ischemia
3.3. Patients with Reduced Left Ventricular Ejection Fraction (LVEF)
3.4. Patients with Acute Coronary Event
4. CTO Revascularization: How?
4.1. Procedural Planning
4.2. Revascularization Strategies
4.3. Anterograde Recanalization
4.3.1. Antegrade Wire Escalation/De-Escalation
4.3.2. Parallel Wiring
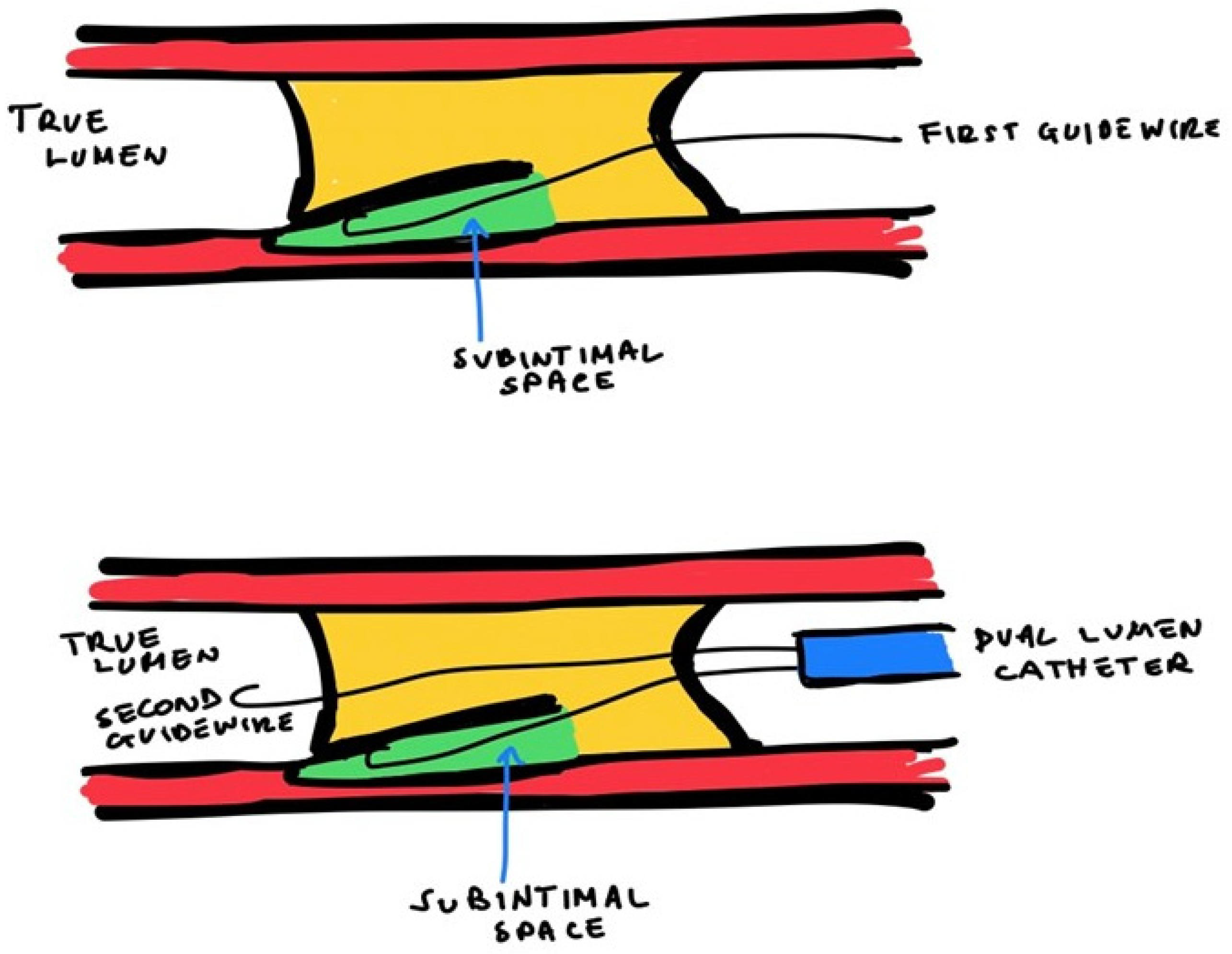
4.3.3. Antegrade Dissection and Re-Entry (ADR)
- -
- If there is a SB at the occlusion site, antegrade IVUS-guided wiring can be helpful. The ultrasound probe is conducted into the SB to visualize the ostium of the CTO, and then the operator can perform the cap puncture under IVUS visualization.
- -
- In case of the absence of a SB, the operator can advance the IVUS in a dissection plan near the cap of the CTO. To make the dissection, the operator safely starts the advancing of the probe into the subintimal space within the unknown arterial course (“move-the-cap” techniques) [48]:
- (1)
- Balloon-assisted subintimal entry (BASE): a balloon is inflated proximally to the CTO cap to have a small disruption of the intimal layer, allowing wire passage in this space [49];
- (2)
- Carlino technique: the tip of a microcatheter proximally to the CTO cap, the operator can perform a microinjection of contrast determining a focal hydraulic dissection [50];
- (3)
- “Scratch-and-go” technique: a puncture the extra plaque space proximally to the cap of the CTO with a high tip-load penetrative wire. Therefore, the high tip-lead guidewire is exchanged via a microcatheter for a polymeric guidewire for subintimal advancement [48].
4.4. Retrograde Recanalization
4.4.1. Retrograde and Anterograde Lumen Connection
4.4.2. Retrograde Dissection Re-Entry (RDR)
4.4.3. Externalization of the Guidewire
5. CTO Complications
5.1. Acute Myocardial Infarction and Periprocedural Myocardial Injury (PMI)
5.2. Coronary Perforation
5.3. Donor Vessel Injury
5.4. Side Branch Occlusion
5.5. Device Loss
5.6. Iatrogenic Aortic Dissection
5.7. Vascular Access Complication
5.8. Contrast-Induced Nephropathy
5.9. Radiation Skin Injury
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fefer, P.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Osherov, A.B.; Yalonetsky, S.; Gannot, S.; Samuel, M.; Weisbrod, M.; Bierstone, D.; et al. Current perspectives on coronary chronic total occlusions: The Canadian Multicenter Chronic Total Occlusions Registry. J. Am. Coll. Cardiol. 2012, 59, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Azzalini, L.; Jolicoeur, E.M.; Pighi, M.; Millán, X.; Picard, F.; Tadros, V.X.; Fortier, A.; L’Allier, P.L.; Ly, H.Q. Epidemiology, Management Strategies, and Outcomes of Patients With Chronic Total Coronary Occlusion. Am. J. Cardiol. 2016, 118, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Råmunddal, T.; Hoebers, L.P.; Henriques, J.P.; Dworeck, C.; Angerås, O.; Odenstedt, J.; Ioanes, D.; Olivecrona, G.; Harnek, J.; Jensen, U.; et al. Chronic total occlusions in Sweden—A report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). PLoS ONE 2014, 9, e103850, Erratum in PLoS ONE 2014, 9, e112370. [Google Scholar] [CrossRef] [PubMed]
- Brilakis, E.S.; Banerjee, S.; Karmpaliotis, D.; Lombardi, W.L.; Tsai, T.T.; Shunk, K.A.; Kennedy, K.F.; Spertus, J.A.; Holmes, D.R., Jr.; Grantham, J.A. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: A report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc. Interv. 2015, 8, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Strauss, B.H.; Shuvy, M.; Wijeysundera, H.C. Revascularization of chronic total occlusions: Time to reconsider? J. Am. Coll. Cardiol. 2014, 64, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, K. A Randomized Trial to Assess Regional Left Ventricular Function after Stent Implantation in Chronic Total Occlusion: The REVASC Trial. JACC Cardiovasc. Interv. 2018, 11, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Weerackody, R.; Rathod, K.; Behar, J.; Gallagher, S.; Knight, C.J.; Kapur, A.; Jain, A.K.; Rothman, M.T.; Thompson, C.A.; et al. Successful recanalization of chronic total occlusions is associated with improved long-term survival. JACC Cardiovasc. Interv. 2012, 5, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Viceconte, N.; Ali, O.; Stuart-Buttle, C.; Saraswathyamma, A.; Parisi, R.; Mirabella, F.; Dimopoulos, K.; Di Mario, C. Improved cardiac survival, freedom from MACE and angina-related quality of life after successful percutaneous recanalization of coronary artery chronic total occlusions. Int. J. Cardiol. 2012, 161, 31–38. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Cockburn, J.; Clayton, T.C.; Ludman, P.; Cotton, J.; Spratt, J.; Redwood, S.; de Belder, M.; de Belder, A.; Hill, J.; et al. Long-term follow-up of elective chronic total coronary occlusion angioplasty: Analysis from the U.K. Central Cardiac Audit Database. J. Am. Coll. Cardiol. 2014, 64, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Claessen, B.E.; Godino, C.; Dangas, G.D.; Obunai, K.; Kanwal, S.; Carlino, M.; Henriques, J.P.; Di Mario, C.; Kim, Y.-H.; et al. Long-term outcome of percutaneous coronary intervention for chronic total occlusions. JACC Cardiovasc. Interv. 2011, 4, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.K.; Khanna, R.; Pandey, C.; Ashfaq, F. Long-term outcomes post chronic total occlusion intervention-implications of completeness of revascularization. J. Interv. Cardiol. 2018, 31, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, S.D.; Boukhris, M.; Giubilato, S.; Marzà, F.; Garbo, R.; Contegiacomo, G.; Marzocchi, A.; Niccoli, G.; Gagnor, A.; Varbella, F.; et al. Management strategies in patients affected by chronic total occlusions: Results from the Italian Registry of Chronic Total Occlusions. Eur. Heart J. 2015, 36, 3189–3198. [Google Scholar] [CrossRef] [PubMed]
- Park, T.K.; Lee, S.H.; Choi, K.H.; Lee, J.M.; Yang, J.H.; Song, Y.B.; Hahn, J.Y.; Choi, J.H.; Gwon, H.C.; Lee, S.H.; et al. Late Survival Benefit of Percutaneous Coronary Intervention Compared With Medical Therapy in Patients With Coronary Chronic Total Occlusion: A 10-Year Follow-Up Study. J. Am. Heart Assoc. 2021, 10, e019022. [Google Scholar] [CrossRef] [PubMed]
- Joyal, D.; Afilalo, J.; Rinfret, S. Effectiveness of recanalization of chronic total occlusions: A systematic review and meta-analysis. Am. Heart J. 2010, 160, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Qintar, M.; Grantham, J.A.; Sapontis, J.; Gosch, K.L.; Lombardi, W.; Karmpaliotis, D.; Moses, J.; Salisbury, A.C.; Cohen, D.J.; Spertus, J.A.; et al. Dyspnea Among Patients With Chronic Total Occlusions Undergoing Percutaneous Coronary Intervention: Prevalence and Predictors of Improvement. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003665. [Google Scholar] [CrossRef] [PubMed]
- Tajti, P.; Burke, M.N.; Karmpaliotis, D.; Alaswad, K.; Werner, G.S.; Azzalini, L.; Carlino, M.; Patel, M.; Mashayekhi, K.; Egred, M.; et al. Update in the Percutaneous Management of Coronary Chronic Total Occlusions. JACC Cardiovasc. Interv. 2018, 11, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Azzalini, L.; Vo, M.; Dens, J.; Agostoni, P. Myths to Debunk to Improve Management, Referral, and Outcomes in Patients With Chronic Total Occlusion of an Epicardial Coronary Artery. Am. J. Cardiol. 2015, 116, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.M.; Hastings, J.L.; Amsavelu, S.; Garcia-Morales, F.; Hendrix, F.; Karatasakis, A.; Danek, B.A.; Karacsonyi, J.; Rangan, B.V.; Roesle, M.; et al. Percutaneous Coronary Intervention of Coronary Chronic Total Occlusions Improves Peak Oxygen Uptake during Cardiopulmonary Exercise Testing. J. Invasive Cardiol. 2017, 29, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, K.; Neuser, H.; Kraus, A.; Zimmer, M.; Dalibor, J.; Akin, I.; Werner, G.; Aurel, T.; Neumann, F.J.; Behnes, M. Successful Percutaneous Coronary Intervention Improves Cardiopulmonary Exercise Capacity in Patients With Chronic Total Occlusions. J. Am. Coll. Cardiol. 2017, 69, 1095–1096. [Google Scholar] [CrossRef] [PubMed]
- Stuijfzand, W.J.; Driessen, R.S.; Raijmakers, P.G.; Rijnierse, M.T.; Maeremans, J.; Hollander, M.R.; Lammertsma, A.A.; van Rossum, A.C.; Dens, J.; Nap, A.; et al. Prevalence of ischaemia in patients with a chronic total occlusion and preserved left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Agrawal, M.; Flynn, S.E.; Werner, G.S.; Uretsky, B.F. The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter. Cardiovasc. Interv. 2014, 83, 9–16. [Google Scholar] [CrossRef]
- Hachamovitch, R.; Hayes, S.W.; Friedman, J.D.; Cohen, I.; Berman, D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003, 107, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Stuijfzand, W.J.; Biesbroek, P.S.; Raijmakers, P.G.; Driessen, R.S.; Schumacher, S.P.; van Diemen, P.; van den Berg, J.; Nijveldt, R.; Lammertsma, A.A.; Walsh, S.J.; et al. Effects of successful percutaneous coronary intervention of chronic total occlusions on myocardial perfusion and left ventricular function. EuroIntervention 2017, 13, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Baks, T.; van Geuns, R.J.; Duncker, D.J.; Cademartiri, F.; Mollet, N.R.; Krestin, G.P.; Serruys, P.W.; de Feyter, P.J. Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusions. J. Am. Coll. Cardiol. 2006, 47, 721–725. [Google Scholar] [CrossRef]
- Camici, P.G.; Wijns, W.; Borgers, M.; De Silva, R.; Ferrari, R.; Knuuti, J.; Lammertsma, A.A.; Liedtke, A.J.; Paternostro, G.; Vatner, S.F. Pathophysiological mechanisms of chronic reversible left ventricular dysfunction due to coronary artery disease (hibernating myocardium). Circulation 1997, 96, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Morimoto, T.; Shiomi, H.; Furukawa, Y.; Nakagawa, Y.; Ando, K.; Kadota, K.; Kimura, T. Chronic total occlusion in a non-infarct-related artery is closely associated with increased five-year mortality in patients with ST-segment elevation acute myocardial infarction undergoing primary percutaneous coronary intervention (from the CREDO-Kyoto AMI registry). EuroIntervention 2017, 12, e1874–e1882. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.P.; Hoebers, L.P.; Råmunddal, T.; Laanmets, P.; Eriksen, E.; Bax, M.; Ioanes, D.; Suttorp, M.J.; Strauss, B.H.; Barbato, E.; et al. Percutaneous Intervention for Concurrent Chronic Total Occlusions in Patients With STEMI: The EXPLORE Trial. J. Am. Coll. Cardiol. 2016, 68, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.; van Dongen, I.M.; Hoebers, L.P.; Ouweneel, D.M.; Claessen BE, P.M.; Råmunddal, T.; Laanmets, P.; Eriksen, E.; van der Schaaf, R.J.; Ioanes, D.; et al. Improved recovery of regional left ventricular function after PCI of chronic total occlusion in STEMI patients: A cardiovascular magnetic resonance study of the randomized controlled EXPLORE trial. J. Cardiovasc. Magn. Reson. 2017, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.B.; Tsuchikane, E.; Ge, L.; Harding, S.A.; Lo, S.; Lim, S.T.; Chen, J.Y.; Lee, S.W.; Qian, J.; Kao, H.L.; et al. Retrograde Versus Antegrade Approach for Coronary Chronic Total Occlusion in an Algorithm-Driven Contemporary Asia-Pacific Multicentre Registry: Comparison of Outcomes. Heart Lung Circ. 2020, 29, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, N.V.; Werner, G.S.; Deftereos, S.; Di Mario, C.; Galassi, A.R.; Buettner, J.H.; Avran, A.; Reifart, N.; Goktekin, O.; Garbo, R.; et al. Temporal Trends in Chronic Total Occlusion Interventions in Europe. Circ. Cardiovasc. Interv. 2018, 11, e006229. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S. Use of coronary computed tomographic angiography to facilitate percutaneous coronary intervention of chronic total occlusions. Circ. Cardiovasc. Interv. 2019, 12, e007387. [Google Scholar] [CrossRef] [PubMed]
- Gurm, H.S.; Dixon, S.R.; Smith, D.E.; Share, D.; Lalonde, T.; Greenbaum, A.; Moscucci, M.; BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium) Registry. Renal function-based contrast dosing to define safe limits of radiographic contrast media in patients undergoing percutaneous coronary interventions. J. Am. Coll. Cardiol. 2011, 58, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Morino, Y.; Abe, M.; Morimoto, T.; Kimura, T.; Hayashi, Y.; Muramatsu, T.; Ochiai, M.; Noguchi, Y.; Kato, K.; Shibata, Y.; et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: The J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc. Interv. 2011, 4, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, G.; Kandzari, D.E.; Yeh, R.W.; Jaffer, F.A.; Karmpaliotis, D.; Wyman, M.R.; Alaswad, K.; Lombardi, W.; Grantham, J.A.; Moses, J.; et al. Development and Validation of a Novel Scoring System for Predicting Technical Success of Chronic Total Occlusion Percutaneous Coronary Interventions: The PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) Score. JACC Cardiovasc. Interv. 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alessandrino, G.; Chevalier, B.; Lefèvre, T.; Sanguineti, F.; Garot, P.; Unterseeh, T.; Hovasse, T.; Morice, M.C.; Louvard, Y. A clinical and angiographic scoring system to predict the probability of successful first-attempt percutaneous coronary intervention in patients with total chronic coronary occlusion. J. Am. Coll. Cardiol. Interv. 2015, 8, 1540–1548. [Google Scholar] [CrossRef]
- Galassi, A.R.; Boukhris, M.; Azzarelli, S.; Castaing, M.; Marzà, F.; Tomasello, S.D. Percutaneous Coronary Revascularization for Chronic Total Occlusions: A Novel Predictive Score of Technical Failure Using Advanced Technologies. JACC Cardiovasc. Interv. 2016, 9, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Maeremans, J.; Spratt, J.C.; Knaapen, P.; Walsh, S.; Agostoni, P.; Wilson, W.; Avran, A.; Faurie, B.; Bressollette, E.; Kayaert, P.; et al. Towards a contemporary, comprehensive scoring system for determining technical outcomes of hybrid percutaneous chronic total occlusion treatment: The RECHARGE score. Catheter. Cardiovasc. Interv. 2018, 91, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.G.; Burke, M.N.; Murad, M.B.; Graham, J.J.; Badawi, R.; Toma, C.; Meltser, H.; Nair, R.; Buller, C.; Whitlow, P.L.; et al. Predictors of Successful Hybrid-Approach Chronic Total Coronary Artery Occlusion Stenting: An Improved Model With Novel Correlates. JACC Cardiovasc. Interv. 2017, 10, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Szijgyarto, Z.; Rampat, R.; Werner, G.S.; Ho, C.; Reifart, N.; Lefevre, T.; Louvard, Y.; Avran, A.; Kambis, M.; Buettner, H.J.; et al. Derivation and Validation of a Chronic Total Coronary Occlusion Intervention Procedural Success Score From the 20,000-Patient EuroCTO Registry: The EuroCTO (CASTLE) Score. JACC Cardiovasc. Interv. 2019, 12, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.B.; Brilakis, E.S.; Mashayekhi, K.; Tsuchikane, E.; Alaswad, K.; Araya, M.; Avran, A.; Azzalini, L.; Babunashvili, A.M.; Bayani, B.; et al. Global Chronic Total Occlusion Crossing Algorithm: JACC State-of-the-Art Review. Am. Coll. Cardiol. 2021, 78, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.R.; Werner, G.S.; Boukhris, M.; Azzalini, L.; Mashayekhi, K.; Carlino, M.; Avran, A.; Konstantinidis, N.V.; Grancini, L.; Bryniarski, L.; et al. Percutaneous recanalisation of chronic total occlusions: 2019 consensus document from the EuroCTO Club. EuroIntervention 2019, 15, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Brilakis, E.S.; Grantham, J.A.; Rinfret, S.; Wyman, R.M.; Burke, M.N.; Karmpaliotis, D.; Lembo, N.; Pershad, A.; Kandzari, D.E.; Buller, C.E.; et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc. Interv. 2012, 5, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Tsuchikane, E.; Muramatsu, T.; Kishi, K.; Muto, M.; Oikawa, Y.; Kawasaki, T.; Hamazaki, Y.; Fujita, T.; Katoh, O. A Novel Algorithm for Treating Chronic Total Coronary Artery Occlusion. J. Am. Coll. Cardiol. 2019, 74, 2392–2404. [Google Scholar] [CrossRef]
- Reddy, S.A.; Pillai, A.A.; Reddy, B.; Rao, V.; Deshpande, A. Knuckle wire technique in percutaneous coronary intervention of chronic total occlusion: Knuckle wire technique. Asia Interv. 2020, 6, 91–101. [Google Scholar] [CrossRef]
- Karacsonyi, J.; Tajti, P.; Rangan, B.V.; Halligan, S.C.; Allen, R.H.; Nicholson, W.J.; Harvey, J.E.; Spaedy, A.J.; Jaffer, F.A.; Grantham, J.A.; et al. Randomized Comparison of a CrossBoss First Versus Standard Wire Escalation Strategy for Crossing Coronary Chronic Total Occlusions: The CrossBoss First Trial. JACC Cardiovasc. Interv. 2018, 11, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Garbo, R.; Iannaccone, M.; Sanchez, J.S.; Oreglia, J.A.; Gagnor, A.; Gasparini, G.L. The ReCross dual-lumen microcatheter versatility during percutaneous coronary interven- tion of chronic total coronary occlusions. REC Interv. Cardiol. 2022, 4, 67–69. (In Spanish) [Google Scholar]
- Vo, M.N.; Karmpaliotis, D.; Brilakis, E.S. “Move the cap” technique for ambiguous or impenetrable proximal cap of coronary total occlusion. Catheter. Cardiovasc. Interv. 2016, 87, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Hill, J.; Spratt, J.C. The “side-BASE technique”: Combined side branch anchor balloon and balloon assisted sub-intimal entry to resolve ambiguous proximal cap chronic total occlusions. Catheter. Cardiovasc. Interv. 2018, 92, E15–E19. [Google Scholar] [CrossRef] [PubMed]
- Carlino, M.; Ruparelia, N.; Thomas, G.; Brooks, M.; Uretsky, B.F.; Brilakis, E.S.; Karmpaliotis, D.; Hanratty, C.; Walsh, S.; Spratt, J.; et al. Modified contrast microinjection technique to facilitate chronic total occlusion recanalization. Catheter. Cardiovasc. Interv. 2016, 87, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, C.; Werner, G.S.; Sianos, G.; Galassi, A.R.; Büttner, J.; Dudek, D.; Chevalier, B.; Lefevre, T.; Schofer, J.; Koolen, J.; et al. European perspective in the recanalisation of Chronic Total Occlusions (CTO): Consensus document from the EuroCTO Club. EuroIntervention 2007, 3, 30–43. [Google Scholar] [PubMed]
- Surmely, J.F.; Tsuchikane, E.; Katoh, O.; Nishida, Y.; Nakayama, M.; Nakamura, S.; Oida, A.; Hattori, E.; Suzuki, T. New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: The CART technique. J. Invasive Cardiol. 2006, 18, 334–338. [Google Scholar] [PubMed]
- Sianos, G.; Barlis, P.; Di Mario, C.; Papafaklis, M.I.; Büttner, J.; Galassi, A.R.; Schofer, J.; Werner, G.; Lefevre, T.; Louvard, Y.; et al. European experience with the retrograde approach for the recanalisation of coronary artery chronic total occlusions. A report on behalf of the EuroCTO club. EuroIntervention 2008, 4, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sumitsuji, S.; Inoue, K.; Ochiai, M.; Tsuchikane, E.; Ikeno, F. Fundamental wire technique and current standard strategy of percutaneous intervention for chronic total occlusion with histopathological insights. JACC Cardiovasc. Interv. 2011, 4, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Allana, S.S.; Rempakos, A.; Kostantinis, S.; Alexandrou, M.; Mutlu, D.; Alaswad, K.; Azzalini, L.; Kearney, K.; Krestyaninov, O.; Khelimskii, D.; et al. The tip-in and rendezvous techniques in retrograde chronic total occlusion percutaneous coronary interventions. EuroIntervention 2023, 19, e856–e859. [Google Scholar] [CrossRef] [PubMed]
- Karacsonyi, J.; Vemmou, E.; Nikolakopoulos, I.D.; Ungi, I.; Rangan, B.V.; Brilakis, E.S. Complications of chronic total occlusion percutaneous coronary intervention. Neth. Heart J. 2021, 29, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Rigger, J.; Hanratty, C.G.; Walsh, S.J. Erratum to: Common and Uncommon CTO complications. Interv. Cardiol. 2019, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Karacsonyi, J.; Vemmou, E.; Nikolakopoulos, I.; Ungi, I.; Abi Rafeh, N.; ElGuindy, A.; Azzalini, L.; Burke, M.N.; Brilakis, E.S. Current challenges and prevention strategies for chronic total occlusion (CTO) complications. Expert Rev. Cardiovasc. Ther. 2021, 19, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Danek, B.A.; Karatasakis, A.; Karmpaliotis, D.; Alaswad, K.; Yeh, R.W.; Jaffer, F.A.; Patel, M.P.; Mahmud, E.; Lombardi, W.L.; Wyman, M.R.; et al. Development and Validation of a Scoring System for Predicting Periprocedural Complications During Percutaneous Coronary Interventions of Chronic Total Occlusions: The Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS CTO) Complications Score. J. Am. Heart Assoc. 2016, 5, e004272. [Google Scholar] [CrossRef] [PubMed]
- Favero, L.; Penzo, C.; Nikas, D.; Pacchioni, A.; Pasquetto, G.; Saccà, S. Cardiac and extracardiac complications during CTO interventions: Prevention and management. Interv. Cardiol. 2010, 2, 355–367. [Google Scholar] [CrossRef]
- Song, L.; Wang, Y.; Guan, C.; Zou, T.; Sun, Z.; Xie, L.; Zhang, R.; Dou, K.; Yang, W.; Wu, Y.; et al. Impact of Periprocedural Myocardial Injury and Infarction Definitions on Long-Term Mortality After Chronic Total Occlusion Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2021, 14, e010923. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Bernardi, A.; Garbo, R. Chronic total occlusion percutaneous coronary intervention complications: Prevention and management. Vessel. Plus 2019, 3, 29. [Google Scholar] [CrossRef]
- Asada, K.; Hosaka, F. CTO PCI Complications: Prevention and Management. In Current Trend and Techniques of Percutaneous Coronary Intervention for Chronic Total Occlusion; Springer: Berlin/Heidelberg, Germany, 2020; pp. 107–115. [Google Scholar]
- Rosseel, L.; Scott, B.; Prihadi, E.; Azzano, A.; Degrauwe, S.; Verheye, S.; Convens, C.; Vermeersch, P. Is a covered stent justifiable in the treatment of coronary artery perforation? An observational analysis of long-term results of two different covered stent types. Catheter. Cardiovasc. Interv. 2019, 93, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Wu, D.K.; Su, H.M.; Chu, C.S.; Voon, W.C.; Lai, W.T.; Sheu, S.H. Septum hematoma: A complication of retrograde wiring in chronic total occlusion. Int. J. Cardiol. 2006, 113, e64–e66. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, M.S.A.; Egred, M. Right Ventricular Wall Hematoma Following Angioplasty to Right Coronary Artery Occlusion. J. Invasive Cardiol. 2019, 31, E66. [Google Scholar] [PubMed]
- Nguyen-Trong, P.K.; Rangan, B.V.; Karatasakis, A.; Danek, B.A.; Christakopoulos, G.E.; Martinez-Parachini, J.R.; Resendes, E.; Ayers, C.R.; Luna, M.; Abdullah, S.; et al. Predictors and Outcomes of Side-Branch Occlusion in Coronary Chronic Total Occlusion Interventions. J. Invasive Cardiol. 2016, 28, 168–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brilakis, E.S. Manual of Chronic Total Occlusion Interventions, a Step-by-Step Approach, 2nd ed.; Elsevier: London, UK, 2018. [Google Scholar]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Aguiar-Souto, P.; Ferrante, G.; Del Furia, F.; Barlis, P.; Khurana, R.; Di Mario, C. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int. J. Cardiol. 2010, 139, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Almendarez, M.; Gurm, H.S.; Mariani, J.; Jr Montorfano, M.; Brilakis, E.S.; Mehran, R.; Azzalini, L. Procedural Strategies to Reduce the Incidence of Contrast-Induced Acute Kidney Injury During Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S. Reducing radiation exposure during PCI of chronic total occlusions—Better is not good enough. EuroIntervention 2018, 14, e496–e498. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J. Avoidance of radiation injuries from medical interventional procedures. Ann. ICRP 2000, 30, 7–67. [Google Scholar] [CrossRef]


| Trial | Intervention | Population and Principal Baseline Characteristics | Endpoints | Results |
|---|---|---|---|---|
| REVASC | CTO PCI vs. OMT | 205 stable patients OMT group (N 104): median age 68 years, N 90 males, N 31 diabetes, N 93 hypertension, N 21 current smokers, N 38 previous MI, N 61 3-vessel disease, median SYNTAX score 16 CTO PCI group (N 101): median age 65 years, N 91 males, N 32 diabetes, N 81 hypertension, N 23 current smokers, N 39 previous MI, N 53 3-vessel disease, median SYNTAX score 14 | Primary: change in SWT in the CTO territory assessed by CMR Secondary: improvement of regional wall motion and changes in left ventricular volumes and ejection fraction; MACE at 12 months | No benefit of CTO PCI in terms of the primary endpoint, SWT, or other indexes of LVF. Significant reduction of MACE at 12 months in CTO PCI group (driven by repeat revascularization) |
| EXPLORE | CTO PCI vs. OMT | 304 STEMI patients with CTO in non IRA artery OMT group (N 154): median age 60 years, N 126 males, N 25 diabetes, N 69 hypertension, N 76 current smokers, N 24 previous MI, N 67 3-vessel disease, MI median SYNTAX score 29 CTO PCI group (N 148): median age 60 years, N 131 males, N 22 diabetes, N 59 hypertension, N 77 current smokers, N 19 previous MI, N 62 3-vessel disease, median SYNTAX score 29 | Primary: LVEF and LVEDV on CMR after 4 months | No differences in terms of LVEF, LVEDV, and 4-month MACE. LVEF significantly higher in LAD CTO subgroup treated with PCI |
| EURO-CTO | CTO PCI vs. OMT | 396 symptomatic patients OMT group (N 137): median age 64.7 years, N 118 males, N 139 diabetes, N 98 hypertension, N 92 smokers, N 25 previous MI, N 24 3-vessel disease CTO PCI group (N 259): median age 65.2 years, N 215 males, N 85 diabetes, N 189 hypertension, N 190 current, N 59 previous MI, N 66 3-vessel disease | Primary: change in QoL at 12 months assessed by SAQ Secondary: MACE, stent thrombosis, cerebrovascular events, hospitalisation for cardiac reasons | Significant improvement of symptoms in CTO PCI group compared to OMT alone. No difference in MACE |
| IMPACTOR-CTO | CTO PCI vs. OMT | 72 patients with isolated RCA CTO and stable angina | Primary: evaluation of ΔMIB (decrease in MIB from baseline to 12 month control) Secondary: changes in 6 min walking test, QoL, and MACE at 12 months. | ΔMIB was significantly higher in the PCI group compared to the OMT group. 6-min walk distance and QoL improved in PCI group. No significant difference in MACE between the two groups |
| DECISION-CTO | CTO PCI vs. OMT | 815 patients with stable angina or ACS (not STEMI) OMT group (N 398): median age 62.9 years, N 319 males, N 134 diabetes, N 238 hypertension, N 102 current smokers, N 34 previous MI, N 128 3-vessel disease, median SYNTAX score 20.8 CTO PCI group (N 417): median age 62.2 years, N 344 males, N 132 diabetes, N 262 hypertension, N 125 Current smokers, N 45 previous MI, N 127 3-vessel disease, median SYNTAX score 20.8 | Primary: composite of death from any cause, MI, stroke or any revascularisation) Secondary: individual components of the primary endpoint, bleeding, stent thrombosis and QoL (EQ-5D and SAQ) | No differences in terms of primary and secondary outcomes in the two groups |
| COMET-CTO | CTO PCI vs. OMT | 100 patients with angina and/or evidence of myocardial ischaemia OMT group (N 50): median age 63 years, N 44 males, N 18 diabetes, N 43 hypertension, N 14 current smokers, N 35 previous MI, median SYNTAX score 9.87 CTO PCI group (N 50): median age 61 years, N 38 males, N 14 diabetes, N 43 hypertension, N 16 current smokers, N 29 previous MI, median SYNTAX score 10.79 | Primary: evaluation of QoL by SAQ Secondary: all-cause mortality and MACE (non-fatal MI, recurrent revascularization with PCI or CABG). | Significant improvement in SAQ in CTO PCI group, while no significant differences in SAQ scores in the OMT group |
| Score | Parameters |
|---|---|
| J-CTO | Blunt stump, calcification, 1 bend >45° within the occlusion, length >20 mm, prior failed CTO PCI attempt |
| PROGRESS-CTO | Proximal cup ambiguity, moderate or severe proximal tortuosity (2 bends >70° or 1 bend >90° proximal to the occlusion), circumflex CTO, absence of interventional collaterals |
| CASTLE | Previous CABG, age ≥70 years, blunt stump, severe tortuosity (≥2 pre-occlusive bends >90° or ≥1 bend >120°), length of the occlusion ≥20 mm, severe calcifications |
| RECHARGE | Previous CABG, blunt stump, calcifications, tortuosity (1 bend ≥45° within the occlusion), length of the occlusion ≥20 mm, diseased distal landing zone |
| CL | Previous CABG, previous MI, severe calcifications, length of the occlusion ≥20 mm, non-LAD location, blunt stump |
| ORA | Age ≥75 years, ostial location, collaterals Rentrop <2 |
| Ellis score | Proximal or retrograde >90° bend, proximal cup ambiguity, moderate-severe calcifications, length of occlusion > 10 mm, poor target vessel, ostial CTO location |
| Wire Categories | Plaque Type | Wire Features |
|---|---|---|
| Tapered polymer-jacketed wires | Soft tissue plaque | Extremely low coefficient of friction |
| Intermediate tip-load | Fibrous and fibrous-calcific plaques | Enhanced torqueability |
| High tip-load guidewires | Severe calcific lesions | Increased penetration force |
| Type | Features |
|---|---|
| 1 | Extraluminal crater without extravasation, or evidence of dissection |
| 2 | Pericardial or myocardial brush without contrast extravasation |
| 3 | Extravasation through a ≥1 mm perforation |
| 3 cavity spilling | Extravasation through a ≥1 mm perforation into a circulatory chamber |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricottini, E.; Coletti, F.; Nusca, A.; Cocco, N.; Corlianò, A.; Appetecchia, A.; Melfi, R.; Mangiacapra, F.; Gallo, P.; Rinaldi, R.; et al. Coronary Chronic Total Occlusion Revascularization: When, Who and How? J. Clin. Med. 2024, 13, 1943. https://doi.org/10.3390/jcm13071943
Ricottini E, Coletti F, Nusca A, Cocco N, Corlianò A, Appetecchia A, Melfi R, Mangiacapra F, Gallo P, Rinaldi R, et al. Coronary Chronic Total Occlusion Revascularization: When, Who and How? Journal of Clinical Medicine. 2024; 13(7):1943. https://doi.org/10.3390/jcm13071943
Chicago/Turabian StyleRicottini, Elisabetta, Federica Coletti, Annunziata Nusca, Nino Cocco, Andrea Corlianò, Alessandro Appetecchia, Rosetta Melfi, Fabio Mangiacapra, Paolo Gallo, Raffaele Rinaldi, and et al. 2024. "Coronary Chronic Total Occlusion Revascularization: When, Who and How?" Journal of Clinical Medicine 13, no. 7: 1943. https://doi.org/10.3390/jcm13071943
APA StyleRicottini, E., Coletti, F., Nusca, A., Cocco, N., Corlianò, A., Appetecchia, A., Melfi, R., Mangiacapra, F., Gallo, P., Rinaldi, R., Grigioni, F., & Ussia, G. P. (2024). Coronary Chronic Total Occlusion Revascularization: When, Who and How? Journal of Clinical Medicine, 13(7), 1943. https://doi.org/10.3390/jcm13071943







