Ventilator Weaning in Prolonged Mechanical Ventilation—A Narrative Review
Abstract
1. Introduction
2. Prolonged MV, Ventilator Dependence and Long-Term Ventilation
3. The Role of Tracheostomy in Prolonged MV
4. Factors Contributing to Prolonged MV
5. Acute Respiratory Failure and Prolonged MV
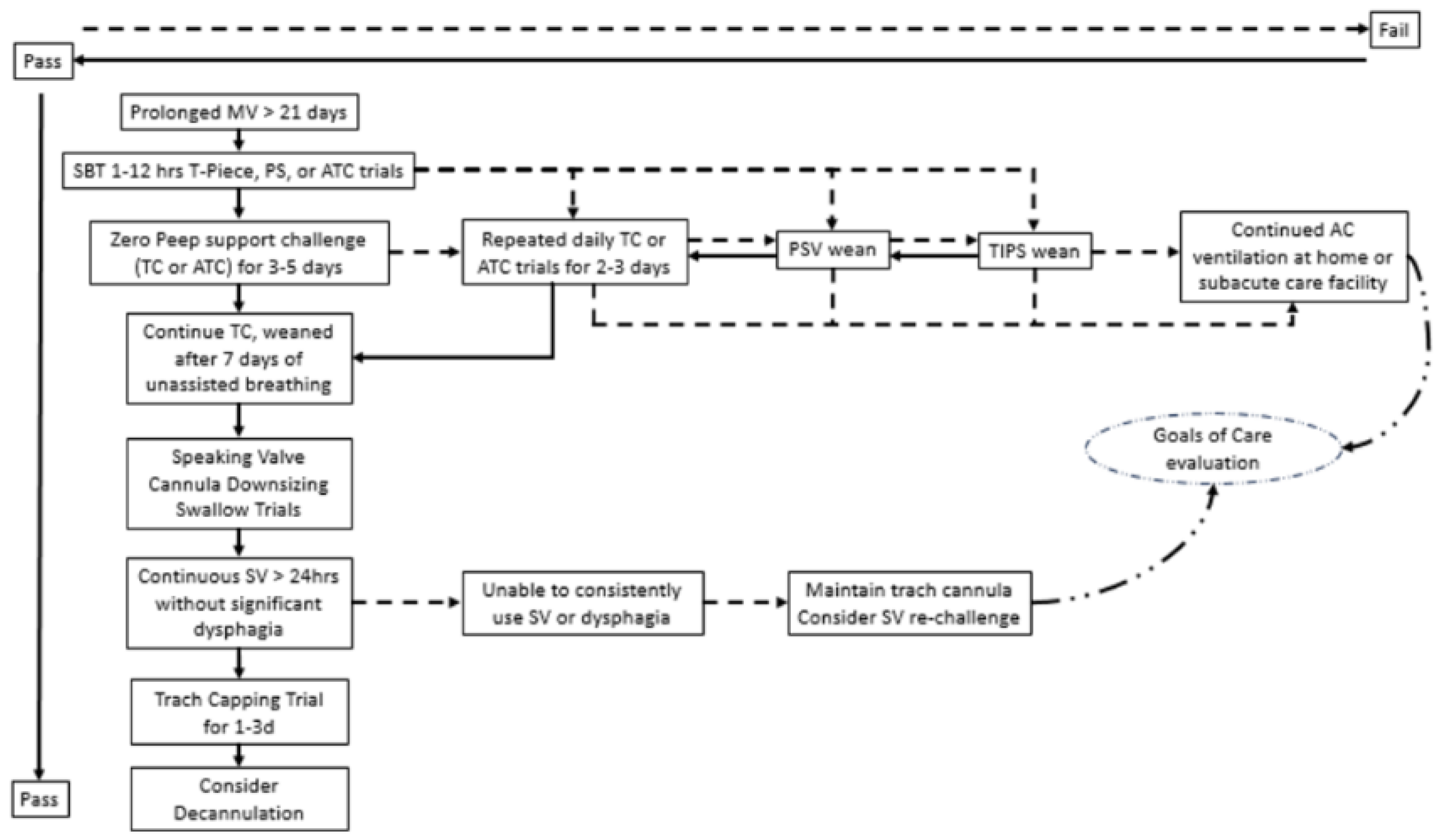
6. Spontaneous Breathing Trials
7. Ventilator Weaning and Liberation
8. Modes of Ventilator Weaning in Prolonged MV
9. The Role of Non-Invasive Ventilation in Prolonged MV Weaning
10. Use of Speaking Valves with MV and Weaning
11. Tracheostomy Decannulation
12. The Role of Complex Rehabilitation
13. Location of Ventilator Weaning
14. Goal-Concordant Care for Patients with Prolonged MV
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wunsch, H.; Linde-Zwirble, W.T.; Angus, D.C.; Hartman, M.E.; Milbrandt, E.B.; Kahn, J.M. The epidemiology of mechanical ventilation use in the United States. Crit. Care Med. 2010, 38, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Jivraj, N.K.; Hill, A.D.; Shieh, M.S.; Hua, M.; Gershengorn, H.B.; Ferrando-Vivas, P.; Harrison, D.; Rowan, K.; Lindenauer, P.K.; Wunsch, H. Use of Mechanical Ventilation across 3 Countries. JAMA Intern. Med. 2023, 183, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Anzueto, A.; Frutos, F.; Alia, I.; Brochard, L.; Stewart, T.E.; Benito, S.; Epstein, S.K.; Apezteguia, C.; Nightingale, P.; et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA 2002, 287, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Brochard, L.; Rauss, A.; Benito, S.; Conti, G.; Mancebo, J.; Rekik, N.; Gasparetto, A.; Lemaire, F. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am. J. Respir. Crit. Care Med. 1994, 150, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Frutos, F.; Tobin, M.J.; Alia, I.; Solsona, J.F.; Valverdu, I.; Fernandez, R.; de la Cal, M.A.; Benito, S.; Tomas, R.; et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N. Engl. J. Med. 1995, 332, 345–350. [Google Scholar] [CrossRef]
- Ely, E.W.; Baker, A.M.; Dunagan, D.P.; Burke, H.L.; Smith, A.C.; Kelly, P.T.; Johnson, M.M.; Browder, R.W.; Bowton, D.L.; Haponik, E.F. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N. Engl. J. Med. 1996, 335, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Boles, J.M.; Bion, J.; Connors, A.; Herridge, M.; Marsh, B.; Melot, C.; Pearl, R.; Silverman, H.; Stanchina, M.; Vieillard-Baron, A.; et al. Weaning from mechanical ventilation. Eur. Respir. J. 2007, 29, 1033–1056. [Google Scholar] [CrossRef]
- Beduneau, G.; Pham, T.; Schortgen, F.; Piquilloud, L.; Zogheib, E.; Jonas, M.; Grelon, F.; Runge, I.; Nicolas, T.; Grange, S.; et al. Epidemiology of Weaning Outcome according to a New Definition. The WIND Study. Am. J. Respir. Crit. Care Med. 2017, 195, 772–783. [Google Scholar] [CrossRef]
- Hill, A.D.; Fowler, R.A.; Burns, K.E.; Rose, L.; Pinto, R.L.; Scales, D.C. Long-Term Outcomes and Health Care Utilization after Prolonged Mechanical Ventilation. Ann. Am. Thorac. Soc. 2017, 14, 355–362. [Google Scholar] [CrossRef]
- Kahn, J.M.; Le, T.; Angus, D.C.; Cox, C.E.; Hough, C.L.; White, D.B.; Yende, S.; Carson, S.S.; ProVent Study Group Investigators. The epidemiology of chronic critical illness in the United States*. Crit. Care Med. 2015, 43, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Jubran, A.; Grant, B.J.B.; Duffner, L.A.; Collins, E.G.; Lanuza, D.M.; Hoffman, L.A.; Tobin, M.J. Long-Term Outcome after Prolonged Mechanical Ventilation. A Long-Term Acute-Care Hospital Study. Am. J. Respir. Crit. Care Med. 2019, 199, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.E.; Carson, S.S.; Govert, J.A.; Chelluri, L.; Sanders, G.D. An economic evaluation of prolonged mechanical ventilation. Crit. Care Med. 2007, 35, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, N.R.; Epstein, S.K.; Carson, S.; Scheinhorn, D.; Christopher, K.; Muldoon, S. Management of patients requiring prolonged mechanical ventilation: Report of a NAMDRC consensus conference. Chest 2005, 128, 3937–3954. [Google Scholar] [CrossRef]
- Make, B.J.; Hill, N.S.; Goldberg, A.I.; Bach, J.R.; Criner, G.J.; Dunne, P.E.; Gilmartin, M.E.; Heffner, J.E.; Kacmarek, R.; Keens, T.G.; et al. Mechanical ventilation beyond the intensive care unit. Report of a consensus conference of the American College of Chest Physicians. Chest 1998, 113, 289S–344S. [Google Scholar] [CrossRef] [PubMed]
- Sahetya, S.; Allgood, S.; Gay, P.C.; Lechtzin, N. Long-Term Mechanical Ventilation. Clin. Chest Med. 2016, 37, 753–763. [Google Scholar] [CrossRef] [PubMed]
- King, A.C. Long-term home mechanical ventilation in the United States. Respir. Care 2012, 57, 921–930, discussion 930–922. [Google Scholar] [CrossRef] [PubMed]
- Scheinhorn, D.J.; Hassenpflug, M.S.; Votto, J.J.; Chao, D.C.; Epstein, S.K.; Doig, G.S.; Knight, E.B.; Petrak, R.A.; Ventilation Outcomes Study Group. Post-ICU mechanical ventilation at 23 long-term care hospitals: A multicenter outcomes study. Chest 2007, 131, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lone, N.I.; Walsh, T.S. Prolonged mechanical ventilation in critically ill patients: Epidemiology, outcomes and modelling the potential cost consequences of establishing a regional weaning unit. Crit. Care 2011, 15, R102. [Google Scholar] [CrossRef]
- Stauffer, J.L.; Olson, D.E.; Petty, T.L. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am. J. Med. 1981, 70, 65–76. [Google Scholar] [CrossRef]
- Cox, C.E.; Carson, S.S.; Holmes, G.M.; Howard, A.; Carey, T.S. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993–2002. Crit. Care Med. 2004, 32, 2219–2226. [Google Scholar] [CrossRef]
- Burns, K.E.A.; Rizvi, L.; Cook, D.J.; Lebovic, G.; Dodek, P.; Villar, J.; Slutsky, A.S.; Jones, A.; Kapadia, F.N.; Gattas, D.J.; et al. Ventilator Weaning and Discontinuation Practices for Critically Ill Patients. JAMA 2021, 325, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.B.; Syeda, S.N.; Bajpayee, L.; Cooke, C.R.; Walkey, A.J.; Wiener, R.S. Trends in Tracheostomy for Mechanically Ventilated Patients in the United States, 1993–2012. Am. J. Respir. Crit. Care Med. 2015, 192, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Chorath, K.; Hoang, A.; Rajasekaran, K.; Moreira, A. Association of Early vs Late Tracheostomy Placement With Pneumonia and Ventilator Days in Critically Ill Patients: A Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 450–459. [Google Scholar] [CrossRef]
- Kollef, M.H.; Levy, N.T.; Ahrens, T.S.; Schaiff, R.; Prentice, D.; Sherman, G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest 1998, 114, 541–548. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Gajic, O.; Afessa, B.; Thompson, B.T.; Frutos-Vivar, F.; Malinchoc, M.; Rubenfeld, G.D.; Esteban, A.; Anzueto, A.; Hubmayr, R.D.; the Second International Study of Mechanical Ventilation and ARDS-net Investigators; et al. Prediction of death and prolonged mechanical ventilation in acute lung injury. Crit. Care 2007, 11, R53. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A.; Network, N.A. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]
- Redaelli, S.; von Wedel, D.; Fosset, M.; Suleiman, A.; Chen, G.; Alingrin, J.; Gong, M.N.; Gajic, O.; Goodspeed, V.; Talmor, D.; et al. Inflammatory subphenotypes in patients at risk of ARDS: Evidence from the LIPS-A trial. Intensive Care Med. 2023, 49, 1499–1507. [Google Scholar] [CrossRef]
- Sinha, P.; Delucchi, K.L.; Chen, Y.; Zhuo, H.; Abbott, J.; Wang, C.; Wickersham, N.; McNeil, J.B.; Jauregui, A.; Ke, S.; et al. Latent class analysis-derived subphenotypes are generalisable to observational cohorts of acute respiratory distress syndrome: A prospective study. Thorax 2022, 77, 13–21. [Google Scholar] [CrossRef]
- Pham, T.; Heunks, L.; Bellani, G.; Madotto, F.; Aragao, I.; Beduneau, G.; Goligher, E.C.; Grasselli, G.; Laake, J.H.; Mancebo, J.; et al. Weaning from mechanical ventilation in intensive care units across 50 countries (WEAN SAFE): A multicentre, prospective, observational cohort study. Lancet Respir. Med. 2023, 11, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Shapiro, S.D.; Silver, P.; St John, R.E.; Prentice, D.; Sauer, S.; Ahrens, T.S.; Shannon, W.; Baker-Clinkscale, D. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit. Care Med. 1997, 25, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Thille, A.W.; Gacouin, A.; Coudroy, R.; Ehrmann, S.; Quenot, J.P.; Nay, M.A.; Guitton, C.; Contou, D.; Labro, G.; Reignier, J.; et al. Spontaneous-Breathing Trials with Pressure-Support Ventilation or a T-Piece. N. Engl. J. Med. 2022, 387, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Subira, C.; Hernandez, G.; Vazquez, A.; Rodriguez-Garcia, R.; Gonzalez-Castro, A.; Garcia, C.; Rubio, O.; Ventura, L.; Lopez, A.; de la Torre, M.C.; et al. Effect of Pressure Support vs T-Piece Ventilation Strategies During Spontaneous Breathing Trials on Successful Extubation Among Patients Receiving Mechanical Ventilation: A Randomized Clinical Trial. JAMA 2019, 321, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Vitacca, M.; Vianello, A.; Colombo, D.; Clini, E.; Porta, R.; Bianchi, L.; Arcaro, G.; Vitale, G.; Guffanti, E.; Lo Coco, A.; et al. Comparison of two methods for weaning patients with chronic obstructive pulmonary disease requiring mechanical ventilation for more than 15 days. Am. J. Respir. Crit. Care Med. 2001, 164, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.A.; Girard, T.D.; Kress, J.P.; Morris, P.E.; Ouellette, D.R.; Alhazzani, W.; Burns, S.M.; Epstein, S.K.; Esteban, A.; Fan, E.; et al. Liberation From Mechanical Ventilation in Critically Ill Adults: Executive Summary of an Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline. Chest 2017, 151, 160–165. [Google Scholar] [CrossRef]
- Esteban, A.; Alia, I.; Gordo, F.; Fernandez, R.; Solsona, J.F.; Vallverdu, I.; Macias, S.; Allegue, J.M.; Blanco, J.; Carriedo, D.; et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am. J. Respir. Crit. Care Med. 1997, 156, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Alia, I.; Tobin, M.J.; Gil, A.; Gordo, F.; Vallverdu, I.; Blanch, L.; Bonet, A.; Vazquez, A.; de Pablo, R.; et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am. J. Respir. Crit. Care Med. 1999, 159, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Jubran, A.; Grant, B.J.; Duffner, L.A.; Collins, E.G.; Lanuza, D.M.; Hoffman, L.A.; Tobin, M.J. Effect of pressure support vs unassisted breathing through a tracheostomy collar on weaning duration in patients requiring prolonged mechanical ventilation: A randomized trial. JAMA 2013, 309, 671–677. [Google Scholar] [CrossRef]
- Wu, C.H.; Lin, F.C.; Jerng, J.S.; Shin, M.H.; Wang, Y.C.; Lee, C.J.; Lin, L.M.; Lin, N.H.; Kuo, Y.W.; Ku, S.C.; et al. Automatic tube compensation for liberation from prolonged mechanical ventilation in tracheostomized patients: A retrospective analysis. J. Formos. Med. Assoc. 2023, 122, 1132–1140. [Google Scholar] [CrossRef]
- Chao, D.C.; Scheinhorn, D.J. Determining the best threshold of rapid shallow breathing index in a therapist-implemented patient-specific weaning protocol. Respir. Care 2007, 52, 159–165. [Google Scholar] [PubMed]
- Yang, T.M.; Chen, L.; Lin, C.M.; Lin, H.L.; Fang, T.P.; Ge, H.; Cai, H.; Hong, Y.; Zhang, Z. Identifying Novel Clusters of Patients With Prolonged Mechanical Ventilation Using Trajectories of Rapid Shallow Breathing Index. Front. Med. 2022, 9, 880896. [Google Scholar] [CrossRef]
- Blackwood, B.; Alderdice, F.; Burns, K.; Cardwell, C.; Lavery, G.; O’Halloran, P. Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta-analysis. BMJ 2011, 342, c7237. [Google Scholar] [CrossRef]
- Kirakli, C.; Ediboglu, O.; Naz, I.; Cimen, P.; Tatar, D. Effectiveness and safety of a protocolized mechanical ventilation and weaning strategy of COPD patients by respiratory therapists. J. Thorac. Dis. 2014, 6, 1180–1186. [Google Scholar] [CrossRef]
- Scheinhorn, D.J.; Chao, D.C.; Stearn-Hassenpflug, M.; Wallace, W.A. Outcomes in post-ICU mechanical ventilation: A therapist-implemented weaning protocol. Chest 2001, 119, 236–242. [Google Scholar] [CrossRef]
- Surani, S.; Sharma, M.; Middagh, K.; Bernal, H.; Varon, J.; Ratnani, I.; Anjum, H.; Khan, A. Weaning from Mechanical Ventilator in a Long-term Acute Care Hospital: A Retrospective Analysis. Open Respir. Med. J. 2020, 14, 62–66. [Google Scholar] [CrossRef]
- Kaufman, M.R.; Bauer, T.; Onders, R.P.; Brown, D.P.; Chang, E.I.; Rossi, K.; Elkwood, A.I.; Paulin, E.; Jarrahy, R. Treatment for bilateral diaphragmatic dysfunction using phrenic nerve reconstruction and diaphragm pacemakers. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 753–760. [Google Scholar] [CrossRef]
- Panelli, A.; Verfuss, M.A.; Dres, M.; Brochard, L.; Schaller, S.J. Phrenic nerve stimulation to prevent diaphragmatic dysfunction and ventilator-induced lung injury. Intensive Care Med. Exp. 2023, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.; Shure, D.; Clark, L.; Criner, G.J.; Dres, M.; de Abreu, M.G.; Laghi, F.; McDonagh, D.; Petrof, B.; Nelson, T.; et al. Temporary transvenous diaphragm pacing vs. standard of care for weaning from mechanical ventilation: Study protocol for a randomized trial. Trials 2019, 20, 60. [Google Scholar] [CrossRef]
- Nava, S.; Ambrosino, N.; Clini, E.; Prato, M.; Orlando, G.; Vitacca, M.; Brigada, P.; Fracchia, C.; Rubini, F. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann. Intern. Med. 1998, 128, 721–728. [Google Scholar] [CrossRef]
- Keenan, S.P.; Powers, C.; McCormack, D.G.; Block, G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: A randomized controlled trial. JAMA 2002, 287, 3238–3244. [Google Scholar] [CrossRef] [PubMed]
- Sancho, J.; Servera, E.; Jara-Palomares, L.; Barrot, E.; Sanchez-Oro-Gomez, R.; Gomez de Terreros, F.J.; Martin-Vicente, M.J.; Utrabo, I.; Nunez, M.B.; Binimelis, A.; et al. Noninvasive ventilation during the weaning process in chronically critically ill patients. ERJ Open Res. 2016, 2, 00061–2016. [Google Scholar] [CrossRef] [PubMed]
- Ceriana, P.; Carlucci, A.; Navalesi, P.; Rampulla, C.; Delmastro, M.; Piaggi, G.; De Mattia, E.; Nava, S. Weaning from tracheotomy in long-term mechanically ventilated patients: Feasibility of a decisional flowchart and clinical outcome. Intensive Care Med. 2003, 29, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Schorr, F.; Genta, P.R.; Gregorio, M.G.; Danzi-Soares, N.J.; Lorenzi-Filho, G. Continuous positive airway pressure delivered by oronasal mask may not be effective for obstructive sleep apnoea. Eur. Respir. J. 2012, 40, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Lebret, M.; Leotard, A.; Pepin, J.L.; Windisch, W.; Ekkernkamp, E.; Pallero, M.; Sanchez-Quiroga, M.A.; Hart, N.; Kelly, J.L.; Patout, M.; et al. Nasal versus oronasal masks for home non-invasive ventilation in patients with chronic hypercapnia: A systematic review and individual participant data meta-analysis. Thorax 2021, 76, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.F.; Benitez, I.; Sanchez-Quiroga, M.A.; Gomez de Terreros, F.J.; Corral, J.; Romero, A.; Caballero-Eraso, C.; Alonso-Alvarez, M.L.; Ordax-Carbajo, E.; Gomez-Garcia, T.; et al. Long-term Noninvasive Ventilation in Obesity Hypoventilation Syndrome Without Severe OSA: The Pickwick Randomized Controlled Trial. Chest 2020, 158, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Leotard, A.; Lebret, M.; Daabek, N.; Prigent, H.; Destors, M.; Saint-Raymond, C.; Sagniez, A.; Leroux, K.; Tamisier, R.; Lofaso, F.; et al. Impact of Interface Type on Noninvasive Ventilation Efficacy in Patients With Neuromuscular Disease: A Randomized Cross-Over Trial. Arch. Bronconeumol. 2021, 57, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.; Storre, J.H.; Schmoor, C.; Windisch, W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: A randomised crossover trial. Thorax 2010, 65, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, P.; Portacci, A.; Carpagnano, G.E.; Banfi, P.; Crimi, C.; Misseri, G.; Gregoretti, C. The right interface for the right patient in noninvasive ventilation: A systematic review. Expert. Rev. Respir. Med. 2022, 16, 931–944. [Google Scholar] [CrossRef]
- Di Mussi, R.; Spadaro, S.; Stripoli, T.; Volta, C.A.; Trerotoli, P.; Pierucci, P.; Staffieri, F.; Bruno, F.; Camporota, L.; Grasso, S. High-flow nasal cannula oxygen therapy decreases postextubation neuroventilatory drive and work of breathing in patients with chronic obstructive pulmonary disease. Crit. Care 2018, 22, 180. [Google Scholar] [CrossRef]
- Tan, D.; Walline, J.H.; Ling, B.; Xu, Y.; Sun, J.; Wang, B.; Shan, X.; Wang, Y.; Cao, P.; Zhu, Q.; et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: A multicenter, randomized controlled trial. Crit. Care 2020, 24, 489. [Google Scholar] [CrossRef] [PubMed]
- Spoletini, G.; Alotaibi, M.; Blasi, F.; Hill, N.S. Heated Humidified High-Flow Nasal Oxygen in Adults: Mechanisms of Action and Clinical Implications. Chest 2015, 148, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Fasano, L.; Corcione, N.; Comellini, V.; Musti, M.A.; Brandao, M.; Bottone, D.; Calderini, E.; Navalesi, P.; Nava, S. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax 2017, 72, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Horie, T.; Chohnabayashi, N.; Jinta, T.; Tsugitomi, R.; Shiraki, A.; Tokioka, F.; Kadowaki, T.; Watanabe, A.; Fukui, M.; et al. Home High-Flow Nasal Cannula Oxygen Therapy for Stable Hypercapnic COPD: A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 206, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- DeVita, M.A.; Spierer-Rundback, L. Swallowing disorders in patients with prolonged orotracheal intubation or tracheostomy tubes. Crit. Care Med. 1990, 18, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Prigent, H.; Lejaille, M.; Terzi, N.; Annane, D.; Figere, M.; Orlikowski, D.; Lofaso, F. Effect of a tracheostomy speaking valve on breathing-swallowing interaction. Intensive Care Med. 2012, 38, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Passy, V.; Baydur, A.; Prentice, W.; Darnell-Neal, R. Passy-Muir tracheostomy speaking valve on ventilator-dependent patients. Laryngoscope 1993, 103, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.A.; Cole, T.D.K.; Percha, C.M.; Asanuma, N.; Mattare, K.; Hager, D.N.; Brenner, M.J.; Pandian, V. Standard versus Accelerated Speaking Valve Placement after Percutaneous Tracheostomy: A Randomized Controlled Feasibility Study. Ann. Am. Thorac. Soc. 2021, 18, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Sutt, A.L.; Anstey, C.M.; Caruana, L.R.; Cornwell, P.L.; Fraser, J.F. Ventilation distribution and lung recruitment with speaking valve use in tracheostomised patient weaning from mechanical ventilation in intensive care. J. Crit. Care 2017, 40, 164–170. [Google Scholar] [CrossRef]
- Freeman-Sanderson, A.L.; Togher, L.; Elkins, M.R.; Phipps, P.R. Return of Voice for Ventilated Tracheostomy Patients in ICU: A Randomized Controlled Trial of Early-Targeted Intervention. Crit. Care Med. 2016, 44, 1075–1081. [Google Scholar] [CrossRef]
- de Mestral, C.; Iqbal, S.; Fong, N.; LeBlanc, J.; Fata, P.; Razek, T.; Khwaja, K. Impact of a specialized multidisciplinary tracheostomy team on tracheostomy care in critically ill patients. Can. J. Surg. 2011, 54, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, M.; Toyama, M.; Imura, H.; Takahashi, Y.; Nakayama, T. Tracheostomy decannulation rates in Japan: A retrospective cohort study using a claims database. Sci. Rep. 2022, 12, 19801. [Google Scholar] [CrossRef] [PubMed]
- Tobin, A.E.; Santamaria, J.D. An intensivist-led tracheostomy review team is associated with shorter decannulation time and length of stay: A prospective cohort study. Crit. Care 2008, 12, R48. [Google Scholar] [CrossRef]
- Christopher, K.L. Tracheostomy decannulation. Respir. Care 2005, 50, 538–541. [Google Scholar]
- Choate, K.; Barbetti, J.; Currey, J. Tracheostomy decannulation failure rate following critical illness: A prospective descriptive study. Aust. Crit. Care 2009, 22, 8–15. [Google Scholar] [CrossRef]
- O’Connor, H.H.; White, A.C. Tracheostomy decannulation. Respir. Care 2010, 55, 1076–1081. [Google Scholar]
- Diaz-Abad, M.; Verceles, A.C.; Brown, J.E.; Scharf, S.M. Sleep-disordered breathing may be under-recognized in patients who wean from prolonged mechanical ventilation. Respir. Care 2012, 57, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Stelfox, H.T.; Crimi, C.; Berra, L.; Noto, A.; Schmidt, U.; Bigatello, L.M.; Hess, D. Determinants of tracheostomy decannulation: An international survey. Crit. Care 2008, 12, R26. [Google Scholar] [CrossRef] [PubMed]
- Sutt, A.L.; Caruana, L.R.; Dunster, K.R.; Cornwell, P.L.; Anstey, C.M.; Fraser, J.F. Speaking valves in tracheostomised ICU patients weaning off mechanical ventilation--do they facilitate lung recruitment? Crit. Care 2016, 20, 91. [Google Scholar] [CrossRef]
- Hernandez Martinez, G.; Rodriguez, M.L.; Vaquero, M.C.; Ortiz, R.; Masclans, J.R.; Roca, O.; Colinas, L.; de Pablo, R.; Espinosa, M.D.; Garcia-de-Acilu, M.; et al. High-Flow Oxygen with Capping or Suctioning for Tracheostomy Decannulation. N. Engl. J. Med. 2020, 383, 1009–1017. [Google Scholar] [CrossRef]
- Winck, J.; Camacho, R.; Ambrosino, N. Multidisciplinary rehabilitation in ventilator-dependent patients: Call for action in specialized inpatient facilities. Rev. Port. Pneumol. 2015, 21, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Dolinay, T.; Jun, D.; Chen, L.; Gornbein, J. Mechanical Ventilator Liberation of Patients With COVID-19 in Long-term Acute Care Hospital. Chest 2022, 161, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Laghi, F.A., Jr.; Brofman, J.; Undevia, N.S.; Shaikh, H. Long-Term Acute Care Hospital Outcomes of Mechanically Ventilated Patients with Coronavirus Disease 2019. Crit. Care Med. 2022, 50, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, D.V.; Bailey, M.J.; Treacher, D.F.; Hamid, S.; Williams, A.J.; Davidson, A.C. Outcomes, cost and long term survival of patients referred to a regional weaning centre. Thorax 2005, 60, 187–192. [Google Scholar] [CrossRef]
- Kahn, J.M.; Davis, B.S.; Le, T.Q.; Yabes, J.G.; Chang, C.H.; Angus, D.C. Variation in mortality rates after admission to long-term acute care hospitals for ventilator weaning. J. Crit. Care 2018, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Herer, B. Outcomes of Prolonged Mechanical Ventilation Before and After Implementation of a Respiratory ICU. Respir. Care 2020, 65, 1011–1018. [Google Scholar] [CrossRef]
- Lu, H.M.; Chen, L.; Wang, J.D.; Hung, M.C.; Lin, M.S.; Yan, Y.H.; Chen, C.R.; Fan, P.S.; Huang, L.C.; Kuo, K.N. Outcomes of prolonged mechanic ventilation: A discrimination model based on longitudinal health insurance and death certificate data. BMC Health Serv. Res. 2012, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, M.; Wijkstra, P.J.; McKim, D.; Benditt, J.; Winck, J.C.; Nasilowski, J.; Borel, J.C. Building a home ventilation programme: Population, equipment, delivery and cost. Thorax 2022, 77, 1140–1148. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Marcus, E.L.; Stessman, J. Prolonged Mechanical Ventilation: A Comparison of Patients Treated at Home Compared With Hospital Long-Term Care. J. Am. Med. Dir. Assoc. 2021, 22, 418–424. [Google Scholar] [CrossRef]
- Ackrivo, J.; Elman, L.; Hansen-Flaschen, J. Telemonitoring for Home-assisted Ventilation: A Narrative Review. Ann. Am. Thorac. Soc. 2021, 18, 1761–1772. [Google Scholar] [CrossRef]
- Boussaid, G.; Lofaso, F.; Santos, D.B.; Vaugier, I.; Pottier, S.; Prigent, H.; Bahrami, S.; Orlikowski, D. Impact of invasive ventilation on survival when non-invasive ventilation is ineffective in patients with Duchenne muscular dystrophy: A prospective cohort. Respir. Med. 2016, 115, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Arcaro, G.; Palmieri, A.; Ermani, M.; Braccioni, F.; Gallan, F.; Soraru, G.; Pegoraro, E. Survival and quality of life after tracheostomy for acute respiratory failure in patients with amyotrophic lateral sclerosis. J. Crit. Care 2011, 26, 329.e7–329.e14. [Google Scholar] [CrossRef] [PubMed]
- Radunovic, A.; Annane, D.; Rafiq, M.K.; Brassington, R.; Mustfa, N. Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 2017, 10, CD004427. [Google Scholar] [CrossRef]
- Mifsud Bonnici, D.; Sanctuary, T.; Warren, A.; Murphy, P.B.; Steier, J.; Marino, P.; Pattani, H.; Creagh-Brown, B.C.; Hart, N. Prospective observational cohort study of patients with weaning failure admitted to a specialist weaning, rehabilitation and home mechanical ventilation centre. BMJ Open 2016, 6, e010025. [Google Scholar] [CrossRef] [PubMed]
- Bornitz, F.; Ewert, R.; Knaak, C.; Magnet, F.S.; Windisch, W.; Herth, F. Weaning from Invasive Ventilation in Specialist Centers Following Primary Weaning Failure. Dtsch. Arztebl. Int. 2020, 117, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ghiani, A.; Tsitouras, K.; Paderewska, J.; Milger, K.; Walcher, S.; Weiffenbach, M.; Neurohr, C.; Kneidinger, N. Incidence, causes, and predictors of unsuccessful decannulation following prolonged weaning. Ther. Adv. Chronic Dis. 2022, 13, 20406223221109655. [Google Scholar] [CrossRef] [PubMed]
- Lanken, P.N.; Terry, P.B.; Delisser, H.M.; Fahy, B.F.; Hansen-Flaschen, J.; Heffner, J.E.; Levy, M.; Mularski, R.A.; Osborne, M.L.; Prendergast, T.J.; et al. An official American Thoracic Society clinical policy statement: Palliative care for patients with respiratory diseases and critical illnesses. Am. J. Respir. Crit. Care Med. 2008, 177, 912–927. [Google Scholar] [CrossRef]
- Nava, S.; Sturani, C.; Hartl, S.; Magni, G.; Ciontu, M.; Corrado, A.; Simonds, A.; on behalf of the European Respiratory Society Task Force on Ethics and Decision-Making in End Stage Lung Disease. End-of-life decision-making in respiratory intermediate care units: A European survey. Eur. Respir. J. 2007, 30, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sumarsono, N.; Sudore, R.L.; Smith, A.K.; Pantilat, S.Z.; Anderson, W.G.; Makam, A.N. Availability of Palliative Care in Long-Term Acute Care Hospitals. J. Am. Med. Dir. Assoc. 2021, 22, 2207–2211. [Google Scholar] [CrossRef]
- Ferrand, E.; Robert, R.; Ingrand, P.; Lemaire, F.; French LATAREA Group. Withholding and withdrawal of life support in intensive-care units in France: A prospective survey. Lancet 2001, 357, 9–14. [Google Scholar] [CrossRef]
- Sprung, C.L.; Cohen, S.L.; Sjokvist, P.; Baras, M.; Bulow, H.H.; Hovilehto, S.; Ledoux, D.; Lippert, A.; Maia, P.; Phelan, D.; et al. End-of-life practices in European intensive care units: The Ethicus Study. JAMA 2003, 290, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Harrington, S.; Sexton, K.; Goyal, A.; Robertson, R.D.; Corwin, H.L. Impact of Palliative Care Utilization for Surgical Patients Receiving Prolonged Mechanical Ventilation: National Trends (2009–2013). Jt. Comm. J. Qual. Patient Saf. 2020, 46, 493–500. [Google Scholar] [CrossRef] [PubMed]
| Classification | ICC | WIND |
|---|---|---|
| Group 1 | Simple weaning: successful extubation after one SBTs | Short weaning: successful separation from MV or death within 24 h |
| Group 2 | Difficult weaning: successful extubation after up to three SBTs in less than 7 days | Difficult weaning: successful separation from MV or death in 1 to 7 days |
| Group 3 | Prolonged weaning: successful extubation after more than three SBTs or more than 7 days | Prolonged weaning: unsuccessful separation 7 days after the first attempt. Subgroup A: eventually separated from MV; B: not separated from MV |
| Group “no weaning” | - | No separation attempt from MV |
| Author | Year | Country | N | Weaned (%) | Wean Time (Day) | LOS (Day) | Inpatient Mortality (%) |
|---|---|---|---|---|---|---|---|
| Bonnici [94] | 2016 | United Kingdom | 168 | 61 | 19 | 31 | 14.5 |
| Bornitz [95] | 2020 | Germany | 65 | 79 | - | 21 | 1.6 |
| Dolinay [82] | 2022 | USA | 165 | 70 | - | 24 | 9.5 |
| Ghiani [96] | 2020 | Germany | 263 | 47.9 | 22 | 52 | 14.4 |
| Herer [86] Cohort 2 | 2020 | France | 103 | 43.8 | 21 | 29 | 7.3 |
| Jubran [39] PSV study arm | 2013 | USA | 152 | 45 | 19 | 41 | 15 |
| Jubran [39] TC study arm | 2013 | USA | 160 | 53 | 15 | 42 | 10 |
| Saad [83] | 2022 | USA | 158 | 70.9 | 11 | 41 | 9.6 |
| Scheinhorn [18] | 2007 | USA | 1419 | 54 | 15 | 40 | 25 |
| Surani [46] | 2022 | USA | 111 | 89 | 8 | - | 21 |
| Wu [40] ATC study arm | 2023 | Taiwan | 157 | 62 | - | - | 13 |
| Wu [40] TC study arm | 2023 | Taiwan | 246 | 71 | - | - | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolinay, T.; Hsu, L.; Maller, A.; Walsh, B.C.; Szűcs, A.; Jerng, J.-S.; Jun, D. Ventilator Weaning in Prolonged Mechanical Ventilation—A Narrative Review. J. Clin. Med. 2024, 13, 1909. https://doi.org/10.3390/jcm13071909
Dolinay T, Hsu L, Maller A, Walsh BC, Szűcs A, Jerng J-S, Jun D. Ventilator Weaning in Prolonged Mechanical Ventilation—A Narrative Review. Journal of Clinical Medicine. 2024; 13(7):1909. https://doi.org/10.3390/jcm13071909
Chicago/Turabian StyleDolinay, Tamás, Lillian Hsu, Abigail Maller, Brandon Corbett Walsh, Attila Szűcs, Jih-Shuin Jerng, and Dale Jun. 2024. "Ventilator Weaning in Prolonged Mechanical Ventilation—A Narrative Review" Journal of Clinical Medicine 13, no. 7: 1909. https://doi.org/10.3390/jcm13071909
APA StyleDolinay, T., Hsu, L., Maller, A., Walsh, B. C., Szűcs, A., Jerng, J.-S., & Jun, D. (2024). Ventilator Weaning in Prolonged Mechanical Ventilation—A Narrative Review. Journal of Clinical Medicine, 13(7), 1909. https://doi.org/10.3390/jcm13071909







