Techniques for Thyroidectomy and Functional Neck Dissection
Abstract
1. Introduction
2. Thyroid and Neck Anatomy
3. Preoperative Planning of Thyroidectomy and Neck Dissection
3.1. Ultrasonography (USG)
3.2. Computed Tomography (CT)
3.3. Magnetic Resonance Imaging (MRI)
3.4. Positron Emission Tomography–Computed Tomography (PET-CT)
4. Techniques for Thyroidectomy
4.1. Technical Details of a Conventional Thyroid Surgery
4.2. Vessel-Sealing Energy Devices
5. Neck Dissection Techniques and the Concept of Functional Neck Dissection
5.1. Central Neck Dissection
5.2. Selective Neck Dissection
5.3. Lateral Neck Dissection
6. Minimally Invasive Operative Techniques
6.1. Minimally Invasive Video-Assisted Thyroidectomy (MIVAT)
6.2. Endoscopic Thyroidectomy
6.3. Robotic Thyroidectomy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Tang, A.L.; Falciglia, M.; Yang, H.; Mark, J.R.; Steward, D.L. Validation of American Thyroid Association Ultrasound Risk Assessment of Thyroid Nodules Selected for Ultrasound Fine-Needle Aspiration. Thyroid 2017, 27, 1077–1082. [Google Scholar] [CrossRef]
- Haddad, R.I.; Bischoff, L.; Ball, D.; Bernet, V.; Blomain, E.; Busaidy, N.L.; Campbell, M.; Dickson, P.; Duh, Q.Y.; Ehya, H.; et al. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 925–951. [Google Scholar] [CrossRef]
- Davies, L.; Welch, H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 317–322. [Google Scholar] [CrossRef]
- Leenhardt, L.; Bernier, M.O.; Boin-Pineau, M.H.; Conte Devolx, B.; Maréchaud, R.; Niccoli-Sire, P.; Nocaudie, M.; Orgiazzi, J.; Schlumberger, M.; Wémeau, J.L.; et al. Advances in diagnostic practices affect thyroid cancer incidence in France. Eur. J. Endocrinol. 2004, 150, 133–139. [Google Scholar] [CrossRef]
- Stack, B.C., Jr.; Ferris, R.L.; Goldenberg, D.; Haymart, M.; Shaha, A.; Sheth, S.; Sosa, J.A.; Tufano, R.P. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid 2012, 22, 501–508. [Google Scholar] [CrossRef]
- Musacchio, M.J.; Kim, A.W.; Vijungco, J.D.; Prinz, R.A. Greater local recurrence occurs with “berry picking” than neck dissection in thyroid cancer. Am. Surg. 2003, 69, 191–196. [Google Scholar] [CrossRef]
- Cheah, W.K.; Arici, C.; Ituarte, P.H.; Siperstein, A.E.; Duh, Q.Y.; Clark, O.H. Complications of neck dissection for thyroid cancer. World J. Surg. 2002, 26, 1013–1016. [Google Scholar] [CrossRef]
- Stewart, W.B.; Rizzolo, L.J. Embryology and Surgical Anatomy of the Thyroid and Parathyroid Glands. In Surgery of the Thyroid and Parathyroid Glands Berlin, 2nd ed.; Oertli, D., Udelsman, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 15–23. [Google Scholar]
- Kim, D.W.; Jung, S.L.; Baek, J.H.; Kim, J.; Ryu, J.H.; Na, D.G.; Park, S.W.; Kim, J.H.; Sung, J.Y.; Lee, Y.; et al. The prevalence and features of thyroid pyramidal lobe, accessory thyroid, and ectopic thyroid as assessed by computed tomography: A multicenter study. Thyroid 2013, 23, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Mohebati, A.; Shaha, A.R. Anatomy of thyroid and parathyroid glands and neurovascular relations. Clin. Anat. 2012, 25, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Pagedar, N.A.; Freeman, J.L. Identification of the External Branch of the Superior Laryngeal Nerve During Thyroidectomy. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 360–362. [Google Scholar] [CrossRef]
- Scheumann, G.F.; Gimm, O.; Wegener, G.; Hundeshagen, H.; Dralle, H. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J. Surg. 1994, 18, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Suárez, O. Le problème chirurgical du cancer du larynx. Ann. D’oto-Laryngol. 1962, 79, 22–34. [Google Scholar]
- Gavilán, J.; Moñux, A.; Herranz, J.; Gavilán, C. Functional neck dissection: Surgical technique. Oper. Tech. Otolaryngol.-Head Neck Surg. 1993, 4, 258–265. [Google Scholar] [CrossRef]
- Cheng, P.T.; Hao, S.P.; Lin, Y.H.; Yeh, A.R. Objective comparison of shoulder dysfunction after three neck dissection techniques. Ann. Otol. Rhinol. Laryngol. 2000, 109 Pt 1, 761–766. [Google Scholar] [CrossRef]
- Carty, S.E.; Cooper, D.S.; Doherty, G.M.; Duh, Q.Y.; Kloos, R.T.; Mandel, S.J.; Randolph, G.W.; Stack, B.C., Jr.; Steward, D.L.; Terris, D.J.; et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid 2009, 19, 1153–1158. [Google Scholar] [CrossRef]
- Yeh, M.W.; Bauer, A.J.; Bernet, V.A.; Ferris, R.L.; Loevner, L.A.; Mandel, S.J.; Orloff, L.A.; Randolph, G.W.; Steward, D.L. American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid 2015, 25, 3–14. [Google Scholar] [CrossRef]
- Kouvaraki, M.A.; Shapiro, S.E.; Fornage, B.D.; Edeiken-Monro, B.S.; Sherman, S.I.; Vassilopoulou-Sellin, R.; Lee, J.E.; Evans, D.B. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery 2003, 134, 946–954. [Google Scholar] [CrossRef]
- Hoang, J.K.; Branstetter, B.F.t.; Gafton, A.R.; Lee, W.K.; Glastonbury, C.M. Imaging of thyroid carcinoma with CT and MRI: Approaches to common scenarios. Cancer Imaging 2013, 13, 128–139. [Google Scholar] [CrossRef]
- Pollard, D.B.; Weber, C.W.; Hudgins, P.A. Preoperative imaging of thyroid goiter: How imaging technique can influence anatomic appearance and create a potential for inaccurate interpretation. AJNR Am. J. Neuroradiol. 2005, 26, 1215–1217. [Google Scholar]
- King, A.D. Imaging for staging and management of thyroid cancer. Cancer Imaging 2008, 8, 57–69. [Google Scholar] [CrossRef]
- Wang, L.Y.; Ganly, I. Post-treatment surveillance of thyroid cancer. Eur. J. Surg. Oncol. 2018, 44, 357–366. [Google Scholar] [CrossRef]
- Haidar, M.; Kassas, M.; Chehade, F.; Chahinian, R.; Abi-Ghosn, J.; Haddad, M.M. The role of 18 F-FDG-PET/CT in the management of differentiated thyroid cancer. Nucl. Med. Commun. 2023, 44, 1046–1052. [Google Scholar] [CrossRef]
- Bogsrud, T.V.; Karantanis, D.; Nathan, M.A.; Mullan, B.P.; Wiseman, G.A.; Kasperbauer, J.L.; Reading, C.C.; Hay, I.D.; Lowe, V.J. 18F-FDG PET in the management of patients with anaplastic thyroid carcinoma. Thyroid 2008, 18, 713–719. [Google Scholar] [CrossRef]
- Agcaoglu, O.; Aliyev, S.; Mitchell, J.; Milas, M.; Siperstein, A.; Berber, E. The use of the harmonic scalpel versus knot tying for modified radical neck dissection. Surg. Innov. 2013, 20, 81–85. [Google Scholar] [CrossRef]
- Kebebew, E.; Clark, O.H. Differentiated thyroid cancer: “complete” rational approach. World J. Surg. 2000, 24, 942–951. [Google Scholar] [CrossRef]
- Bliss, R.D.; Gauger, P.G.; Delbridge, L.W. Surgeon’s approach to the thyroid gland: Surgical anatomy and the importance of technique. World J. Surg. 2000, 24, 891–897. [Google Scholar] [CrossRef]
- Calò, P.G.; Pisano, G.; Medas, F.; Pittau, M.R.; Gordini, L.; Demontis, R.; Nicolosi, A. Identification alone versus intraoperative neuromonitoring of the recurrent laryngeal nerve during thyroid surgery: Experience of 2034 consecutive patients. J. Otolaryngol. Head Neck Surg. 2014, 43, 16. [Google Scholar] [CrossRef]
- Demarchi, M.S.; Seeliger, B.; Lifante, J.C.; Alesina, P.F.; Triponez, F. Fluorescence Image-Guided Surgery for Thyroid Cancer: Utility for Preventing Hypoparathyroidism. Cancers 2021, 13, 3792. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, H.S.; Lee, K.D. Intraoperative real-time localization of parathyroid gland with near infrared fluorescence imaging. Gland Surg. 2017, 6, 516–524. [Google Scholar] [CrossRef]
- Fanaropoulou, N.M.; Chorti, A.; Markakis, M.; Papaioannou, M.; Michalopoulos, A.; Papavramidis, T. The use of Indocyanine green in endocrine surgery of the neck: A systematic review. Med. Baltim. 2019, 98, e14765. [Google Scholar] [CrossRef]
- Lennquist, S.; Cahlin, C.; Smeds, S. The superior laryngeal nerve in thyroid surgery. Surgery 1987, 102, 999–1008. [Google Scholar] [PubMed]
- Delbridge, L.; Reeve, T.S.; Khadra, M.; Poole, A.G. Total thyroidectomy: The technique of capsular dissection. ANZ J. Surg. 1992, 62, 96–99. [Google Scholar] [CrossRef]
- Schietroma, M.; Pessia, B.; Bianchi, Z.; De Vita, F.; Carlei, F.; Guadagni, S.; Amicucci, G.; Clementi, M. Thyroid Surgery: To Drain or Not to Drain, That Is the Problem—A Randomized Clinical Trial. ORL J. Otorhinolaryngol. Relat. Spec. 2017, 79, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Khairi, F.; Wijerathne, S. Electrosurgery and Energy Devices. In Mastering Endo-Laparoscopic and Thoracoscopic Surgery: ELSA Manual; Lomanto, D., Chen, W.T.-L., Fuentes, M.B., Eds.; Springer Nature: Singapore, 2023; pp. 19–24. [Google Scholar]
- Janssen, P.F.; Brölmann, H.A.M.; Huirne, J.A.F. Effectiveness of electrothermal bipolar vessel-sealing devices versus other electrothermal and ultrasonic devices for abdominal surgical hemostasis: A systematic review. Surg. Endosc. 2012, 26, 2892–2901. [Google Scholar] [CrossRef] [PubMed]
- Manouras, A.; Lagoudianakis, E.E.; Antonakis, P.T.; Filippakis, G.M.; Markogiannakis, H.; Kekis, P.B. Electrothermal bipolar vessel sealing system is a safe and time-saving alternative to classic suture ligation in total thyroidectomy. Head Neck 2005, 27, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Van Slycke, S.; Gillardin, J.-P.; Van Den Heede, K.; Minguet, J.; Vermeersch, H.; Brusselaers, N. Comparison of the harmonic focus and the thunderbeat for open thyroidectomy. Langenbeck’s Arch. Surg. 2016, 401, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, N.C.; de Kleijn, B.J.; Wedman, J.; van der Laan, B.; Plaat, B.E.C.; Halmos, G.B. A comparison of the Thunderbeat and standard electrocautery devices in head and neck surgery: A prospective randomized controlled trial. Eur. Arch. Otorhinolaryngol. 2021, 278, 4987–4996. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, Y.; Soh, E.Y. Central Lymph Node Metastasis Is an Important Prognostic Factor in Patients with Papillary Thyroid Microcarcinoma. J. Korean Med. Sci. 2014, 29, 48–52. [Google Scholar] [CrossRef]
- Robbins, K.T. Neck Dissection: Classification and Incisions; Shockley, V.Y., Pillsbury, H.C., Eds.; The neck, diagnosis and surgery; Mosby: St. Louis, MO, USA, 1994; pp. 381–389. [Google Scholar]
- Robbins, K.T.; Clayman, G.; Levine, P.A.; Medina, J.; Sessions, R.; Shaha, A.; Som, P.; Wolf, G.T. Neck dissection classification update: Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology–Head and Neck Surgery. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 751–758. [Google Scholar] [CrossRef]
- Crile, G. Excision of cancer of the head and neck. with special reference to the plan of dissection based on one hundred and thirty-two operations. J. Am. Med. Assoc. 1906, 47, 1780–1786. [Google Scholar] [CrossRef]
- Suarez, O. El problema de las metastasis linfaticas y alejadas del cancer de laringe e hipofaringe. Rev. De Otorrinolaringol. 1963, 23, 83–99. [Google Scholar]
- Gavilan, J.; Castro, A.; Rodrigánez, L.; Herranz-González, J. Functional and Selective Neck Dissection, 2nd ed.; Thieme: Stuttgart, Germany, 2020. [Google Scholar]
- Robbins, K.T.; Medina, J.E.; Wolfe, G.T.; Levine, P.A.; Sessions, R.B.; Pruet, C.W. Standardizing neck dissection terminology. Official report of the Academy’s Committee for Head and Neck Surgery and Oncology. Arch. Otolaryngol. Head Neck Surg. 1991, 117, 601–605. [Google Scholar] [CrossRef]
- Gršić, K.; Bumber, B.; Curić Radivojević, R.; Leović, D. Prophylactic Central Neck Dissection in Well-differentiated Thyroid Cancer. Acta Clin. Croat. 2020, 59 (Suppl. S1), 87–95. [Google Scholar] [CrossRef]
- Roh, J.L.; Kim, J.M.; Park, C.I. Central lymph node metastasis of unilateral papillary thyroid carcinoma: Patterns and factors predictive of nodal metastasis, morbidity, and recurrence. Ann. Surg. Oncol. 2011, 18, 2245–2250. [Google Scholar] [CrossRef]
- Townsend, C.M.; Beauchamp, R.D.; Evers, B.M.; Mattox, K.L. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Park, J.H.; Lee, Y.S.; Kim, B.W.; Chang, H.S.; Park, C.S. Skip lateral neck node metastases in papillary thyroid carcinoma. World J. Surg. 2012, 36, 743–747. [Google Scholar] [CrossRef]
- Cobin, R.H.; Gharib, H.; Bergman, D.A.; Clark, O.H.; Cooper, D.S.; Daniels, G.H.; Dickey, R.A.; Duick, D.S.; Garber, J.R.; Hay, I.D.; et al. AACE/AAES medical/surgical guidelines for clinical practice: Management of thyroid carcinoma. American Association of Clinical Endocrinologists. American College of Endocrinology. Endocr. Pract. 2001, 7, 202–220. [Google Scholar] [CrossRef]
- Stulak, J.M.; Grant, C.S.; Farley, D.R.; Thompson, G.B.; van Heerden, J.A.; Hay, I.D.; Reading, C.C.; Charboneau, J.W. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch. Surg. 2006, 141, 489–494. [Google Scholar] [CrossRef]
- Porterfield, J.R.; Factor, D.A.; Grant, C.S. Operative technique for modified radical neck dissection in papillary thyroid carcinoma. Arch. Surg. 2009, 144, 567–574. [Google Scholar] [CrossRef]
- Caron, N.R.; Tan, Y.Y.; Ogilvie, J.B.; Triponez, F.; Reiff, E.S.; Kebebew, E.; Duh, Q.Y.; Clark, O.H. Selective modified radical neck dissection for papillary thyroid cancer-is level I, II and V dissection always necessary? World J. Surg. 2006, 30, 833–840. [Google Scholar] [CrossRef]
- Neiderman, N.N.C.; Baris, H.; Duek, I.; Warshavsky, A.; Ringel, B.; Izkhakov, E.; Horowitz, G.; Fliss, D.M. Lateral Neck Dissection for Well-Differentiated Thyroid Carcinoma: Is Prophylactic Level V Neck Dissection Necessary? A Retrospective Cohort Study. Ear Nose Throat J. 2023, 102, NP349–NP357. [Google Scholar] [CrossRef]
- Mulholland, M.W.; Albo, D.; Dalman, R.; Hawn, M.; Hughes, S.; Sabel, M. Operative Techniques in Surgery; Wolters Kluwer Health: Philadelphia, PA, USA, 2014. [Google Scholar]
- Tae, K.; Ji, Y.B.; Song, C.M.; Ryu, J. Robotic and Endoscopic Thyroid Surgery: Evolution and Advances. Clin. Exp. Otorhinolaryngol. 2019, 12, 1. [Google Scholar] [CrossRef]
- Terris, D.J.; Singer, M.C. Minimally Invasive and Robotic Thyroid and Parathyroid Surgery; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Pisanu, A.; Podda, M.; Reccia, I.; Porceddu, G.; Uccheddu, A. Systematic review with meta-analysis of prospective randomized trials comparing minimally invasive video-assisted thyroidectomy (MIVAT) and conventional thyroidectomy (CT). Langenbecks Arch. Surg. 2013, 398, 1057–1068. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.W.; Han, X.D.; Di, J.Z.; Zheng, Q. Meta-analysis of comparison between minimally invasive video-assisted thyroidectomy and conventional thyroidectomy. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1381–1387. [Google Scholar]
- Miccoli, P.; Fregoli, L.; Rossi, L.; Papini, P.; Ambrosini, C.E.; Bakkar, S.; De Napoli, L.; Aghababyan, A.; Matteucci, V.; Materazzi, G. Minimally invasive video-assisted thyroidectomy (MIVAT). Gland Surg. 2020, 9, S1–S5. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, X.; Wang, X.; Xiang, J.; Chen, Z. Endoscopic thyroidectomy for differentiated thyroid cancer. Sci. World J. 2012, 2012, 456807. [Google Scholar] [CrossRef]
- Li, X.; Ding, W.; Zhang, H. Surgical outcomes of endoscopic thyroidectomy approaches for thyroid cancer: A systematic review and network meta-analysis. Front. Endocrinol. 2023, 14, 1256209. [Google Scholar] [CrossRef]
- Chen, X.D.; Peng, B.; Gong, R.X.; Wang, L.; Liao, B.; Li, C.L. Endoscopic thyroidectomy: An evidence-based research on feasibility, safety and clinical effectiveness. Chin. Med. J. Engl. 2008, 121, 2088–2094. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.M.; Makay, Ö.; Chang, H.; Kim, B.W.; Lee, Y.S.; Park, C.S.; Chang, H.S. Transoral endoscopic thyroidectomy using the vestibular approach with an endoscopic retractor in thyroid cancer: Experience with the first 132 patients. Surg. Endosc. 2020, 34, 5414–5420. [Google Scholar] [CrossRef]
- Chang, E.H.E.; Kim, H.Y.; Koh, Y.W.; Chung, W.Y. Overview of robotic thyroidectomy. Gland Surg. 2017, 6, 218–228. [Google Scholar] [CrossRef]
- Taskin, H.E.; Arslan, N.C.; Aliyev, S.; Berber, E. Robotic endocrine surgery: State of the art. World J. Surg. 2013, 37, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Chai, Y.J.; Dionigi, G.; Berber, E.; Tufano, R.P.; Kim, H.Y. Transoral Robotic Thyroidectomy for Papillary Thyroid Carcinoma: Perioperative Outcomes of 100 Consecutive Patients. World J. Surg. 2019, 43, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Shin, W.Y.; Yi, J.W. Single Surgeon Experience with 500 Cases of the Robotic Bilateral Axillary Breast Approach (BABA) for Thyroid Surgery Using the Da-Vinci Xi System. J. Clin. Med. 2021, 10, 4048. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.H.; Hong, H. Comparison of Various Thyroidectomy Approaches: A Retrospective Cross-sectional Study. Surg. Laparosc. Endosc. Percutan. Tech. 2023, 33, 632–639. [Google Scholar] [CrossRef]
- Tae, K.; Ji, Y.B.; Jeong, J.H.; Kim, K.R.; Choi, W.H.; Ahn, Y.H. Comparative study of robotic versus endoscopic thyroidectomy by a gasless unilateral axillo-breast or axillary approach. Head Neck 2013, 35, 477–484. [Google Scholar] [CrossRef]

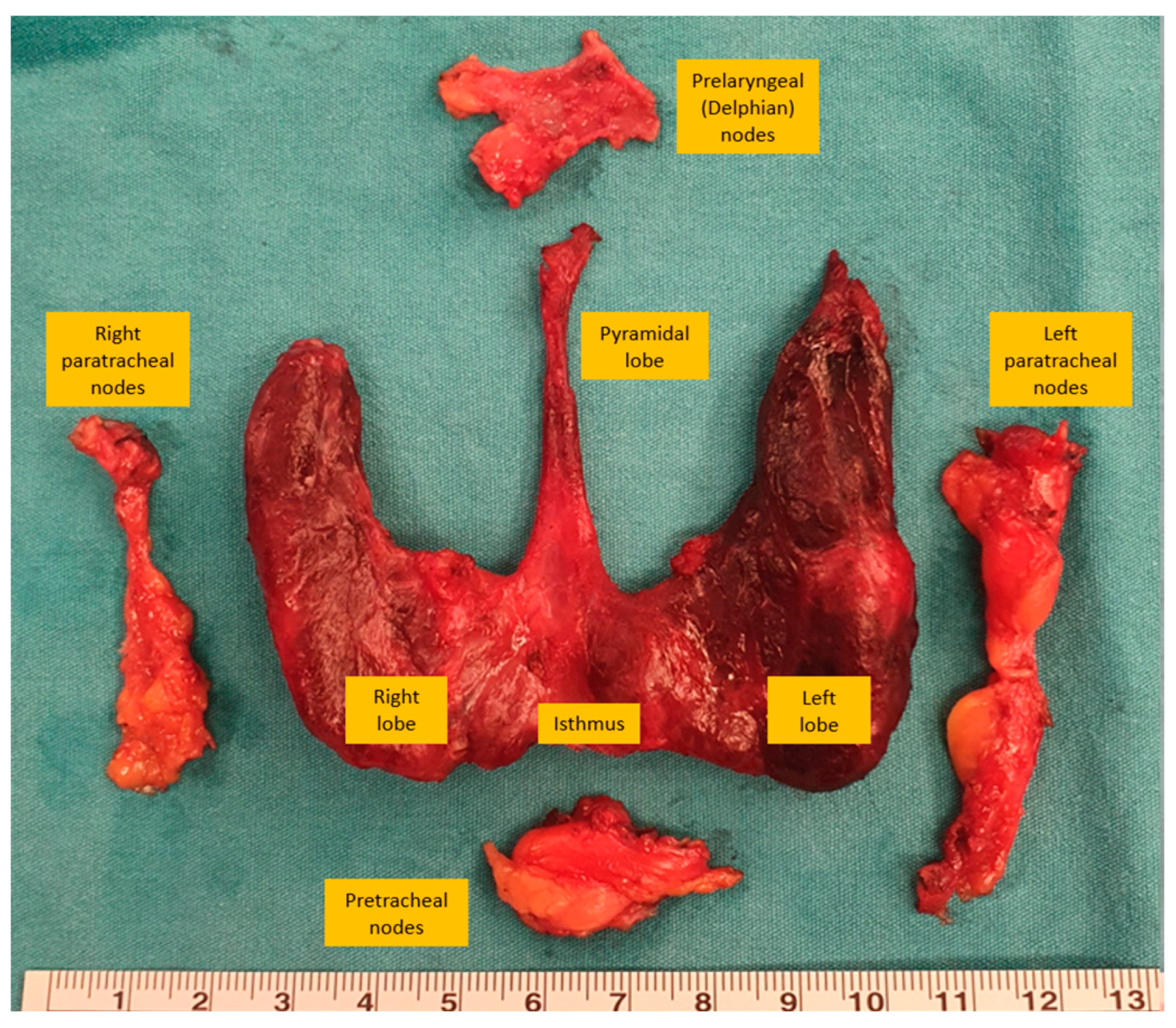

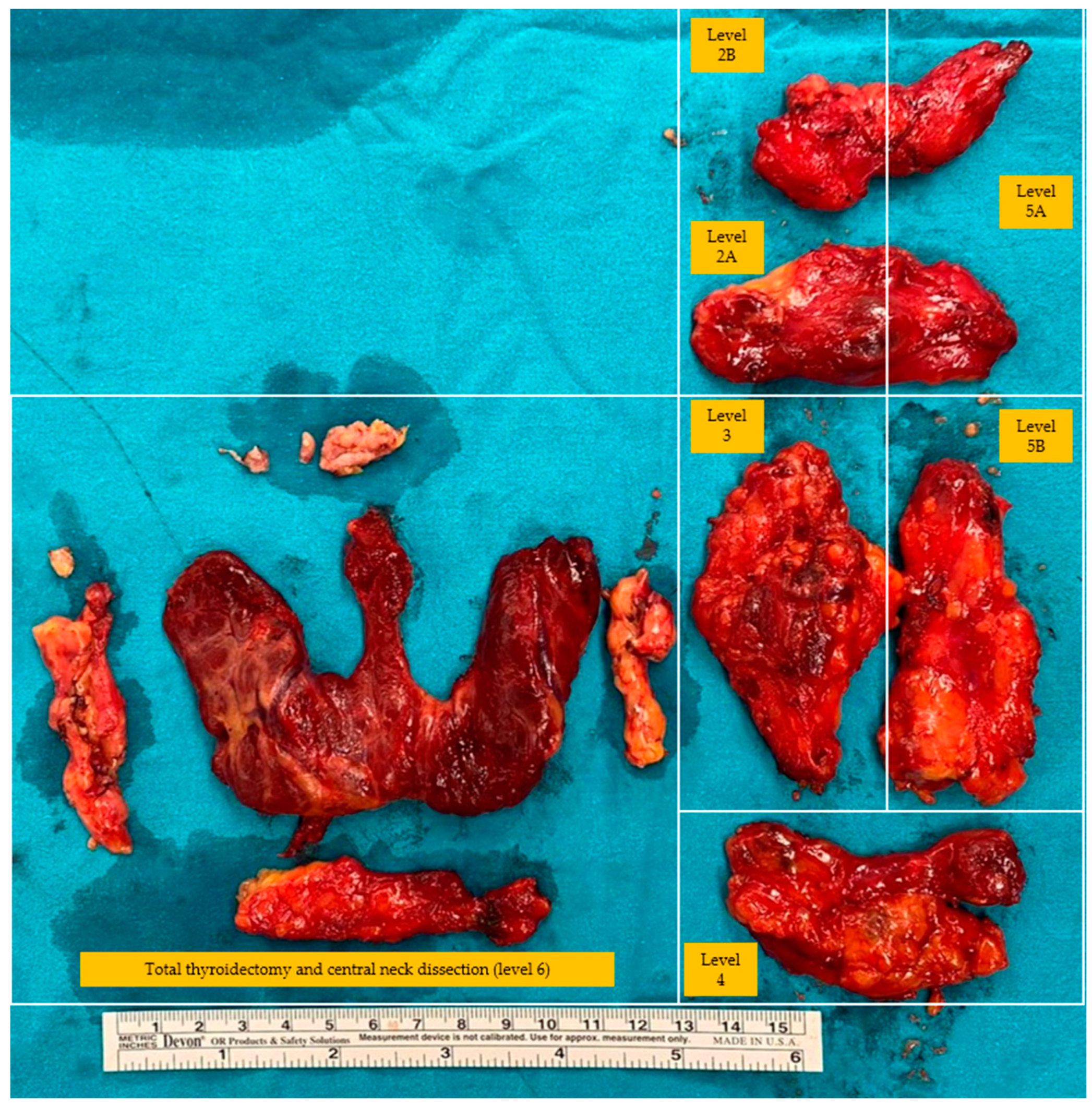
| Type of Neck Dissection | Description |
|---|---|
| Radical Neck Dissection | Excision of all ipsilateral cervical lymph node clusters (level I–level V). In addition, the spinal accessory nerve, internal jugular vein, and sternocleidomastoid muscle are excised. |
| Modified Radical Neck Dissection | Removal of all lymph nodes typically excised during the radical neck dissection with the retention of one or more nonlymphatic structures (i.e., the spinal accessory nerve, internal jugular vein, sternocleidomastoid muscle). |
| Selective Neck Dissection | Indicates a cervical lymphadenectomy in which there is the preservation of one or more of the lymph node clusters that are commonly excised in radical neck dissection. |
| Extended Neck Dissection | Refers to the excision of one or more additional lymph node clusters or nonlymphatic entities, or both, that are not covered by the radical neck dissection (examples of these lymph node clusters include parapharyngeal, superior mediastinal, perifacial, paratracheal; examples of nonlymphatic entities include the carotid artery, hypoglossal nerve, vagus nerve, and paraspinal muscles). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agcaoglu, O.; Sucu, S.; Toprak, S.; Tezelman, S. Techniques for Thyroidectomy and Functional Neck Dissection. J. Clin. Med. 2024, 13, 1914. https://doi.org/10.3390/jcm13071914
Agcaoglu O, Sucu S, Toprak S, Tezelman S. Techniques for Thyroidectomy and Functional Neck Dissection. Journal of Clinical Medicine. 2024; 13(7):1914. https://doi.org/10.3390/jcm13071914
Chicago/Turabian StyleAgcaoglu, Orhan, Serkan Sucu, Safa Toprak, and Serdar Tezelman. 2024. "Techniques for Thyroidectomy and Functional Neck Dissection" Journal of Clinical Medicine 13, no. 7: 1914. https://doi.org/10.3390/jcm13071914
APA StyleAgcaoglu, O., Sucu, S., Toprak, S., & Tezelman, S. (2024). Techniques for Thyroidectomy and Functional Neck Dissection. Journal of Clinical Medicine, 13(7), 1914. https://doi.org/10.3390/jcm13071914






