1. Introduction
Endometriosis is a chronic benign estrogen-dependent disease. It is characterized by the presence of endometrial glands and stroma outside of the uterus. It is a common public health problem [
1], affecting up to 10% of reproductive-aged women and 30% of infertile patients [
2,
3,
4,
5]. Pelvic endometriotic implants tends to spread towards surrounding tissues, leading to fibrosis and tissue adherence. In some cases, endometriosis may metastasize to lymph nodes or beyond the abdominal cavity. The commonest site of disease is the ovary, often affected by a distinct ovarian cyst known as “endometrioma”.
Although endometriosis is widely deemed as a benign disease, affected patients inherently have an increased risk of developing malignancy, especially these presenting with endometriomas [
6]. Back in 1925, Sampson proposed an association between endometriosis and ovarian cancer, describing the development of an ovarian endometrioid carcinoma (EnOC) from ectopically implanted endometrial tissue [
7]. This finding was later confirmed by Scott, who focused on the malignant changes in endometriotic tissues, pointing out that benign endometriosis (BE) might be contiguous with endometriosis-associated ovarian cancer (EAOC) [
8,
9].
The available evidence points to a direct relationship between specific subtypes of epithelial ovarian cancer and endometriosis. EAOC refers to a type of ovarian cancer that is believed to arise from or be influenced by endometriosis [
9]. The term highlights the observed association between endometriosis and certain ovarian cancer subtypes, suggesting that endometriosis may predispose individuals to the development of these cancers or share common underlying factors.
This association has been validated through molecular pathology, demonstrating common mutations in cancer-associated genes. Although atypical endometriosis may precede these cancers, it is not consistently present in all cases of endometriosis-related ovarian cancer.
Approximately 0.5–1% of cases of endometriosis are affected by different types of ovarian neoplasia, and EAOC occurs in 0.14–2.9% of individuals with endometriomas [
10,
11,
12,
13,
14]. Among EAOCs, the most common histotypes are clear cell ovarian carcinoma (CCC), EnOC, and low-grade serous ovarian carcinoma [
15]. Several studies have indicated atypical endometriosis (AE)—i.e., the histological finding of cytologic atypia and architectural atypia or hyperplasia—as the direct precursor to these specific tumor histotypes: AE is present in 12–35% of ovarian endometriosis cases, and approximately 60–80% of EAOC occurs with AE [
9,
16]. The mechanisms underlying the malignant transformation from BE to cancer are currently not well established, although various alternatives have been suggested, including excessive oxidative stress, altered cytokine production, genetic mutation occurrence, and the presence of a hyperestrogenic environment. These molecular mechanisms might become useful diagnostic targets for the early detection of endometriosis-related cancers. However, the clinical application of these novel biomarkers may pose challenges as they all require molecular analysis [
9].
Therefore, considering the limited but existing risk of association and/or neoplastic degeneration of endometriomas, it is of fundamental importance to establish a diagnostic-therapeutic pathway aimed at investigating and identifying the presence of potentially malignant endometriotic ovarian lesions. Ultrasound imaging may be considered the first diagnostic tool that is useful in differentiating typical endometriosis from AE and EAOC, even though the only definitive diagnosis is the histological one. A correct diagnostic classification to identify “high-risk” disease would allow for the most appropriate treatment. A conservative pharmacological approach can usually be adopted to treat endometriotic lesions; however, surgery may be the first-line treatment option in cases where the risk of neoplastic degeneration is deemed concrete. Preoperative suspicion of a malignant transformation may lead to increased intraoperative care and efforts toward the prevention of intrabdominal cyst cell dissemination.
The aim of this narrative review is to present the current state of the art of EAOC, offering a general view of the available data. The pathogenetic mechanisms of EAOC are reported, including the supposed precursory role of AE, as well as its challenging diagnostic and therapeutic pathways. We also propose a pragmatical clinical flowchart to optimize the available therapeutic options, favoring patient quality of life.
2. Materials and Methods
An electronic literature search was conducted to evaluate the existing literature on EAOC, encompassing the hypotheses for pathogenesis, the diagnostic assessment, and the potential therapeutic approaches. The search was performed using the online medical MEDLINE database (accessed via PubMed). A set of predefined search terms was employed, including the following: “Adenocarcinoma, Clear Cell” (MeSH Unique ID: D018262); “Atypia”; “Atypical”; “Atypical endometriosis”, “Biomarkers, Tumor” (MeSH Unique ID: D014408); “Carcinoma, Endometrioid” (MeSH Unique ID: D018269); “Diagnosis”; “Diagnostic Imaging” (MeSH Unique ID: D003952); “Endometrioma”; “Endometriosis” (MeSH Unique ID: D004715); “Genital Neoplasms, Female” (MeSH Unique ID: D005833); “Gynecologic Surgical Procedures” (MeSH Unique ID: D013509); “Pathogenesis”; “Therapy” (MESH unique ID: D013812); “Treatment”; “Ultrasonography” (MeSH Unique ID: D014463).
The investigation included articles from inception to January 2023. Original articles, including randomized and non-randomized clinical trials, prospective observational studies, retrospective cohort studies, and case–control studies, review articles, and case reports, were considered eligible for the purpose of this review. The research selection process began with a careful examination of articles’ titles and abstracts, ensuring that their content included elements relevant to our research question. Furthermore, we conducted a thorough examination of the bibliography of the selected articles, identifying additional papers for further scrutiny. The identified articles underwent a rigorous screening process conducted by three independent reviewers (G.C., L.L., and E.P.), who meticulously evaluated the content for relevance and scientific merit. Articles that deviated from the predetermined theme or lacked substantial scientific contributions were excluded. To ensure a more contemporary perspective on the subject matter, more dated articles were included only if historically relevant.
This methodological approach was used to compile a cohesive and high-quality collection of studies, providing a nuanced understanding of the specified topics within the context of EAOC research.
Eighty-nine articles were included for the purpose of our narrative review, i.e., to present the current state of the art regarding EAOC.
3. Epidemiology and Pathogenesis of Endometriosis and Endometriosis-Associated Ovarian Cancers: How Can Endometriosis Progress into Cancer?
Different theories have been proposed to explain the pathogenesis of endometriosis, including retrograde menstruation, coelomic metaplasia, lymphatic or vascular dissemination, immune system dysfunction, genetic predisposition, and environmental impacts. These theories are not mutually exclusive, and it is likely that a combination of factors contributes to the development of endometriosis. However, a sequence of bleeding, inflammation, fibrin deposition, adhesion formation, and scarring and distortion of the peritoneal surfaces of organs and pelvic anatomy constitute the natural history of this disease [
17].
Endometriosis is deemed a benign disease but has some features in common with the malignant ones. Indeed, it may have a metastatic behavior with attachment to the surrounding tissues, and in some cases, it metastasizes to distant organs. However, endometrioma represents the most common presentation of the disease and is considered a benign ovarian cyst [
5,
6]. Nevertheless, the presence of endometrioma determines an increased risk, albeit an overall limited risk, of concomitant ovarian malignancy in the affected patient [
9], with these malignancies often arising from the endometriotic tissue itself. Indeed, two different scenarios have been proposed to explain the malignant progression of endometrioma into EAOC.
The first one is a cyclic hemorrhage occurring inside the endometriotic cyst that leads to the accumulation of blood components (i.e., extracellular hemoglobin, iron, and heme), inducing cellular oxidative damage by elevating reactive oxygen species. This oxidative stress induces DNA damage and subsequent oncological mutations.
The second scenario revolves around the continuous production of antioxidants: endometriotic cells adapt to oxidative stress with the aid of macrophages, enhancing antioxidative defenses and influencing redox signaling, energy metabolism, and the tumor immune microenvironment, potentially leading to malignant transformation [
18]. Additionally, some specific molecular alterations have also been noted, such as
ARIDA1/BAF250a,
PIK3CA,
CTNNB1, and
PTEN mutation, as well as microsatellite instability and the loss of heterozygosity [
19,
20,
21,
22,
23].
Given that endometriosis is typically not associated with cancer, we can hypothesize that the mechanisms of cellular oxidative damage are self-restricting in the majority of patients. This phenomenon maintains a pro-inflammatory environment characterized by a delicate equilibrium between oxidative stressors and antioxidant mediators [
18,
19,
20,
21,
22,
23,
24]. Nevertheless, there is no established molecular mechanism that can be used to predict, with certainty, the oncological progression of the disease in these patients, making it challenging to identify at-risk patients early on. The role of cancer-driving mutations and the correlation between genotypes and clinical outcomes are still to be elucidated [
25].
However, some clinical risk factors for the development of EAOC among patients with endometriosis have been identified: older age at the time of diagnosis, presence of a solid component inside the cyst, postmenopausal status, large-sized (≥9 cm) endometrioma, nulliparity, and hyperestrogenism [
26].
Epidemiological and Prognostic Characteristics of the Main Endometriosis-Associated Ovarian Cancers Histotypes
EAOC typically impacts women in the age range of 35–55 years. Approximately 0.5–1% of cases of endometriosis are complicated by neoplasia, and EAOC is observed in 0.14–2.9% of individuals with endometriomas [
8,
9,
10,
11,
12]. Among EAOCs, the most prevalent histotype is CCC, which accounts for 5–12% of cases; it exhibits geographical variability, being more common in certain Asian countries. It is characterized as a high-grade ovarian carcinoma and is associated with a poor prognosis in advanced stages due to its early resistance to platinum-based treatments. The second most common histotype is represented by EnOC, which constitutes about 10% of EAOCs; the third most frequent EAOC is low-grade serous ovarian carcinoma [
15,
27]. The majority of CCCs and EnOCs fall under the category of Type I ovarian tumors, originating from benign lesions implanted on the ovary and undergoing subsequent malignant transformation (within benign ovarian endometriotic cysts in the case of endometriosis). Type I ovarian tumors are typically clinically indolent and characterized as low-grade carcinomas [
21,
22] (
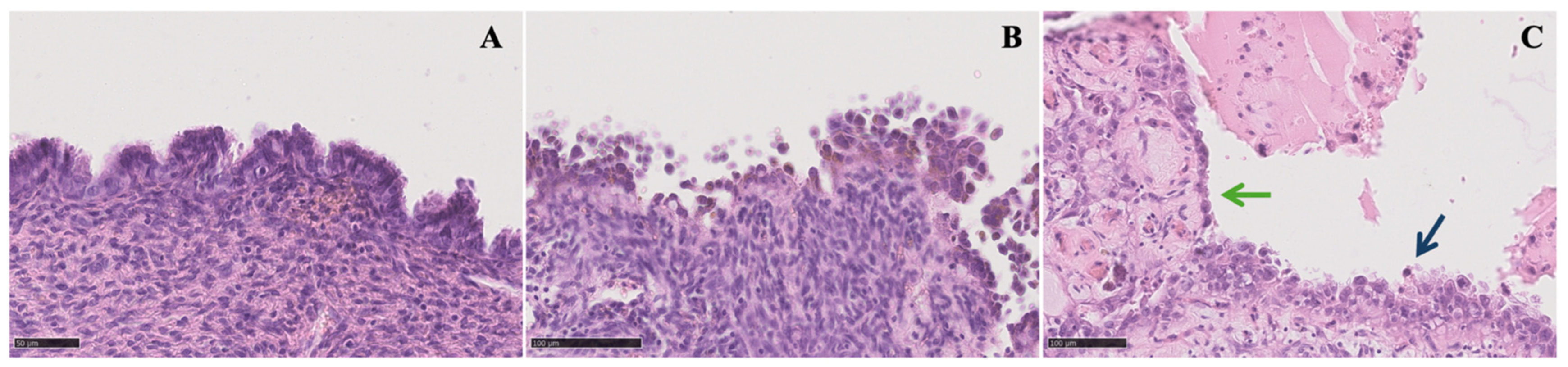
Figure 1).
4. Atypical Endometriosis
AE is present in 12–35% of ovarian endometriosis [
16], and approximately 60–80% of EAOC occurs with the concomitant presence of AE (in 23% of EnOCs and in 36% of CCCs) [
28], often in direct continuity with the tumor [
29]. The high variability of the incidence might be attributed to its difficult histological diagnosis, which lacks worldwide uniformity. Therefore, it is clear that there is a need to revise the classification in order to identify histologically “high-risk” diseases.
Several studies have considered AE as a direct precursor to CCC and EnOC. This hypothesis is based on specific histologic criteria detected on AE specimens, including large nuclei, significant pleomorphism, an increased nucleus-to-cytoplasmic ratio, cellular crowding, and stratification [
18,
19,
28,
29].
We may consider the hypothesis that premalignant lesions like AE may act as an intermediary stage along the pathway to cancer, reflecting genetic changes that occur prior to the onset of malignant behavior. The presence of frequent mutations in cancer-associated genes confirms this association at the molecular pathologic level. In EnOC, mutations in
CTNNB1,
PTEN, and
ARID1A are common, while in CCC,
PIK3CA and
ARID1A mutations are prevalent [
30]. Even if AE may be the precursor of these cancers, it is not systematically found in all cases of EAOC [
31].
Three distinct scenarios may arise: ovarian cancers with histological evidence confirming the transition from endometriosis to ovarian cancer, as defined by Sampson and Scott; the coexistence of ovarian cancers with endometriosis in the same ovary, lacking histological proof of transition; and the occurrence of ovarian cancers alongside concurrent endometriosis at any pelvic location [
16].
AE includes two distinct histologic findings: cytologic atypia and architectural atypia or hyperplasia [
20]. The term “cytologic atypia” refers to the presence of abnormal nuclear features in the epithelial lining of endometriotic cysts, whereas “architectural atypia or hyperplasia” represents the same range of abnormal cell growth found in the endometrium [
30]. The identification of architectural atypia in endometriosis is important because patients with hyperplastic AE may be at an increased risk of developing EAOC [
31].
Tanase et al. highlighted the need to recognize the potential malignant transformation, strongly advising the diligent monitoring of AE when it is detected. They reported the case of a 33-year-old woman whose condition evolved over the span of 10 years and three laparoscopic surgeries from typical endometriosis to AE and eventually to EnOC [
32].
Nevertheless, there is a scarcity of evidence about clinical characteristics, risk factors, and the likelihood of AE recurring. Hence, given that AE is characterized by histological premalignant changes, it is acknowledged as having the potential for precancerous progression, but some mechanisms driving malignant transformation remain uncertain, though several pathways from BE to cancer have been proposed. These pathways implicate factors such as oxidative stress, cytokine activity, genetic mutations, and exposure to a hyperestrogenic environment.
Furthermore, it should be noted that while some studies explain AE as reactivity to severe local inflammation or superficial ulceration with regenerative activity, possibly leading to dysplasia, the association with severe stromal inflammation remains contentious. Conversely, other authors indicate that epithelial atypia can manifest independently of inflammation, implying an intrinsic precancerous potential [
33].
Notably, AE and EAOC share common molecular/genetic alterations, including somatic
ARID1A [
32,
34],
PTEN [
35], and
PIK3CA mutations [
36];
HNF-1b up-regulation [
37]; the loss of the estrogen and progesterone receptor [
38]; and, rarely,
TP53 mutations [
39]. These mutations delineate a spectrum of tumor progression, evolving from benign cystic neoplasms to their corresponding carcinomas. This progression is frequently evident through precursor lesions such as AE (
Table 1). It is worth noting that while atypical endometriosis poses an elevated risk of malignant progression, instances of transformation into carcinoma are infrequent and warrant further investigation.
Several potential targets, such as these molecular changes, have been suggested for the early identification of cancers linked to endometriosis. Yet, the practical use of these new biomarkers could present hurdles, as they all necessitate molecular analysis.
5. EAOC and Endometrial Cancer
Fifty percent of individuals with EnOC show concomitant endometrial adenocarcinoma, making detailed ultrasonographic endometrial assessment imperative when EnOC diagnosis is suspected. In 2–8% of patients affected by endometrial adenocarcinomas, there is the potential for synchronous ovarian carcinoma, necessitating thorough ovarian evaluation in conservative endometrial adenocarcinoma treatments. Notably, approximately 90% of concomitant tumors involving both the ovary and the endometrium display endometrioid histology. Research indicates a substantial prevalence of coexisting endometriosis in cases presenting with simultaneous ovarian and endometrial carcinoma. Patients with EAOC display a higher rate of synchronous endometrial cancer, reporting a reduced recurrence rate and enhanced 5-year disease-free survival, although this phenomenon does not translate into a discernible difference in overall survival [
42].
6. Clinical Approach: How to Make a Diagnosis and Issues for Early Cancer Detection
In patients with endometriotic cysts, preoperative suspicion of malignant transformation is essential to prevent the intraoperative dissemination of malignant cells. To achieve this goal, a considerable level of proficiency in transvaginal sonography (TVS) is necessary, as it remains the most advantageous and easily accessible approach for preoperative evaluation, being a real time dynamic assessment with a sensitivity ranging from 79 to 94% and a specificity of 94% [
43]. MRI may provide supplementary information in specific instances, but it is not a routine method for the preoperative evaluation of endometrioma. The tumor marker CA-125 may be of assistance, although its diagnostic value for early-stage EAOC is restricted due to its lack of specificity. Notably, it is worth mentioning that women with BE often experience slightly increased levels of CA-125, even in the absence of any signs of EAOC [
25,
27,
44,
45,
46].
Sonography continues to be the major technique for evaluating the risk of cancer. Distinguishing between endometriomas and early-stage cystic CCC or EnOC through sonography can be quite challenging. In some cases, a carcinoma may develop from an abnormal epithelial spot within an endometrioma, being difficult to recognize by sonography in its early stage [
47,
48,
49,
50,
51].
Even though endometriosis is increasingly prevalent among young women, EAOC primarily occurs in elderly women [
52] and is characterized by the presence of solid components and larger tumor sizes [
10,
26,
44]. Nevertheless, as individuals grow older, the occurrence of endometriomas containing blood clots miming solid components becomes more common [
25,
44,
53]. During follow-up, these lesions may develop additional atypical features, such as a larger size or multi-cystic formations [
25,
53]. The Ovarian Tumor Analysis (IOTA) database has shown that approximately 21% of endometriomas in women who are 45 years or older may contain solid components [
53]. Furthermore, although ovarian CCC is commonly detected in its early stages, conventional imaging indicators for malignancy may have restricted diagnostic significance [
54,
55]. The lack of guidance on differentiating endometriomas from CCCs is a critical matter that requires immediate attention [
27].
6.1. Ultrasound
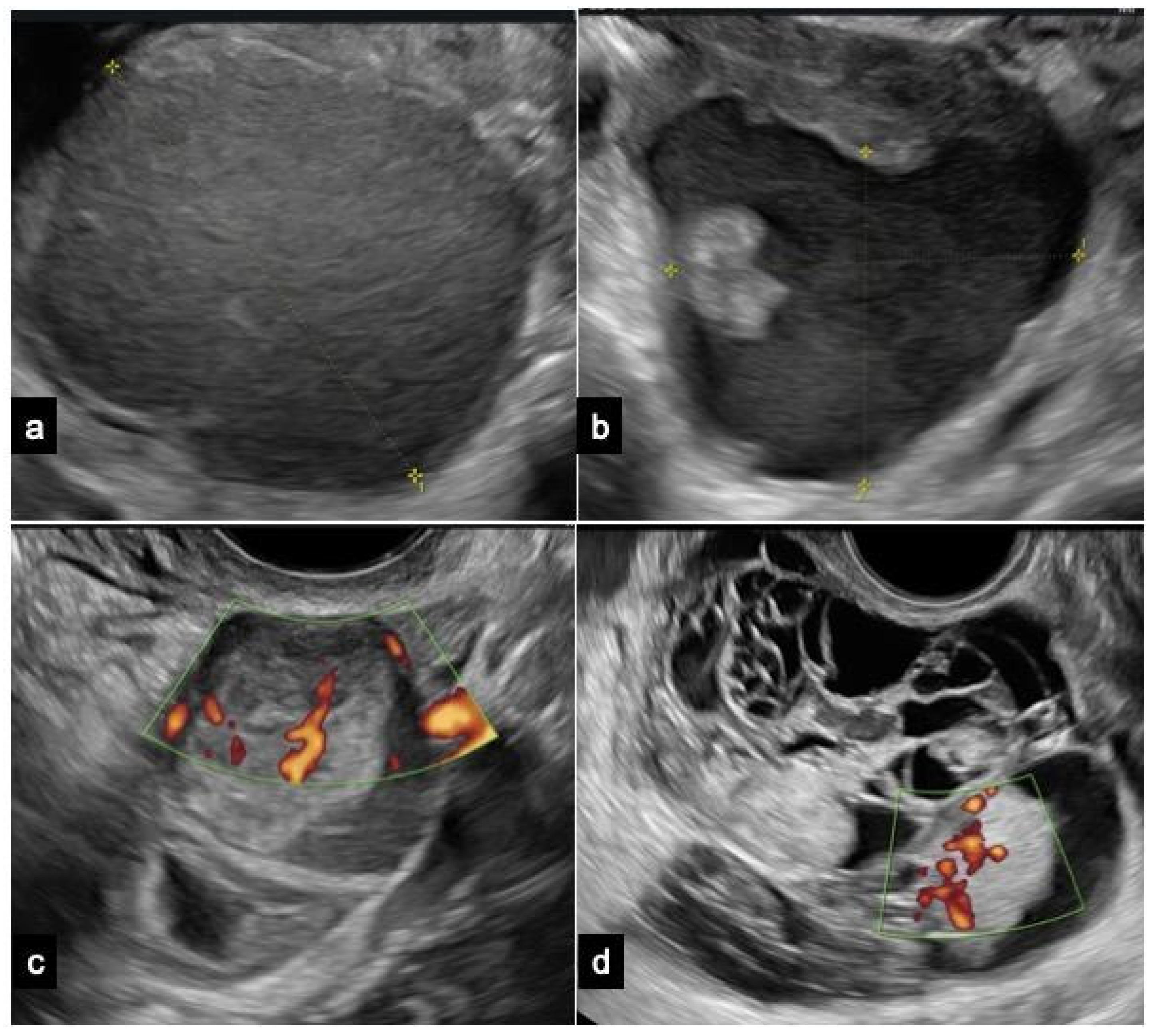
Endometrioma is predominantly a unilocular cyst displaying a uniform “ground-glass” echogenicity without observable solid or vascularized papillary components, facilitating its diagnosing in non-experienced hands (
Figure 2).
However, endometrioma can be defined as atypical when at least one of the following sonographic characteristics is observed: cyst diameters of 10 ± 1 cm, multi-cystic formations, the presence of any solid component or papillary structure—defined by IOTA as a protrusion of solid tissue into a cyst cavity with a minimum height of 3 mm—and the detection of blood flow at any level [
56,
57]. The IOTA risk score considers unilocular cysts with small solid components (less than 7 mm of maximum diameters), acoustic shadows, uniform multilocular tumors with maximum diameters less than 10 cm, and the absence of blood flow as benign features. Malignant features encompass irregular solid tumors, ascites, four or more papillary structures, irregular multilocular solid tumors with a maximum diameter ≥10 cm, and strong blood flow. Typically, obtaining a histological evaluation post intervention for atypical endometriomas deemed at neoplastic risk is recommended. However, additional considerations, including patient’s age or fertility desires, are essential for determining the most appropriate management approach [
56,
58].
Ovarian CCC is typically diagnosed in its early stages, presenting as a sizable unilateral mass with solid components. Patients with CCC arising from endometriosis tend to be younger compared to those with non-endometriosis-associated clear cell carcinoma. Moreover, CCC originating from endometriosis may frequently display a ground-glass echogenicity in the cyst fluid [
59].
EnOCs typically present as larger, unilateral, multilocular-solid, or solid tumors. The ultrasound features of EnOC originating from endometriosis exhibit distinctions from those not associated with endometriosis [
52].
Borderline tumors and carcinomas originating from endometriomas typically show a vascularized solid component. An age of 45 years or older and endometrioma size of 8 cm or more are significant factors that independently predict the development of ovarian cancer in women with endometriomas [
10,
22,
31].
A promising approach for the early diagnosis of endometrial and ovarian cancer seems to be represented by investigating mutation analyses in endocervical or, preferably, intrauterine cell samples. Nevertheless, additional research is needed to determine the accuracy and reliability of these approaches in a clinical setting. Even if, in the future, certain methods may be successful for a peri/postmenopausal population, they may not necessarily be applicable to younger patients with endometriosis, due to the frequency of occurrence of genetic mutations in both eutopic and ectopic endometrium being greater previously assumed [
31,
60,
61,
62,
63]. Currently, there are no effective screening options for epithelial ovarian cancer, and this holds true for women with endometriosis as well [
64,
65,
66,
67].
Early-stage disease with subtle morphological changes poses a challenge for the current diagnostic modalities, and histological examination, though definitive, is invasive.
6.2. Other Instrumental Exams: MRI and CT
In the event of uncertainty following an expert sonographic assessment, additional instrumental examinations are available. Consideration may be given to performing magnetic resonance imaging (MRI) with contrast to differentiate benign ovarian formations from borderline and malignant ones. If neoplastic suspicion is confirmed, a computed tomography (CT) scan of the thorax–abdomen–pelvis at the earliest opportunity is imperative. The objective is to assess the intraperitoneal diffusion of the suspicious mass, lymphadenopathy, and ureteral stenosis in the retroperitoneal space, along with potential thoracic diffusion involving pleural, parenchymal, or mediastinal nodules.
CT is recommended for the staging of ovarian cancer. Contrast-enhanced CT provides clinically relevant information, including the size of the primary tumor and the size and location of any peritoneal and lymph node implants. This information is integral for predicting resectability. The overall accuracy of contrast-enhanced CT in diagnosing malignant neoplasms reaches 89%. CT can visualize tumor implants larger than 1 cm with a sensitivity ranging from 85 to 93% and a specificity of 91–96%. However, the sensitivity diminishes to 25–50% when detecting implants 1 cm or smaller [
68].
MRI offers excellent tissue differentiation and serves as a valuable tool for characterizing lesions that may contain fat observed upon CT or ultrasound with indeterminate significance. Overall, MRI exhibits an accuracy of 83–91% in distinguishing between benign and malignant ovarian masses. The staging accuracy of MRI is comparable to that of conventional CT [
43]. Due to its superior resolution in soft tissue contrast, MRI can precisely identify the invasion of pelvic organs. In predicting resectability, MRI demonstrates a sensitivity of 94% and specificity of 77%, in contrast to 55%, 86%, and 63%, respectively, for CT.
6.3. Molecular Biomarkers
Molecular biomarkers may also be used for further evaluation when the histopathological investigation is not sufficient for assessing the presence of EAOC. For instance,
Ki-67 specifically binds to a nuclear nonhistone protein that is present in the nuclei of actively dividing cells. It can forecast the likelihood of AE developing into a precancerous condition. Statistical differences were discovered by Ogawa and colleagues in the
Ki-67 indices of typical endometriosis, AE, and ovarian cancer [
69]. Their findings indicate that AE shows a level of proliferation activity that falls between typical endometriosis and ovarian carcinoma, positioning it as a precancerous condition (with
Ki-67 indices of 2.7 ± 0.90, 9.9 ± 1.73, and 23.1 ± 3.29, respectively).
In addition,
CD10 can assist in identifying endometriosis in cases where it is challenging to detect through histology, assessing endometriotic tissue characterized by the presence of endometriotic stromal cells [
70].
BE often experiences slightly increased levels of CA-125, even in the absence of any signs of EAOC, but its value usually stays below 100 U/mL. Despite extensive study, CA-125’s diagnostic efficacy for endometriosis remains limited due to its low sensitivity (20–50%). Current international guidelines do not recommend routine CA-125 measurement in endometriosis diagnostics. Elevated CA-125 levels are associated with severe forms of endometriosis and the progression of endometriosis, particularly in ovarian endometriomas (stages three and four). Moreover, CA-125 levels tend to decrease following both medical and surgical interventions for endometriosis.
Patient education regarding the significance of an elevated tumor marker without malignancy is crucial, considering the emotional impact. The decision to categorize the condition as benign or potentially malignant requires careful discussion. Theories propose that CA-125-rich fluid within an endometriotic cyst, particularly after leakage, contributes to elevated levels. This fluid, upon absorption into the peripheral circulation, can induce peritoneal inflammation, leading to an elevated CA-125 level. Additionally, heightened peritoneal fluid in mild endometriosis, with CA-125 concentrations surpassing serum levels, may contribute to elevated serum CA-125 measurements [
71,
72,
73].
In 2008, Yamaguchi et al. identified elevated iron levels in endometriotic cysts, proposing a potential diagnostic marker [
74]. Recent advances include the non-invasive quantification of iron levels using MRI relaxometry. This technique, demonstrating high sensitivity and specificity, holds promise for the early detection of malignant transformation in endometriosis, offering valuable insights for disease management strategies [
75].
7. Common Pathogenic Features of Endometriosis and EAOC, Novel Biomarkers, and Potential Target Therapy
Certainly, AE and EAOC share common molecular/genetic alterations, including somatic
ARID1A and
PTEN mutations,
PIK3CA mutations,
HNF-1b up-regulation, the loss of the estrogen receptor and progesterone receptor, and, occasionally,
TP53 mutations. These mutations illustrate the spectrum of tumor progression from benign cystic neoplasms to corresponding carcinomas like EnOC and CCC, often evolving through precursor lesions such as AE. While various targets have been proposed for the early detection of endometriosis-related cancers, implementing these novel biomarkers in clinical practice may be challenging due to the requirement for molecular analysis [
19].
The occurrence of frequent mutations in
ARID1A, associated with the
SWI/SNF complex, has led to extensive research on their involvement in ovarian CCC, EnOC, and their precursor lesions [
7,
27,
76].
ARID1A mutations, found in approximately 60% of CCCs and 30% of EnOCs, lead to a loss of function, primarily seen in CCC and BE cases. Significantly, endometriosis is the sole benign condition where a deficiency in
ARID1A expression has been detected, even in situations where there is no indication of malignancy [
29,
52,
77,
78,
79,
80,
81].
Although
ARID1A inactivation occurs early in the process, it is not enough to cause cancerous transformation. Additional mechanisms, such as
PIK3CA-activating mutations, are necessary for this purpose [
27,
82,
83]. Sequencing-based detection is difficult, therefore making
ARID1A immunohistochemistry a valuable substitute indicator. It is important to note that there are currently no specific genetic mutations that can be used to differentiate between CCC and EnOC, even though they have different physical and clinical characteristics. CCC exhibits genomic features in 26% of cases, while microsatellite instability is prevalent (28%) in EnOC [
27,
84].
Targeting
ARID1A mutations directly for therapeutic purposes is not feasible [
77], thus driving the investigation of the synthetic lethality strategy to target cancers with
ARID1A deficiency [
85,
86].
ARID1A mutations frequently coincide with
PI3K/AKT pathway activation in CCC, indicating a collaborative function in the process of malignant transformation. Preclinical studies demonstrate synthetic lethality using inhibitors like MK-2206, perifosine, buparlisib, AZD8055, and the
HDAC6 inhibitor ACY1215. Synthetic lethality in
ARID1A-mutated tumors can be induced by targeting
ARID1B [
76,
77]. Chronic inflammation, driven by
ARID1A loss and
PIK3CA mutations, contributes to CCC development through sustained IL-6 production. Anti-IL-6 therapies may be effective. Immune checkpoint inhibitors successful in
SWI/SNF-related cancers show promise in CCC. The
SWI/SNF complex is crucial for DNA damage repair and oxidative stress resistance, making
ARID1A-mutant tumors more sensitive to reactive oxygen species-inducing agents, leading to apoptosis. Dasatinib, a multi-inhibitor, selectively targets
ARID1A-mutated CCC, causing cell cycle arrest and apoptosis [
78,
79].
Potential therapies for
ARID1A-deficient CCC involve targeting proliferative pathways (
PI3K/AKT/mTOR,
YES1/SRC) and metabolic alterations (glutathione biogenesis). Clinical trials explore agents demonstrating synthetic lethality. While
ARID1A mutations show promise as predictive biomarkers, their role in early cancer detection and other biomarker studies is under investigation. Conflicting results in studies on
ARID1A as a prognostic marker in ovarian cancer highlight the need for further research [
27,
80,
81,
82,
83,
84,
85,
86]. Clinical trials explore new therapeutic options for ARID1A-mutated tumors, including
ATR and
PI3K/Akt/mTOR pathway inhibitors. Everolimus plus bevacizumab shows potential benefits, particularly in ARID1A-mutated ovarian cancer. Dasatinib, already approved for leukemia, is currently being investigated for its potential use in treating different solid tumors. Ongoing trials are assessing novel
EZH2,
HDAC, and
BET inhibitors.
ARID1A status may guide chemotherapy decisions, with gemcitabine showing effectiveness in platinum-resistant CCC, especially in
ARID1A-deficient cases.
Furthermore, Moga et al. investigated circulating miRNAs as potential non-invasive diagnostic biomarkers for endometriosis and EAOC, as they are key regulators of cellular processes. Despite their inherent limitations, miRNAs guarantee simplicity, tissular specificity, and steadiness in biological fluids [
87].
MiR-200 family (
miR-200a,
miR-200b,
miR-141) dysregulation in endometriosis suggests a significant role in the disease’s pathophysiology. Noteworthy miRNAs, including
miR-20a and
miR-143, show potential implications for lesion growth and cellular invasion.
MiR-199a, downregulated in endometriosis, has controversial diagnostic potential, likely
miR-145 regulation, with conflicting reports across studies and disease stages.
8. Surgery
A conservative pharmacological approach is usually preferred for the treatment of endometriotic lesions according to symptoms, age, patient desire, and contraindications [
5]. However, it is essential to identify cases that warrant surgery even if asymptomatic, thereby minimizing the risk of neoplastic degeneration associated with certain types of lesions. Detecting the rare cases with an elevated risk of malignant transformation before surgery is crucial in patients with presumed endometriotic cysts in order to prevent the intraoperative dissemination of malignant cells [
25].
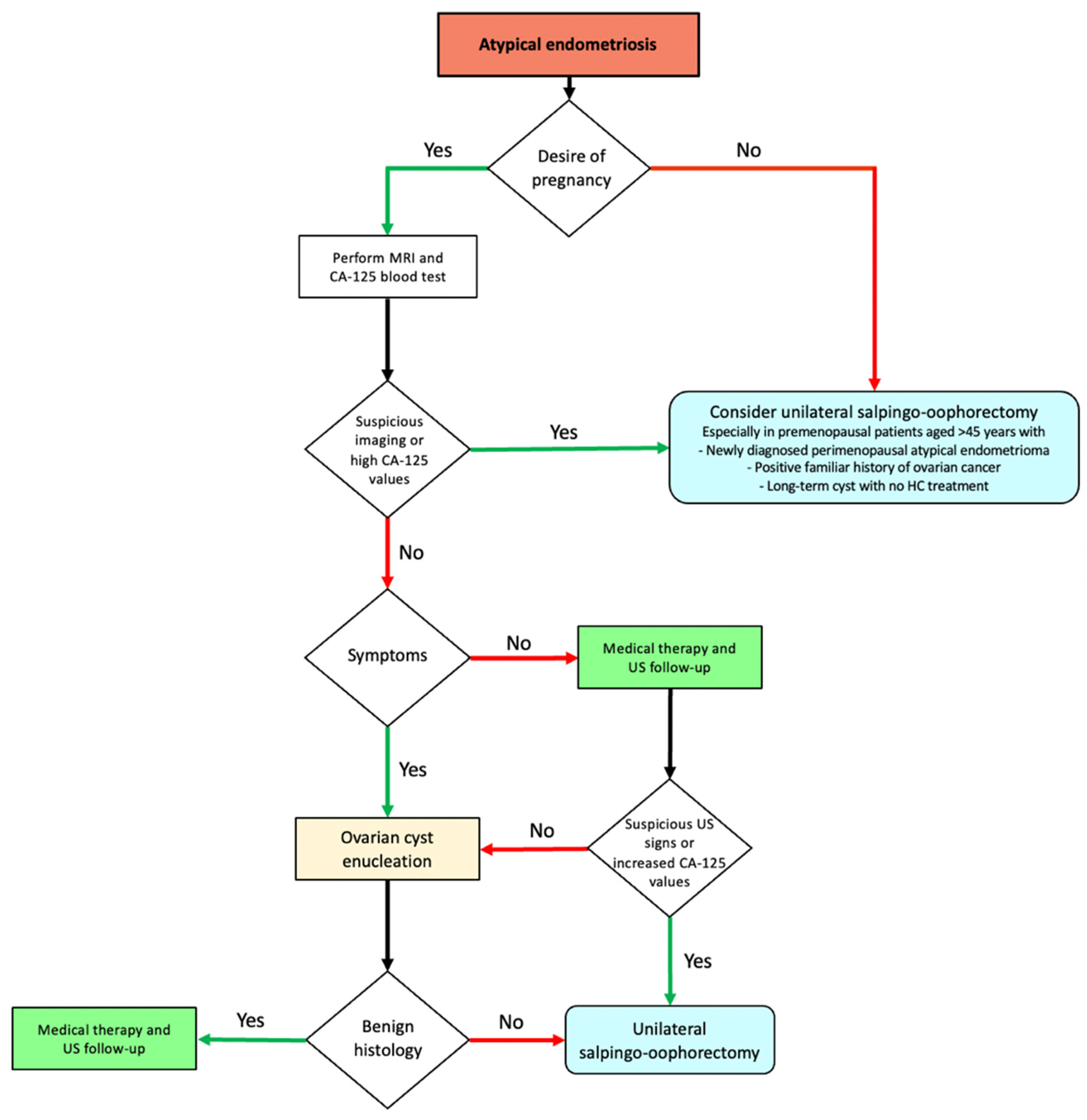
The indication for the surgical management of AE is not straightforward and poses decisional challenges, with the need to evaluate several clinical factors to assess the risk of malignancy, including age, medical history, pregnancy desire, endometriotic cyst history, non-reassuring sonographic characteristics (papillary projections, septa, positive Color Doppler), and tumor biomarkers.
In favor of the surgical approach, Melin et al. showed a significant reduction in future ovarian cancer risk, leading to the complete surgical removal of endometriosis lesions, including endometriomas [
24]. As for any case of adnexal mass with the suspicion of cancer, when AE is coexistent or followed by suspected EAOC, a more aggressive approach might appear to be the safest route. However, the line between under- and over-treatment is very thin, with there being a high risk of crossing it.
Due to the relatively low incidence of neoplastic association and/or the transformation of endometriomas, unilateral salpingo-oophorectomy (USO) cannot be universally recommended for all cases. Such a practice may reflect an excessive level of caution and could easily lead to over-treatment. This is even more true considering that premenopausal oophorectomy is associated with increased all-cause mortality and significant menopause-related morbidity, decreasing life expectancy [
88]. Furthermore, based on Melin’s analysis, performing systematic USO on women with endometriomas would prevent only one case of ovarian cancer every 62 interventions [
24]. It is worth mentioning that their estimates referred to the removal of every endometriotic cyst—including benign endometriomas—not just the atypical ones that are the basis of our assessments.
The risks and benefits of USO should be thoroughly discussed with the patient affected by AE and evaluated independently from her age. Although not recommended in general due to the associated health consequences, USO may be a viable option in adequately informed women approaching menopause with no desire of pregnancy. It may be offered especially when EAOC risk is higher due to patient history (positive family history of ovarian cancer or previous history of infertility), cyst history (premenopausal newly diagnosed atypical endometrioma, endometriotic cyst size increase, long-term cyst with no previous hormonal contraceptive treatment), and/or suspicious imaging characteristics. According to Vercellini et al., 45 years of age could be considered a valuable age cut-off for estimating one’s premenopausal status irrespective of symptoms suggestive of climaterium [
25].
Conversely, USO should not be considered the first choice for young women desiring pregnancy. Since CA-125 is already increased in BE, it may not effectively distinguish atypical endometriomas from EAOC; in these cases, monitoring ultrasound characteristics is crucial for optimal decision making. MRI may offer additional value in specific cases: in instances where atypical sonographic features are detected, the exclusion of malignancy-associated elements may be achieved through MRI. A fertility-sparing approach may include either a conservative cyst excision or a wait-and-see follow-up, provided that the clinical characteristics of the cyst permit it (for example, if the cyst does not increase in size over time and the atypical ultrasound findings remain stable). The optimal follow-up timing is still unknown for atypical endometriomas, probably due to the scarcity of data. Further information is needed to shed light on the unclear aspects regarding EAOC (
Figure 3).
Keeping the patient informed through up-to-date and comprehensive counseling is essential for effective management. Physicians must carefully weigh the risks of over- and under-treatment, providing patients with the necessary information to make informed decisions about their care.
Surgery must always be followed by careful follow-up. Indeed, according to some studies, women experiencing a recurrence of endometriotic lesions after excision have an elevated risk of ovarian neoplasia. This particular patient population must be offered close personalized follow-up and a possible second operation [
89].
Finally, in consideration of the most recent data, it would be interesting and desirable in the future to investigate possible therapeutic alternatives that could be effective in reducing recurrence after surgery in order to further reduce the neoplastic risk.
9. Conclusions
Our narrative review summarizes the current knowledge on EAOC, proposing a flowchart dedicated to the management of the disease. Endometriosis patients appear to have an increased risk of developing EAOC, particularly the EnOC and CCC subtypes. However, the scarcity of data currently prevents definitive conclusions.
Researchers still need to understand some unclear aspects, such as early detection, risk factor identification, and risk stratification. Advances in the SWI/SNF complex and ARID1A alterations provide insights into endometriosis and EAOC carcinogenesis. The success of PARP inhibitors in ovarian cancer therapy fuels the exploration of new targeted therapies. Inflammatory and epigenetic processes, prominent in the CCC and EnOC subtypes of EAOC, suggest potential treatments with immune checkpoint inhibitors, PI3K pathway targeting, and epigenetic approaches. Clinical research tailored to the molecular features of these subtypes will be crucial.
Finally, it is worth underlining that TVS diagnosis remains the cornerstone of early detection, guiding the patient towards the most appropriate therapy, follow-up, and treatment, while MRI aids in characterizing TVS-indeterminate lesions. Malignancy indicators, such as increasing tumor size, rapid growth, papillary excrescences, and thick septations, are crucial for suspicion.
Author Contributions
Conceptualization, G.C., G.S., L.L., R.S. and E.Z.; methodology, M.G., G.S., N.H. and G.C.; validation, E.Z. and G.C.; investigation, G.S., F.G.M., E.P. and F.F.; data curation, G.S., M.G., E.P., V.M. and D.R.; writing—original draft preparation, G.C., F.G.M., E.P., G.S., V.M. and R.S.; writing—review and editing, D.R., M.G., L.L., G.C., R.S. and E.Z.; visualization, F.F., N.H., M.G. and L.L.; supervision, G.C., E.Z., D.R., R.S. and L.L.; project administration, G.C., E.Z. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Centini, G.; Lazzeri, L.; Dores, D.; Pianigiani, L.; Iannone, P.; Luisi, S.; Petraglia, F.; Zupi, E. Chronic Pelvic Pain and Quality of Life in Women with and without Endometriosis. J. Endometr. Pelvic Pain Disord. 2013, 5, 27–33. [Google Scholar] [CrossRef]
- Huang, K.J.; Li, Y.X.; Wu, C.J.; Chang, W.C.; Wei, L.H.; Sheu, B.C. Sonographic features differentiating early-stage ovarian clear cell carcinoma from endometrioma with atypical features. J. Ovarian Res. 2022, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Fuldeore, M.; Yang, H.; Du, E.X.; Soliman, A.M.; Wu, E.Q.; Winkel, C. Healthcare utilization and costs in women diagnosed with endometriosis before and after diagnosis: A longitudinal analysis of claims databases. Fertil. Steril. 2015, 103, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Fuldeore, M.J.; Soliman, A.M. Prevalence and Symptomatic Burden of Diagnosed Endometriosis in the United States: National Estimates from a Cross-Sectional Survey of 59,411 Women. Gynecol. Obstet. Investig. 2017, 82, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Falcone, T.; Flyckt-Rebecca, R. Clinical management of endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Capmas, P.; Suarthana, E.; Tulandi, T. Further evidence that endometriosis is related to tubal and ovarian cancers: A study of 271,444 inpatient women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch. Surg. 1925, 10, 1–72. [Google Scholar] [CrossRef]
- Scott, R.B. Malignant changes in endometriosis. Obstet. Gynecol. 1953, 2, 283–289. [Google Scholar]
- Bartiromo, L.; Schimberni, M.; Villanacci, R.; Mangili, G.; Ferrari, S.; Ottolina, J.; Salmeri, N.; Dolci, C.; Tandoi, I.; Candiani, M. A Systematic Review of Atypical Endometriosis-Associated Biomarkers. Int. J. Mol. Sci. 2022, 23, 4425. [Google Scholar] [CrossRef]
- He, Z.X.; Shi, H.H.; Fan, Q.B.; Zhu, L.; Leng, J.H.; Sun, D.W.; Li, Z.F.; Shen, K.; Wang, S.; Lang, J.H. Predictive factors of ovarian carcinoma for women with ovarian endometrioma aged 45 years and older in China. J. Ovarian Res. 2017, 10, 45. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, T.H.; Chung, H.H.; Song, Y.S. Risk and prognosis of ovarian cancer in women with endometriosis: A meta-analysis. Br. J. Cancer 2014, 110, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Sumimoto, K.; Kitanaka, T.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; et al. Ovarian endometrioma-Risks factors of ovarian cancer development. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Vigano’, P.; Parazzini, F.; Stoppelli, S.; Giambattista, E.; Vercellini, P. Association between endometriosis and cancer: A comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol. Oncol. 2006, 101, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.H.; Huang, C.Y.; Ueng, S.H.; Huang, K.G.; Lee, C.L.; Yen, C.F. Unexpected epithelial ovarian cancers arising from presumed endometrioma: A 10-year retrospective analysis. Taiwan J. Obstet. Gynecol. 2017, 56, 55–61. [Google Scholar] [CrossRef]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, T.; Amant, F.; Neven, P.; Vergote, I.; Moerman, P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Lamceva, J.; Uljanovs, R.; Strumfa, I. The Main Theories on the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 4254. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, H.; Tsunemi, T.; Haruta, S.; Kajihara, H.; Yoshizawa, Y.; Tanase, Y.; Furukawa, N.; Yoshida, S.; Sado, T.; Kobayashi, H. Molecular Mechanisms Linking Endometriosis Under Oxidative Stress with Ovarian Tumorigenesis and Therapeutic Modalities. Cancer Investig. 2012, 30, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A Mutations in Endometriosis-Associated Ovarian Carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef]
- McConechy, M.K.; Ding, J.; Senz, J.; Yang, W.; Melnyk, N.; Tone, A.A.; Prentice, L.M.; Wiegand, K.C.; McAlpine, J.N.; Shah, S.P.; et al. Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod. Pathol. 2014, 27, 128–134. [Google Scholar] [CrossRef]
- Matsumoto, T.; Yamazaki, M.; Takahashi, H.; Kajita, S.; Suzuki, E.; Tsuruta, T.; Saegusa, M. Distinct β-Catenin and PIK3CA Mutation Profiles in Endometriosis-Associated Ovarian Endometrioid and Clear Cell Carcinomas. Am. J. Clin. Pathol. 2015, 144, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Catasús, L.; Bussaglia, E.; Rodríguez, I.; Gallardo, A.; Pons, C.; Irving, J.A.; Prat, J. Molecular genetic alterations in endometrioid carcinomas of the ovary: Similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum. Pathol. 2004, 35, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Tsunoda, H.; Nishida, M.; Morishita, Y.; Takimoto, Y.; Kubo, T.; Noguchi, M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: Possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000, 60, 7052–7056. [Google Scholar] [PubMed]
- Melin, A.S.; Lundholm, C.; Malki, N.; Swahn, M.L.; Sparèn, P.; Bergqvist, A. Hormonal and surgical treatments for endometriosis and risk of epithelial ovarian cancer. Acta Obstet. Gynecol. Scand. 2013, 92, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Buggio, L.; Makieva, S.; Scarfone, G.; Cribiù, F.M.; Parazzini, F.; Somigliana, E. Perimenopausal management of ovarian endometriosis and associated cancer risk: When is medical or surgical treatment indicated? Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.H.; Schnack, T.H.; Buchardi, K.; Hummelshoj, L.; Missmer, S.A.; Forman, A.; Blaakaer, J. Risk factors of epithelial ovarian carcinomas among women with endometriosis: A systematic review. Acta Obstet. Gynecol. Scand. 2017, 96, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, E.P.; Labidi-Galy, S.I.; Moschetta, M.; Uccello, M.; Kalaitzopoulos, D.R.; Perez-Fidalgo, J.A.; Boussios, S. Endometriosis-associated ovarian carcinomas: Insights into pathogenesis, diagnostics, and therapeutic targets—A narrative review. Ann. Transl. Med. 2020, 8, 1712. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Nomura, K.; Ishikawa, E.; Ushigome, S. Ovarian atypical endometriosis: Its close association with malignant epithelial tumours. Histopathology 1997, 30, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Rollason, T.; Gupta, J.K.; Maher, E.R. Endometriosis and the neoplastic process. Reproduction 2004, 127, 293–304. [Google Scholar] [CrossRef]
- Mikhaleva, L.M.; Davydov, A.I.; Patsap, O.I.; Mikhaylenko, E.V.; Nikolenko, V.N.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. Malignant Transformation and Associated Biomarkers of Ovarian Endometriosis: A Narrative Review. Adv. Ther. 2020, 37, 2580–2603. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Yong, P.J. Endometriosis-associated Ovarian Cancers. Clin. Obstet. Gynecol. 2017, 60, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Bayramolu, H.; Düzcan, E. Atypical epithelial changes and mutant p53 gene expression in ovarian endometriosis. Pathol. Oncol. Res. 2001, 7, 33–38. [Google Scholar] [CrossRef]
- Tanase, Y.; Furukawa, N.; Kobayashi, H.; Matsumoto, T. Malignant Transformation from Endometriosis to Atypical Endometriosis and Finally to Endometrioid Adenocarcinoma within 10 Years. Case Rep. Oncol. 2013, 6, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Roman, R.A.; Rambhatla, A.; Nezhat, F. Reproductive and oncologic outcomes after fertility-sparing surgery for early stage cervical cancer: A systematic review. Fertil. Steril. 2020, 113, 685–703. [Google Scholar] [CrossRef]
- Del Carmen, M.G.; Smith Sehdev, A.E.; Fader, A.N.; Zahurak, M.L.; Richardson, M.; Fruehauf, J.P.; Montz, F.J.; Bristow, R.E. Endometriosis-associated ovarian carcinoma. Cancer 2003, 98, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, S.; Sekizawa, A.; Otsuka, J.; Tachikawa, T.; Saito, H.; Okai, T. Malignant transformation of endometriosis and genetic alterations of K-ras and microsatellite instability. Int. J. Gynecol. Obstet. 2004, 86, 371–376. [Google Scholar] [CrossRef]
- Sáinz De La Cuesta, R.; Izquierdo, M.; Cañamero, M.; Granizo, J.J.; Manzarbeitia, F. Increased prevalence of p53 overexpression from typical endometriosis to atypical endometriosis and ovarian cancer associated with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, 87–93. [Google Scholar] [CrossRef]
- Akahane, T.; Sekizawa, A.; Okuda, T.; Kushima, M.; Saito, H.; Okai, T. Disappearance of steroid hormone dependency during malignant transformation of ovarian clear cell cancer. Int. J. Gynecol. Pathol. 2005, 24, 369–376. [Google Scholar] [CrossRef]
- Ali-Fehmi, R.; Khalifeh, I.; Bandyopadhyay, S.; Lawrence, W.D.; Silva, E.; Liao, D.; Sarkar, F.H.; Munkarah, A.R. Patterns of loss of heterozygosity at 10q23.3 and microsatellite instability in endometriosis, atypical endometriosis, and ovarian carcinoma arising in association with endometriosis. Int. J. Gynecol. Pathol. 2006, 25, 223–229. [Google Scholar] [CrossRef]
- Hollis, R.L. Molecular characteristics and clinical behaviour of epithelial ovarian cancers. Cancer Lett. 2023, 555, 216057. [Google Scholar] [CrossRef]
- Romero, I.; Leskelä, S.; Mies, B.P.; Velasco, A.P.; Palacios, J. Morphological and molecular heterogeneity of epithelial ovarian cancer: Therapeutic implications. Eur. J. Cancer Suppl. 2020, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Rauh-Hain, J.A.; Andrade, C.; Boruta, D.M., II; Schorge, J.O.; Horowitz, N.S.; May, T.; Del Carmen, M.G. Comparison of clinical outcomes of patients with clear cell and endometrioid ovarian cancer associated with endometriosis to papillary serous carcinoma of the ovary. Gynecol. Oncol. 2014, 132, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Vigueras Smith, A.; Cabrera, R.; Trippia, C.; Zomer, M.T.; Kondo, W.; Ferreira, H.; Da Silva, L.C.; Sumak, R. Indirect and atypical imaging signals of endometriosis: A wide range of manifestations. Facts Views Vis. ObGyn 2021, 13, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Tanase, Y.; Kawaguchi, R.; Takahama, J.; Kobayashi, H. Factors that Differentiate between Endometriosis-associated Ovarian Cancer and Benign Ovarian Endometriosis with Mural Nodules. Magn. Reson. Med. Sci. 2018, 17, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Foster, W. Diagnosing endometriosis: CA125 rules in, but not out. BJOG 2016, 123, 1769. [Google Scholar] [CrossRef] [PubMed]
- Moro, F.; Magoga, G.; Pasciuto, T.; Mascilini, F.; Moruzzi, M.C.; Fischerova, D.; Savelli, L.; Giunchi, S.; Mancari, R.; Franchi, D.; et al. Imaging in gynecological disease (13): Clinical and ultrasound characteristics of endometrioid ovarian cancer. Ultrasound Obstet. Gynecol. 2018, 52, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kamura, T.; Kigawa, J.; Terakawa, N.; Kikuchi, Y.; Kita, T.; Suzuki, M.; Sato, I.; Taguchi, K. Clinical characteristics of clear cell carcinoma of the ovary: A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 2000, 88, 2584–2589. [Google Scholar] [CrossRef]
- Köbel, M.; Kalloger, S.E.; Huntsman, D.G.; Santos, J.L.; Swenerton, K.D.; Seidman, J.D.; Gilks, C.B. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int. J. Gynecol. Pathol. 2010, 29, 203–211. [Google Scholar] [CrossRef]
- Park, H.K.; Ruterbusch, J.J.; Cote, M.L. Recent trends in ovarian cancer incidence and relative survival in the United States by race/ethnicity and histologic subtypes. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1511–1518. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, L.; Lang, J.H.; Shen, K.; Huang, H.F.; Pan, L.Y.; Wu, M.; Yang, J.X.; Guo, L.N. Prognostic analysis of endometrioid epithelial ovarian cancer with or without endometriosis: A 12-year cohort study of Chinese patients. Am. J. Obstet. Gynecol. 2013, 209, 241.e1–241.e9. [Google Scholar] [CrossRef]
- Chan, J.K.; Teoh, D.; Hu, J.M.; Shin, J.Y.; Osann, K.; Kapp, D.S. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol. Oncol. 2008, 109, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Martire, F.G.; Russo, C.; Selntigia, A.; Nocita, E.; Soreca, G.; Lazzeri, L.; Zupi, E.; Exacoustos, C. Early noninvasive diagnosis of endometriosis: Dysmenorrhea and specific ultrasound findings are important indicators in young women. Fertil. Steril. 2023, 119, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Van Calster, B.; Somigliana, E.; Ajossa, S.; Froyman, W.; De Cock, B.; Coosemans, A.; Fischerova, D.; Van Holsbeke, C.; Alcazar, J.L.; et al. Age-related differences in the sonographic characteristics of endometriomas. Hum. Reprod. 2016, 31, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, F.R.; Apostol, R.; Nezhat, C.; Pejovic, T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am. J. Obstet. Gynecol. 2015, 213, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Scarfone, G.; Bergamini, A.; Noli, S.; Villa, A.; Cipriani, S.; Taccagni, G.; Candiani, M.; Parazzini, F.; Mangili, G. Characteristics of clear cell ovarian cancer arising from endometriosis: A two center cohort study. Gynecol. Oncol. 2014, 133, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ameye, L.; Jurkovic, D.; Van Holsbeke, C.; Paladini, D.; Van Calster, B.; Vergote, I.; Van Huffel, S.; et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. 2008, 31, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Ameye, L.; Fischerova, D.; Epstein, E.; Melis, G.B.; Guerriero, S.; Van Holsbeke, C.; Savelli, L.; Fruscio, R.; Lissoni, A.A.; et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: Prospective validation by IOTA group. BMJ 2010, 341, 94. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Van Calster, B.; Testa, A.; Savelli, L.; Fischerova, D.; Froyman, W.; Wynants, L.; Van Holsbeke, C.; Epstein, E.; Franchi, D.; et al. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am. J. Obstet. Gynecol. 2016, 214, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Pozzati, F.; Moro, F.; Pasciuto, T.; Gallo, C.; Ciccarone, F.; Franchi, D.; Mancari, R.; Giunchi, S.; Timmerman, D.; Landolfo, C.; et al. Imaging in gynecological disease (14): Clinical and ultrasound characteristics of ovarian clear cell carcinoma. Ultrasound Obstet. Gynecol. 2018, 52, 792–800. [Google Scholar] [CrossRef]
- Moore, L.; Leongamornlert, D.; Coorens, T.H.; Sanders, M.A.; Ellis, P.; Dentro, S.C.; Dawson, K.J.; Butler, T.; Rahbari, R.; Mitchell, T.J.; et al. The mutational landscape of normal human endometrial epithelium. Nature 2020, 580, 640–646. [Google Scholar] [CrossRef]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Lac, V.; Nazeran, T.M.; Tessier-Cloutier, B.; Aguirre-Hernandez, R.; Albert, A.; Lum, A.; Khattra, J.; Praetorius, T.; Mason, M.; Chiu, D.; et al. Oncogenic mutations in histologically normal endometrium: The new normal? J. Pathol. 2019, 249, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lac, V.; Verhoef, L.; Aguirre-Hernandez, R.; Nazeran, T.M.; Tessier-Cloutier, B.; Praetorius, T.; Orr, N.L.; Noga, H.; Lum, A.; Khattra, J.; et al. Iatrogenic endometriosis harbors somatic cancer-driver mutations. Hum. Reprod. 2019, 34, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Douville, C.; Cohen, J.D.; Yen, T.T.; Kinde, I.; Sundfelt, K.; Kjær, S.K.; Hruban, R.H.; Shih, I.M.; et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci. Transl. Med. 2018, 10, eaap8793. [Google Scholar] [CrossRef]
- Kinde, I.; Bettegowda, C.; Wang, Y.; Wu, J.; Agrawal, N.; Shih, I.M.; Kurman, R.; Dao, F.; Levine, D.A.; Giuntoli, R.; et al. Evaluation of DNA from the papanicolaou test to detect ovarian and endometrial cancers. Sci. Transl. Med. 2013, 5, 167ra4. [Google Scholar] [CrossRef] [PubMed]
- Maritschnegg, E.; Heitz, F.; Pecha, N.; Bouda, J.; Trillsch, F.; Grimm, C.; Vanderstichele, A.; Agreiter, C.; Harter, P.; Obermayr, E.; et al. Uterine and Tubal Lavage for Earlier Cancer Detection Using an Innovative Catheter: A Feasibility and Safety Study. Int. J. Gynecol. Cancer 2018, 28, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Maritschnegg, E.; Wang, Y.; Pecha, N.; Horvat, R.; Van Nieuwenhuysen, E.; Vergote, I.; Heitz, F.; Sehouli, J.; Kinde, I.; Diaz, L.A., Jr.; et al. Lavage of the uterine cavity for molecular detection of Müllerian duct carcinomas: A proof-of-concept study. J. Clin. Oncol. 2015, 33, 4293–4300. [Google Scholar] [CrossRef]
- Javadi, S.; Ganeshan, D.M.; Qayyum, A.; Iyer, R.B.; Bhosale, P. Ovarian cancer, the revised FIGO staging system, and the role of imaging. Am. J. Roentgenol. 2016, 206, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Kaku, T.; Amada, S.; Kobayashi, H.; Hirakawa, T.; Ariyoshi, K.; Kamura, T.; Nakano, H. Ovarian endometriosis associated with ovarian carcinoma: A clinicopathological and immunohistochemical study. Gynecol. Oncol. 2000, 77, 298–304. [Google Scholar] [CrossRef]
- Wei, J.J.; William, J.; Bulun, S. Endometriosis and ovarian cancer: A review of clinical, pathologic, and molecular aspects. Int. J. Gynecol. Pathol. 2011, 30, 553–568. [Google Scholar] [CrossRef]
- Molina, R.; Ojeda, B.; Filella, X.; Borras, G.; Jo, J.; Mas, E.; Lopez, J.J.; Ballesta, A. A Prospective Study of Tumor Markers CA 125 and CA 19.9 in Patients with Epithelial Ovarian Carcinomas. Tumor Biol. 1992, 13, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, N.; Miyagi, E.; Nomura, A.; Morita, E.; Ino, Y.; Ohtake, N.; Miyagi, Y.; Hirahara, F.; Hirano, H. Secretome-Based identification of TFPI2, A novel serum biomarker for detection of ovarian clear cell adenocarcinoma. J. Proteome Res. 2013, 12, 4340–4350. [Google Scholar] [CrossRef] [PubMed]
- Blackman, A.; Mitchell, J.; Rowswell-Turner, R.; Singh, R.; Kim, K.K.; Eklund, E.; Skates, S.; Bast, R.C.; Messerlian, G.; Miller, M.C.; et al. Analysis of serum HE4 levels in various histologic subtypes of epithelial ovarian cancer and other malignant tumors. Tumor Biol. 2021, 43, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Mandai, M.; Toyokuni, S.; Hamanishi, J.; Higuchi, T.; Takakura, K.; Fujii, S. Contents of Endometriotic Cysts, Especially the High Concentration of Free Iron, Are a Possible Cause of Carcinogenesis in the Cysts through the Iron-Induced Persistent Oxidative Stress. Clin. Cancer Res. 2008, 14, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, C.; Takahama, J.; Iwabuchi, T.; Uchikoshi, M.; Shigetomi, H.; Kobayashi, H. Transverse Relaxation Rate of Cyst Fluid Can Predict Malignant Transformation of Ovarian Endometriosis. Magn. Reson. Med. Sci. 2017, 16, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tsuda, H.; Takano, M.; Tamai, S.; Matsubara, O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod. Pathol. 2012, 25, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Bitler, B.G.; Aird, K.M.; Garipov, A.; Li, H.; Amatangelo, M.; Kossenkov, A.V.; Schultz, D.C.; Liu, Q.; Shih, I.M.; Conejo-Garcia, J.R.; et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat. Med. 2015, 21, 231–238. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Mak, T.W. Glutathione Metabolism: An Achilles’ Heel of ARID1A-Deficient Tumors. Cancer Cell 2019, 35, 161–163. [Google Scholar] [CrossRef]
- Chandler, R.L.; Damrauer, J.S.; Raab, J.R.; Schisler, J.C.; Wilkerson, M.D.; Didion, J.P.; Starmer, J.; Serber, D.; Yee, D.; Xiong, J.; et al. Coexistent ARID1A–PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat. Commun. 2015, 6, 6118. [Google Scholar] [CrossRef]
- Katagiri, A.; Nakayama, K.; Rahman, M.T.; Rahman, M.; Katagiri, H.; Nakayama, N.; Ishikawa, M.; Ishibashi, T.; Iida, K.; Kobayashi, H.; et al. Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod. Pathol. 2012, 25, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, A.; Matsumoto, T.; Watanabe, G.; Tochimoto, M.; Saegusa, M. Immunophenotypic profile as a predictor of prognosis in advanced ovarian carcinoma. Hum. Pathol. 2019, 85, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Heckl, M.; Schmoeckel, E.; Hertlein, L.; Rottmann, M.; Jeschke, U.; Mayr, D. The ARID1A, p53 and ß-Catenin statuses are strong prognosticators in clear cell and endometrioid carcinoma of the ovary and the endometrium. PLoS ONE 2018, 13, e0192881. [Google Scholar] [CrossRef] [PubMed]
- Jelinic, P.; Ricca, J.; Van Oudenhove, E.; Olvera, N.; Merghoub, T.; Levine, D.A.; Zamarin, D. Immune-Active Microenvironment in Small Cell Carcinoma of the Ovary, Hypercalcemic Type: Rationale for Immune Checkpoint Blockade. JNCI J. Natl. Cancer Inst. 2018, 110, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Matsushita, Y.; Shigeto, T.; Futagami, M.; Mizunuma, H. Decreased ARID1A expression is correlated with chemoresistance in epithelial ovarian cancer. J. Gynecol. Oncol. 2014, 25, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Oda, K.; Ikeda, Y.; Koso, T.; Tsuji, S.; Yamamoto, S.; Asada, K.; Sone, K.; Kurikawa, R.; Makii, C.; et al. Integrated Copy Number and Expression Analysis Identifies Profiles of Whole-Arm Chromosomal Alterations and Subgroups with Favorable Outcome in Ovarian Clear Cell Carcinomas. PLoS ONE 2015, 10, e0128066. [Google Scholar] [CrossRef]
- Moga, M.A.; Bălan, A.; Dimienescu, O.G.; Burtea, V.; Dragomir, R.M.; Anastasiu, C.V. Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer—An Overview. J. Clin. Med. 2019, 8, 735. [Google Scholar] [CrossRef]
- Asfour, V.; Jakes, A.D.; McMicking, J.; Szetho, W.Z.; Sayasneh, A.; Diab, Y.; Mascarenhas, L.; Rymer, J. Oophorectomy or ovarian conservation at the time of hysterectomy for benign disease. Obstet. Gynaecol. 2022, 24, 131–136. [Google Scholar] [CrossRef]
- Haraguchi, H.; Koga, K.; Takamura, M.; Makabe, T.; Sue, F.; Miyashita, M.; Urata, Y.; Izumi, G.; Harada, M.; Hirata, T.; et al. Development of ovarian cancer after excision of endometrioma. Fertil. Steril. 2016, 106, 1432–1437.e2. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).