Risk Factors Associated with Adverse Events Leading to Methotrexate Withdrawal in Elderly Rheumatoid Arthritis Patients: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Clinical Setting
2.2. Inclusion, Exclusion and Elimination Criteria
2.3. Ethics
2.4. Study Development
- (a)
- Sociodemographic variables: gender, age, type of insurance, body mass index (BMI), alcohol consumption, smoking;
- (b)
- Comorbid diseases: hypertension, diabetes mellitus type 2, obesity, depression and other comorbid diseases;
- (c)
- Disease characteristics: disease duration, articular and extraarticular manifestations, functional classification;
- (d)
- Pharmacological treatment (cs-DMARDS use, Methotrexate, Sulfasalazine, Antimalarials, other cs-DMARDS), persistence of treatment (years), combined cs-DMARD therapy—use of 2 or more cs-DMARDs simultaneously—and any other drug prescribed, such as glucocorticoids, non-steroidal anti-inflammatory drugs, analgesics, painkillers, antiacids, or antihypertensives, alongside folic acid supplementation;
- (e)
- Safety: adverse events that led to MTX discontinuation alongside MTX dose and usage time at the time of their appearance. Only adverse events that led patients to stop their MTX treatment for more than 90 days were counted. Additionally, these were classified based on the organ and system affected and the specific type of event.
- (f)
- Adverse events were considered as reported by the rheumatologist in the clinical charts at the time of each visit. Because this is a retrospective cohort, we were unable to identify the adverse events using prespecified definitions; however, the guidelines/recommendations to identify adverse events associated with MTX in our institution are described briefly as follows:
- Gastritis, gastropathies and gastrointestinal manifestations. These included gastric or duodenal mucosal injury, nausea, vomiting, mucosal ulcers, loss of appetite and epigastralgia.
- Transaminitis (elevated transaminases): presence of alanine transaminase (ALT) and aspartate transaminase (AST), higher than upper limits of normal (ULN) cutoff values of the reference laboratory; the normal values in our laboratory were as follows: ALT: normal range 5–50 IU/L; AST: normal range 10 to 34 IU/L. Severe transaminitis was considered an increase of ALT or AST > 3-fold ULN in two consecutive visits.
- Diagnosis of bleeding diverticulitis was performed by gastroenterologist based on symptoms of recurrent mild abdominal pain and distension plus rectal bleeding, corroborated with diverticula images in abdominal CT scan.
- Oral ulcers: symptoms of painful and observation of well-defined small ulcers (yellow or white rounded by erythema) in mouth and throat plus diverse difficulty in swallowing food with presence of small mucosal erosions/ulcerations on oral mucosa and/or tongue. Chronologically associated with MTX use and disappearing after this DMARD withdrawn (corroborated by dermatologist).
- Alopecia and hair loss: hair loss temporally correlated with the use of MTX, disappeared when MTX was withdrawn (corroborated by dermatologist).
- Abnormal blood counts; definitions:
- ○
- Anemia: hemoglobin level of <115 g/L;
- ○
- Leukopenia: peripheral blood leukocyte count < 3.0 × 109/L;
- ○
- Neutropenia: neutrophil count of <1.8 × 109/L;
- ○
- Lymphopenia: lymphocytes count < 1.1 × 109/L;
- ○
- Thrombocytopenia: platelet count of <100 × 109/L;
- ○
- Thrombocytosis: platelet count of >450 × 109/L.
- Interstitial pneumopathy: persistent symptoms of dyspnea and dry cough, plus findings of scattered or diffuse and patchy, ground glass opacity with images of reticular involvement identified by high resolution computed tomography (HRCT) and corroborated by a pneumologist. Pulmonary fibrosis was diagnosed by findings of honeycombing images (clustered cystic airspaces) located in subpleural region, with well-defined walls and diameters >0.5 cm, observed in HRCT corroborated by radiologist and pneumologist.
- Dermatosis attributable to MTX: cutaneous lesions of erythematous indurated papules located on proximal areas of the extremities with a direct chronologic correlation with MTX therapy corroborated by dermatologist. These lesions disappeared when MTX was withdrawn and had response to corticosteroids (topical or systemics).
- Weakness as persistent symptoms of fatigue or tiredness.
- Weight loss: >10% in kilograms, obtained from the difference between weight in the last visit (index visit)—weight in the previous visit.
- Recurrent infections: corroborated by persistent positive cultures or other accepted method.
- Urinary lithiasis: presence of stones observed in kidney or urinary tract using ultrasound or computed tomography (CT).
2.5. Statistical Analysis
3. Results
3.1. Pharmacological Treatment
3.2. Changes of MTX Monotherapy to MTX Combined Therapy during the Follow-Up
3.3. Adverse Events Leading to Suspension of MTX
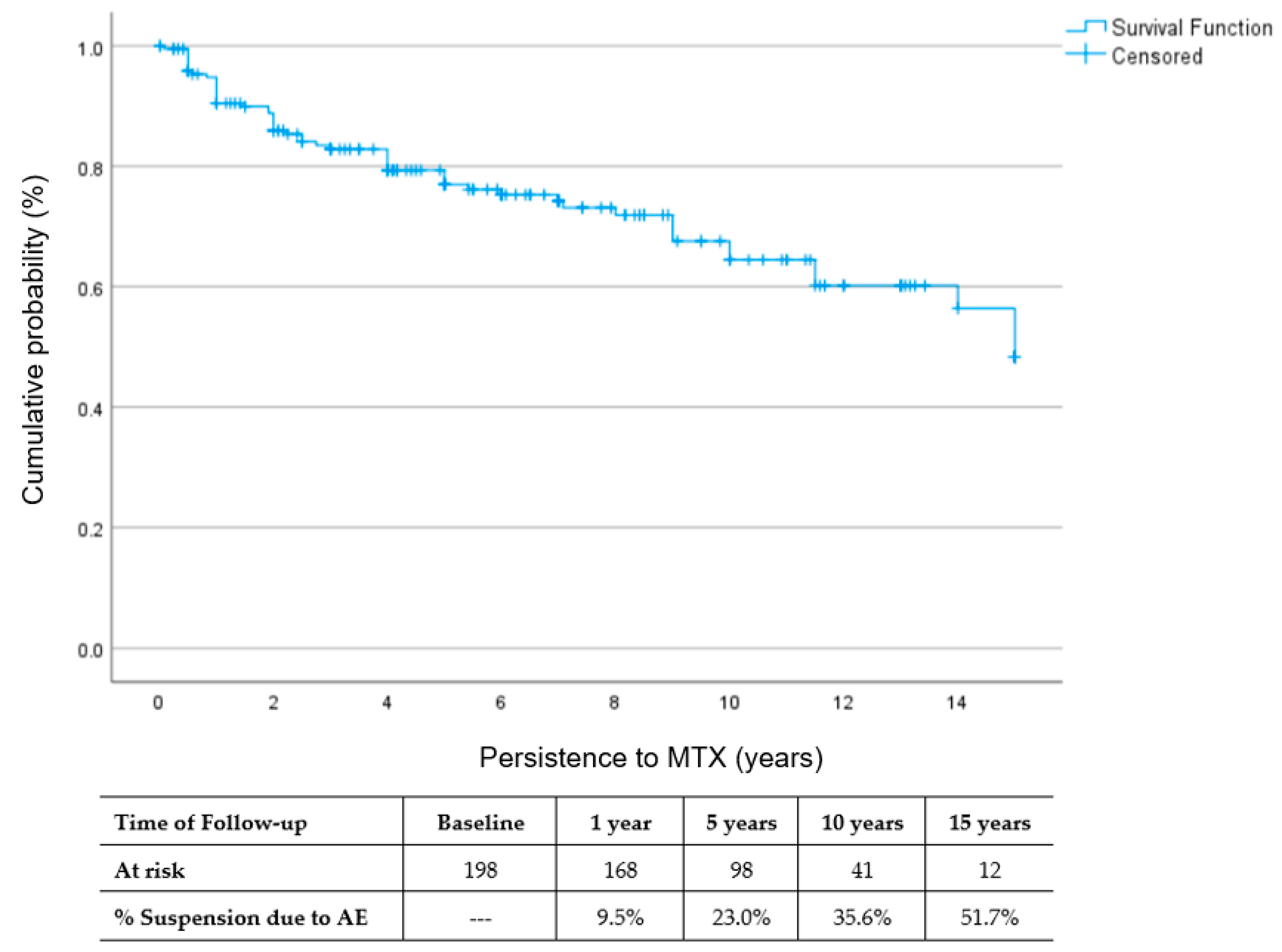
3.4. Rate of MTX Withdrawal
4. Discussion
4.1. MTX Persistence
4.2. Adverse Events of MTX That Led to Suspension
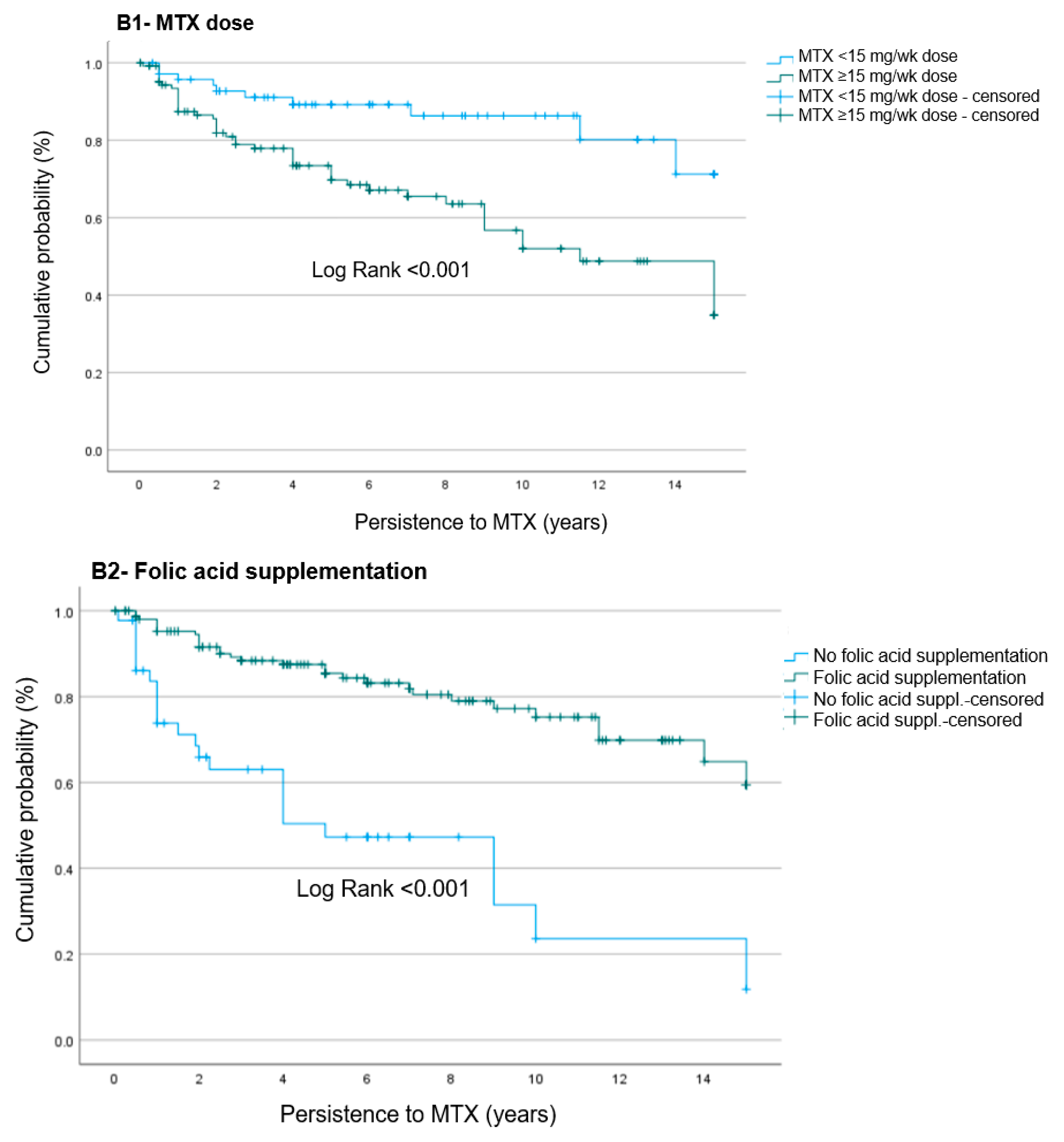
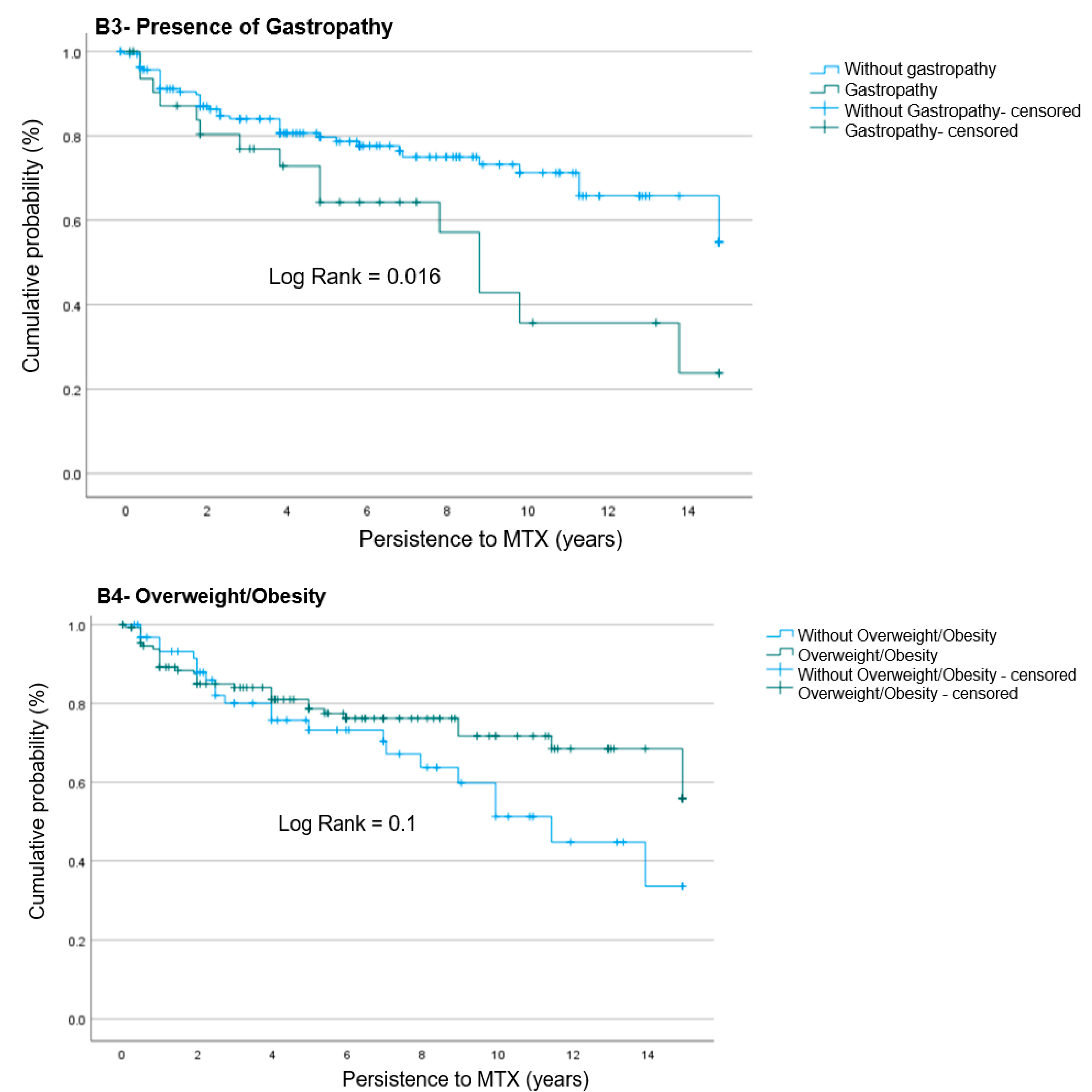
4.3. Risk Factors Associated with MTX Suspension Due to AEs
4.4. Folate Supplementation as Protective Factor for MTX Suspension Due to AEs
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid Arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Silman, A.J.; Pearson, J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002, 4 (Suppl. S3), S265–S272. [Google Scholar] [CrossRef]
- NICE. Rheumatoid Arthritis in Adults: Management. 2018. Available online: www.nice.org.uk/guidance/ng100 (accessed on 15 February 2024).
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Zhao, Z.; Hua, Z.; Luo, X.; Li, Y.; Yu, L.; Li, M.; Lu, C.; Zhao, T.; Liu, Y. Application and pharmacological mechanism of methotrexate in rheumatoid arthritis. Biomed. Pharmacother. 2022, 150, 113074. [Google Scholar] [CrossRef]
- Goycochea-Robles, M.V.; Arce-Salinas, C.A.; Guzmán-Vázquez, S.; Cardiel-Ríos, M.H. Prescription Rheumatology Practices Among Mexican Specialists. Arch. Med Res. 2007, 38, 354–359. [Google Scholar] [CrossRef]
- Moura, C.S.; Schieir, O.; Valois, M.; Thorne, C.; Bartlett, S.J.; Pope, J.E.; Hitchon, C.A.; Boire, G.; Haraoui, B.; Hazlewood, G.S.; et al. Treatment Strategies in Early Rheumatoid Arthritis Methotrexate Management: Results from a Prospective Cohort. Arthritis Care Res. 2020, 72, 1104–1111. [Google Scholar] [CrossRef]
- Alarcon, G.S.; Tracy, I.C.; Strand, G.M.; Singh, K.; Macaluso, M. Survival and drug discontinuation analyses in a large cohort of methotrexate treated rheumatoid arthritis patients. Ann. Rheum. Dis. 1995, 54, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Alarcóan, G.S.; Tracy, I.C.; Blackburn, W.D. Methotrexate in rheumatoid arthritis. Toxic effects as the major factor in limiting long-term treatment. Arthritis Rheum. 1989, 32, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M.; Laar, M.A.F.J.V.D.; Moens, H.J.B.; Kruijsen, M.W.M.; Haagsma, C.J. Longterm observational study of methotrexate use in a Dutch cohort of 1022 patients with rheumatoid arthritis. J. Rheumatol. 2003, 30, 2325–2329. [Google Scholar] [PubMed]
- Sevillano Gutierrez, J.; Capelusnik, D.; Schneeberger, E.; Citera, G. Tolerancia, sobrevida y adherencia al tratamiento con Metotrexato en pacientes con artritis reumatoidea. Rev. Argent. De Reumatol. 2019, 30, 13–17. [Google Scholar] [CrossRef]
- Sherbini, A.A.; Sharma, S.D.; Gwinnutt, J.M.; Hyrich, K.L.; Verstappen, S.M.M. Prevalence and predictors of adverse events with methotrexate mono- and combination-therapy for rheumatoid arthritis: A systematic review. Rheumatology 2021, 60, 4001–4017. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, S.M.M.; Bakker, M.F.; Heurkens, A.H.M.; van der Veen, M.J.; A Kruize, A.; Geurts, M.A.W.; Bijlsma, J.W.J.; Jacobs, J.W.G. Adverse events and factors associated with toxicity in patients with early rheumatoid arthritis treated with methotrexate tight control therapy: The CAMERA study. Ann. Rheum. Dis. 2010, 69, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Ideguchi, H.; Ohno, S.; Ishigatsubo, Y. Risk Factors Associated with the Cumulative Survival of Low-Dose Methotrexate in 273 Japanese Patients with Rheumatoid Arthritis. J. Clin. Rheumat. 2007, 13, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, H.; Eriksen, S.A.; Christensen, R.; Lorenzen, T.; Hansen, M.S.; Østergaard, M.; Dreyer, L.; Luta, G.; Vestergaard, P. Adherence to Methotrexate in Rheumatoid Arthritis: A Danish Nationwide Cohort Study. Arthritis 2015, 2015, 915142. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health#:~:text=By%202050%2C%20the%20world's%20population,2050%20to%20reach%20426%20million (accessed on 15 February 2024).
- Morales-Romero, J.; Cázares-Méndez, J.M.; Gámez-Nava, J.I.; Triano-Páez, M.; Villa-Manzano, A.; López-Olivo, M.; Rodríguez-Arreola, B.; González-López, L. La atención médica en reumatología en un hospital de segundo nivel de atención [Patterns of health care in an out patient rheumatologic clinic]. Reumatol. Clin. 2005, 1, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis. Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Tutuncu, Z.; Reed, G.; Kremer, J.; Kavanaugh, A. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Ann. Rheum. Dis. 2006, 65, 1226–1229. [Google Scholar] [CrossRef]
- Mathieu, S.; Pereira, B.; Saraux, A.; Richez, C.; Combe, B.; Soubrier, M. Disease-modifying drug retention rate according to patient age in patients with early rheumatoid arthritis: Analysis of the ESPOIR cohort. Rheumatol. Int. 2021, 41, 879–885. [Google Scholar] [CrossRef]
- Scully, C.J.; Anderson, C.J.; Cannon, G.W. Long-term methotrexate therapy for rheumatoid arthritis. Semin. Arthritis Rheum. 1991, 20, 317–331. [Google Scholar] [CrossRef]
- Nikiphorou, E.; Negoescu, A.; Fitzpatrick, J.D.; Goudie, C.T.; Badcock, A.; Östör, A.J.K.; Malaviya, A.P. Indispensable or intolerable? Methotrexate in patients with rheumatoid and psoriatic arthritis: A retrospective review of discontinuation rates from a large UK cohort. Clin. Rheumatol. 2014, 33, 609–614. [Google Scholar] [CrossRef]
- Nagafuchi, H.; Goto, Y.; Kiyokawa, T.; Kawahata, K. Reasons for discontinuation of methotrexate in the treatment of rheumatoid arthritis and challenges of methotrexate resumption: A single-center, retrospective study. Egypt. Rheumatol. Rehabil. 2022, 49, 63. [Google Scholar] [CrossRef]
- Singal, V.; Chaturvedi, V.; Brar, K. Efficacy and Toxicity Profile of Methotrexate Chloroquine Combination in Treatment of Active Rheumatoid Arthritis. Med. J. Armed Forces India 2005, 61, 29–32. [Google Scholar] [CrossRef]
- Sherbini, A.A.; Gwinnutt, J.M.; Hyrich, K.L.; RAMS Co-Investigators; Verstappen, S.M.M.; Adebajo, A.; Ahmed, K.; Al-Ansari, A.; Amarasena, R.; Bukhari, M.; et al. Rates and predictors of methotrexate-related adverse events in patients with early rheumatoid arthritis: Results from a nationwide UK study. Rheumatology 2022, 61, 3930–3938. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Kaneko, Y.; Okano, Y.; Taguchi, H.; Oshima, H.; Izumi, K.; Yamaoka, K.; Takeuchi, T. Association of erythrocyte methotrexate-polyglutamate levels with the efficacy and hepatotoxicity of methotrexate in patients with rheumatoid arthritis: A 76-week prospective study. RMD Open 2017, 3, e000363. [Google Scholar] [CrossRef] [PubMed]
- Cummins, L.; Katikireddi, V.S.; Shankaranarayana, S.; Su, K.Y.C.; Duggan, E.; Videm, V.; Pahau, H.; Thomas, R. Safety and retention of combination triple disease-modifying anti-rheumatic drugs in new-onset rheumatoid arthritis. Intern. Med. J. 2015, 45, 1266–1273. [Google Scholar] [CrossRef]
- Asai, S.; Nagai, K.; Takahashi, N.; Watanabe, T.; Matsumoto, T.; Asai, N.; Sobue, Y.; Ishiguro, N.; Kojima, T. Influence of methotrexate on gastrointestinal symptoms in patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2019, 22, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Shoda, H.; Inokuma, S.; Yajima, N.; Tanaka, Y.; Oobayashi, T.; Setoguchi, K. Higher maximal serum concentration of methotrexate predicts the incidence of adverse reactions in Japanese rheumatoid arthritis patients. Mod. Rheumatol. 2007, 17, 311–316. [Google Scholar] [CrossRef]
- Banal, F.; Dougados, M.; Combescure, C.; Gossec, L. Sensitivity and specificity of the American College of Rheumatology 1987 criteria for the diagnosis of rheumatoid arthritis according to disease duration: A systematic literature review and meta-analysis. Ann. Rheum. Dis. 2009, 68, 1184–1191. [Google Scholar] [CrossRef]
- Sulaiman, F.N.; Wong, K.K.; Ahmad, W.A.W.; Ghazali, W.S.W. Anti-cyclic citrullinated peptide antibody is highly associated with rheumatoid factor and radiological defects in rheumatoid arthritis patients. Medicine 2019, 98, e14945. [Google Scholar] [CrossRef]
- Korkmaz, C.; Us, T.; Kaşifoğlu, T.; Akgün, Y. Anti-cyclic citrullinated peptide (CCP) antibodies in patients with long-standing rheumatoid arthritis and their relationship with extra-articular manifestations. Clin. Biochem. 2006, 39, 961–965. [Google Scholar] [CrossRef] [PubMed]
| n = 198 (100.0) | |
|---|---|
| Female gender, n (%) | 168 (84.8) |
| Age (yrs), mean ± SD | 66.8 ± 5.6 |
| BMI *, mean ± SD | 27.5 ± 5.7 |
| Smoking, n (%) | 6 (3.0) |
| Alcohol abuse, n (%) | 4 (2.0) |
| Comorbidities, n (%) | 153 (77.3) |
| Number of comorbidities, median (range) | 1 (0.0, 4.0) |
| Overweight or Obesity, n (%) | 118 (59.6) |
| Arterial Hypertension, n (%) | 91 (46.0) |
| Osteoporosis, n (%) | 57 (28.8) |
| Type 2 Diabetes Mellitus, n (%) | 35 (17.1) |
| Clinical depression, n (%) | 19 (9.6) |
| RA duration (yrs) up to onset of MTX treatment, median (range) | 1.0 (0.0, 39.0) |
| Steinbroker’s functional class, n (%) | |
| - Functional class I, n (%) | 49 (24.7) |
| - Functional class II–IV, n (%) | 149 (75.3) |
| Pain score (VAS ** 0–100), mean ± DS | 72.5 ± 15.7 |
| Morning stiffness (>1 h), n (%) | 108 (54.5) |
| Positive RF ***, n (%) | 81 (40.9) |
| Extraarticular manifestations, n (%): | 130 (65.7) |
| Anemia, n (%) | 70 (35.4) |
| Sjogren syndrome, n (%) | 69 (34.8) |
| Rheumatic nodules, n (%) | 47 (23.7) |
| Neuropathies, n (%) | 39 (19.7) |
| Pneumopathies, n (%) | 4 (2.0) |
| n = 198 (100.0) | |
|---|---|
| Methotrexate as monotherapy, n (%) | 120 (60.6) |
| Combined therapy, n (%) | 78 (39.3) |
| MTX dose (mg)/wk, mean ± SD | 13.6 ± 3.6 |
| Usage duration (yrs), median (min., max.) | 5 (0.01, 15.00) |
| Combined therapy MTX plus: | |
| Sulfasalazine, n (%) | 38 (19.2) |
| Azathioprine, n (%) | 17 (8.6) |
| Leflunomide, n (%) | 9 (4.5) |
| Chloroquine, n (%) | 4 (2.0) |
| SSZ + CHQ, n (%) | 5 (2.5) |
| AZA + LEF, n (%) | 2 (1.0) |
| Other combinations *, n (%) | 3 (1.5) |
| Glucocorticoids, n (%) | 169 (85.4) |
| NSAIDs, n (%) | 189 (95.5) |
| Analgesics, n (%) | 156 (78.8) |
| Other drugs: | |
| Omeprazole, n (%) | 130 (65.7) |
| Antiresorptive, n (%) | 46 (23.2) |
| Antihypertensives, n (%) | 43 (21.7) |
| Antidiabetic drugs, n (%) | 16 (8.1) |
| Num. of drugs used at the same time, mean ± SD | 8.3 ± 2.6 |
| Polypharmacy (≥5 or more drugs), n (%) | 188 (94.9) |
| Folic acid supplementation, n (%) | 154 (77.8) |
| Patients Who Discontinued MTX Due to AEs | n = 54 (100.0) |
|---|---|
| Main organs and systems with AEs | |
| Gastrointestinal, n (%) | 31 (57.4) |
| Mucocutaneous, n (%) | 6 (11.1) |
| Hepatic, n (%) | 5 (9.2) |
| Constitutional symptoms, n (%) | 4 (7.4) |
| Recurrent Infections, n (%) | 3 (5.5) |
| Hematologic, n (%) | 2 (3.7) |
| Pulmonary, n (%) | 2 (3.7) |
| Renal, n (%) | 1 (1.8) |
| Specific adverse event | |
| Epigastralgia and/or Gastritis | 18 (33.3) |
| Nausea, vomiting, gastric intolerance and/or diarrhea | 10 (18.5) |
| Transaminitis | 5 (9.2) |
| Bleeding diverticulitis and upper digestive tract bleeding | 5 (9.2) |
| Oral ulcers and/or Alopecia/hair loss | 5 (9.2) |
| Leukopenia and/or lymphopenia | 2 (3.7) |
| Interstitial pneumopathy/pulmonary fibrosis | 2 (3.7) |
| Weakness and weight loss | 2 (3.7) |
| Dermatosis | 1 (1.8) |
| Tuberculosis infection. | 1 (1.8) |
| Hepatitis C infection | 1 (1.8) |
| Recurrent infections | 1 (1.8) |
| Urinary lithiasis | 1 (1.8) |
| Variable, n (%) | MTX Withdrawals * n = 54 (100.0) | MTX (Non-Withdrawals) n = 144 (100.0) | p |
|---|---|---|---|
| Age (yrs), mean ± SD | 65.6 ± 5.0 | 67.2 ± 5.7 | 0.08 |
| BMI, mean ± SD | 28.2 ± 7.5 | 27.2 ± 4.5 | 0.3 |
| Num. comorbidities, mean ± SD | 1.2 ± 1.1 | 1.3 ± 0.9 | 0.6 |
| Arterial Hypertension, n (%) | 25 (46.3) | 66 (45.8) | 0.9 |
| RA duration before MTX, mean ± DS | 5.6 ± 8.7 | 5.3 ± 7.9 | 0.8 |
| Pain score (VAS 0–100 mm), mean ± DS | 74.2 ± 14.6 | 73.3 ± 14.0 | 0.7 |
| Tender joints count, mean ± DS | 10.3 ± 5.9 | 10.4 ± 6.0 | 0.8 |
| Swollen joints count, mean ± DS | 9.9 ± 4.4 | 9.3 ± 4.1 | 0.4 |
| Morning stiffness (>1 h), n (%) | 30 (55.6) | 78 (54.2) | 0.8 |
| Extraarticular manifestations, n (%): | 40 (74.1) | 90 (62.5) | 0.1 |
| Sjogren syndrome, n (%) | 14 (9.7) | 7 (13.0) | 0.5 |
| Rheumatic nodules, n (%) | 13 (24.1) | 34 (23.6) | 0.9 |
| RF, n (%) | 23 (42.6) | 58 (40.3) | 0.7 |
| MTX dose (mg/wk), mean ± SD | 14.3 ± 3.1 | 12.9 ± 3.2 | 0.006 |
| Time of using MTX (years), mean ± SD | 4.1 ± 4.0 | 6.4 ± 4.4 | <0.001 |
| Combined therapy **, n (%) | 21 (38.9) | 57 (39.6) | 0.9 |
| Glucocorticoids, n (%) | 50 (92.6)) | 119 (82.6) | 0.07 |
| Polypharmacy (≥5 or more drugs), n (%) | 50 (92.6) | 138 (95.8) | 0.3 |
| Folic acid supplementation, n (%) | 30 (55.6) | 124 (86.1) | <0.001 |
| MTX Treatment Suspension Due to Adverse Events | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| Enter Method | Stepwise Method | |||||
| HR | 95% CI | p-Value | aHR | 95% CI | p-Value | |
| Female sex | 1.03 | 0.47–2.28 | 0.9 | -- | -- | -- |
| Overweight/obesity | 0.75 | 0.41–1.36 | 0.3 | -- | -- | -- |
| ≥2 Comorbidities | 0.70 | 0.33–1.46 | 0.3 | -- | -- | -- |
| Tender joints | 0.98 | 0.93–1.03 | 0.6 | -- | -- | -- |
| Swollen joints | 1.05 | 0.98–1.13 | 0.1 | -- | -- | -- |
| ≥2 DMARDs * | 0.78 | 0.43–1.41 | 0.4 | -- | -- | -- |
| MTX dose ≥ 15 mg/wk | 2.76 | 1.33–5.74 | 0.006 | 2.46 | 1.22–4.96 | 0.012 |
| Folic acid usage | 0.27 | 0.15–0.49 | <0.001 | 0.28 | 0.16–0.49 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avalos-Salgado, F.A.; Gonzalez-Lopez, L.; Gonzalez-Vazquez, S.; Ponce-Guarneros, J.M.; Santiago-Garcia, A.P.; Amaya-Cabrera, E.L.; Arellano-Cervantes, R.; Gutiérrez-Aceves, J.A.; Alcaraz-Lopez, M.F.; Nava-Valdivia, C.A.; et al. Risk Factors Associated with Adverse Events Leading to Methotrexate Withdrawal in Elderly Rheumatoid Arthritis Patients: A Retrospective Cohort Study. J. Clin. Med. 2024, 13, 1863. https://doi.org/10.3390/jcm13071863
Avalos-Salgado FA, Gonzalez-Lopez L, Gonzalez-Vazquez S, Ponce-Guarneros JM, Santiago-Garcia AP, Amaya-Cabrera EL, Arellano-Cervantes R, Gutiérrez-Aceves JA, Alcaraz-Lopez MF, Nava-Valdivia CA, et al. Risk Factors Associated with Adverse Events Leading to Methotrexate Withdrawal in Elderly Rheumatoid Arthritis Patients: A Retrospective Cohort Study. Journal of Clinical Medicine. 2024; 13(7):1863. https://doi.org/10.3390/jcm13071863
Chicago/Turabian StyleAvalos-Salgado, Felipe Alexis, Laura Gonzalez-Lopez, Sergio Gonzalez-Vazquez, Juan Manuel Ponce-Guarneros, Aline Priscilla Santiago-Garcia, Edna Lizeth Amaya-Cabrera, Reynaldo Arellano-Cervantes, J. Ahuixotl Gutiérrez-Aceves, Miriam Fabiola Alcaraz-Lopez, Cesar Arturo Nava-Valdivia, and et al. 2024. "Risk Factors Associated with Adverse Events Leading to Methotrexate Withdrawal in Elderly Rheumatoid Arthritis Patients: A Retrospective Cohort Study" Journal of Clinical Medicine 13, no. 7: 1863. https://doi.org/10.3390/jcm13071863
APA StyleAvalos-Salgado, F. A., Gonzalez-Lopez, L., Gonzalez-Vazquez, S., Ponce-Guarneros, J. M., Santiago-Garcia, A. P., Amaya-Cabrera, E. L., Arellano-Cervantes, R., Gutiérrez-Aceves, J. A., Alcaraz-Lopez, M. F., Nava-Valdivia, C. A., Gonzalez-Ponce, F., Rodriguez-Jimenez, N. A., Macias-Islas, M. A., Valdivia-Tangarife, E. R., Saldaña-Cruz, A. M., Cardona-Muñoz, E. G., & Gamez-Nava, J. I., on behalf of Research Group for Factors Related to Therapeutic Outcomes in Autoimmune Diseases. (2024). Risk Factors Associated with Adverse Events Leading to Methotrexate Withdrawal in Elderly Rheumatoid Arthritis Patients: A Retrospective Cohort Study. Journal of Clinical Medicine, 13(7), 1863. https://doi.org/10.3390/jcm13071863






