Continuous Superior Trunk Block versus Single-Shot Superior Trunk Block with Intravenous Dexmedetomidine for Postoperative Analgesia in Arthroscopic Shoulder Surgery: A Prospective Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Randomization and Study Protocol
2.2. Nerve Block Procedure
2.3. Postoperative Management
2.4. Outcome Assessments
2.5. Statistical Analysis
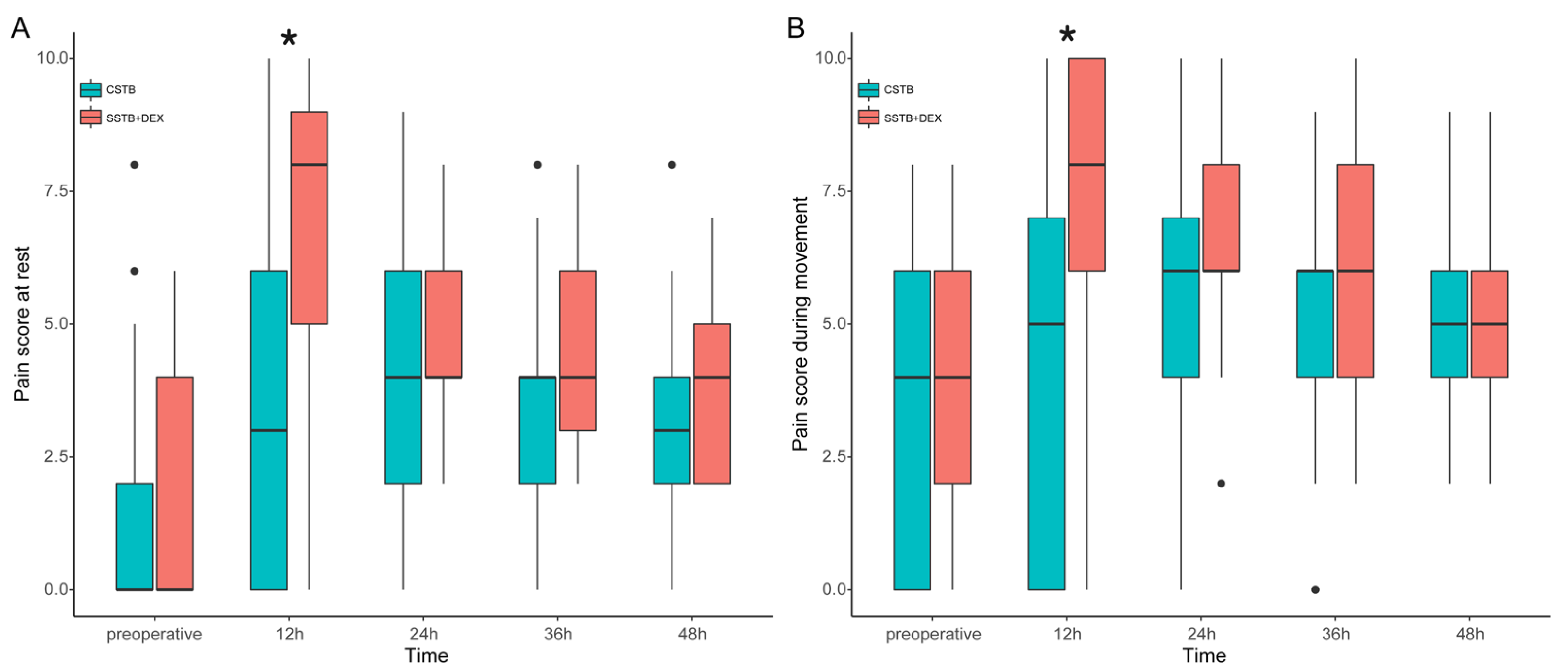
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vorobeichik, L.; Brull, R.; Bowry, R.; Laffey, J.G.; Abdallah, F.W. Should continuous rather than single-injection interscalene block be routinely offered for major shoulder surgery? A meta-analysis of the analgesic and side-effects profiles. Br. J. Anaesth. 2018, 120, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Leyva, F.; Cubillos, J.; Chin, K.J. Managing rebound pain after regional anesthesia. Korean J. Anesthesiol. 2020, 73, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Jeong, J.S.; Chin, K.J.; Yoo, J.C.; Lee, J.H.; Choi, S.J.; Gwak, M.S.; Hahm, T.S.; Ko, J.S. Superior trunk block provides noninferior analgesia compared with interscalene brachial plexus block in arthroscopic shoulder surgery. Anesthesiology 2019, 131, 1316–1326. [Google Scholar] [CrossRef]
- Kim, D.H.; Lin, Y.; Beathe, J.C.; Liu, J.; Oxendine, J.A.; Haskins, S.C.; Ho, M.C.; Wetmore, D.S.; Allen, A.A.; Wilson, L.; et al. Superior trunk block: A phrenic-sparing alternative to the interscalene block: A randomized controlled trial. Anesthesiology 2019, 131, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Robles, C.; Berardone, N.; Orebaugh, S. Effect of superior trunk block on diaphragm function and respiratory parameters after shoulder surgery. Reg. Anesth. Pain Med. 2022, 47, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, M.K.; Pakpirom, J.; Songthamwat, B.; Areeruk, P. High definition ultrasound imaging of the individual elements of the brachial plexus above the clavicle. Reg. Anesth. Pain Med. 2020, 45, 344–350. [Google Scholar] [CrossRef]
- Nielsen, S.; Degenhardt, L.; Hoban, B.; Gisev, N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol. Drug Saf. 2016, 25, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Bojaxhi, E.; Lumermann, L.A.; Mazer, L.S.; Howe, B.L.; Ortiguera, C.J.; Clendenen, S.R. Interscalene brachial plexus catheter versus single-shot interscalene block with periarticular local infiltration analgesia for shoulder arthroplasty. Minerva Anestesiol. 2019, 85, 840–845. [Google Scholar] [CrossRef]
- Farrar, J.T.; Portenoy, R.K.; Berlin, J.A.; Kinman, J.L.; Strom, B.L. Defining the clinically important difference in pain outcome measures. Pain 2000, 88, 287–294. [Google Scholar] [CrossRef]
- Abdallah, F.W.; Halpern, S.H.; Aoyama, K.; Brull, R. Will the real benefits of single-shot interscalene block please stand up? A systematic review and meta-analysis. Anesth. Analg. 2015, 120, 1114–1129. [Google Scholar] [CrossRef]
- Yun, S.; Jo, Y.; Sim, S.; Jeong, K.; Oh, C.; Kim, B.; Lee, W.Y.; Park, S.; Kim, Y.H.; Ko, Y.; et al. Comparison of continuous and single interscalene block for quality of recovery score following arthroscopic rotator cuff repair. J. Orthop. Surg. 2021, 29, 23094990211000142. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Koh, H.J.; Kim, D.K.; Lee, H.J.; Kwon, K.H.; Lee, K.Y.; Kim, Y.S. Interscalene brachial plexus bolus block versus patient-controlled interscalene indwelling catheter analgesia for the first 48 hours after arthroscopic rotator cuff repair. J. Shoulder Elb. Surg. 2018, 27, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Salviz, E.A.; Xu, D.; Frulla, A.; Kwofie, K.; Shastri, U.; Chen, J.; Shariat, A.N.; Littwin, S.; Lin, E.; Choi, J.; et al. Continuous interscalene block in patients having outpatient rotator cuff repair surgery: A prospective randomized trial. Anesth. Analg. 2013, 117, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Jeong, J.S.; Yoo, J.C.; Lee, J.H.; Choi, S.J.; Gwak, M.S.; Hahm, T.S.; Huh, J.; Ko, J.S. Effective dose of intravenous dexmedetomidine to prolong the analgesic duration of interscalene brachial plexus block: A single-center, prospective, double-blind, randomized controlled trial. Reg. Anesth. Pain Med. 2018, 43, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Lee, H.J.; Oh, H.W.; Lee, J.W.; Baik, H.J.; Kim, Y.J. Perineural dexamethasone reduces rebound pain after ropivacaine single injection interscalene block for arthroscopic shoulder surgery: A randomized controlled trial. Reg. Anesth. Pain Med. 2021, 46, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Shi, Y.; Du, F.; Xue, Z.; Cang, J.; Miao, C.; Zhang, X. The effect of perineural dexamethasone on rebound pain after ropivacaine single-injection nerve block: A randomized controlled trial. BMC Anesthesiol. 2021, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.A.; Jeong, J.S.; Yoo, J.C.; Lee, J.H.; Gwak, M.S.; Choi, S.J.; Hahm, T.S.; Cho, H.S.; Ko, J.S. Improvement in postoperative pain control by combined use of intravenous dexamethasone with intravenous dexmedetomidine after interscalene brachial plexus block for arthroscopic shoulder surgery: A randomised controlled trial. Eur. J. Anaesthesiol. 2019, 36, 360–368. [Google Scholar] [CrossRef]
- Abdallah, F.W.; Dwyer, T.; Chan, V.W.; Niazi, A.U.; Ogilvie-Harris, D.J.; Oldfield, S.; Patel, R.; Oh, J.; Brull, R. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: A randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology 2016, 124, 683–695. [Google Scholar] [CrossRef]
- Kirksey, M.A.; Haskins, S.C.; Cheng, J.; Liu, S.S. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: A systematic qualitative review. PLoS ONE. 2015, 10, e0137312. [Google Scholar] [CrossRef]
- Hussain, N.; Grzywacz, V.P.; Ferreri, C.A.; Atrey, A.; Banfield, L.; Shaparin, N.; Vydyanathan, A. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: A systematic review and meta-analysis of 18 randomized controlled trials. Reg. Anesth. Pain Med. 2017, 42, 184–196. [Google Scholar] [CrossRef]
- Vorobeichik, L.; Brull, R.; Abdallah, F.W. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: A systematic review and meta-analysis of randomized controlled trials. Br. J. Anaesth. 2017, 118, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Weerink, M.A.S.; Struys, M.M.R.F.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef] [PubMed]
- Stiglitz, Y.; Gosselin, O.; Sedaghatian, J.; Sirveaux, F.; Molé, D. Pain after shoulder arthroscopy: A prospective study on 231 cases. Orthop. Traumatol. Surg. Res. 2011, 97, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Rolf, R.H.; Sympson, A.N.; Eten, K.; Elsass, T.R. Single-shot versus continuous interscalene block for postoperative pain control after shoulder arthroplasty: A prospective randomized clinical trial. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2019, 3, e014. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.; Mass, D.; Cohn, S. Postoperative analgesia in a prolonged continuous interscalene block versus single-shot block in outpatient arthroscopic rotator cuff repair: A prospective randomized study. Arthroscopy 2016, 32, 1544–1550.e1. [Google Scholar] [CrossRef] [PubMed]
- Barry, G.S.; Bailey, J.G.; Sardinha, J.; Brousseau, P.; Uppal, V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br. J. Anaesth. 2021, 126, 862–871. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, D.C.; Cheah, J.W.; Aleshi, P.; Zhang, A.L.; Ma, C.B.; Feeley, B.T. Multimodal analgesia decreases opioid consumption after shoulder arthroplasty: A prospective cohort study. J. Shoulder Elb. Surg. 2018, 27, 686–691. [Google Scholar] [CrossRef]
- Ebert, T.J.; Hall, J.E.; Barney, J.A.; Uhrich, T.D.; Colinco, M.D. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000, 93, 382–394. [Google Scholar] [CrossRef]


| CSTB Group (n = 45) | SSTB + DEX Group (n = 45) | p Value | |
|---|---|---|---|
| Age (years) | 65 ± 7 | 62 ± 8 | 0.126 |
| Female/Male | 20/25 | 19/26 | >0.999 |
| Height (cm) | 160.6 ± 8.5 | 160.8 ± 8.9 | 0.931 |
| Weight (kg) | 64.5 (59.0–70.5) | 62.5 (57.0–69.0) | 0.345 |
| Body mass index (kg/m2) | 24.9 (23.5–26.2) | 24.6 (22.8–26.1) | 0.399 |
| ASA class (I/II/III) | 22/16/7 | 17/25/3 | 0.121 |
| Surgical procedure | |||
| Arthroscopic rotator cuff repair | 45 (100) | 45 (100) | |
| Subpectoral biceps tenodesis | 30 (67) | 22 (49) | 0.135 |
| Operation time (min) | 90 (70–115) | 90 (70–105) | 0.539 |
| Anesthesia time (min) | 148.7 ± 29.9 | 152.9 ± 31.4 | 0.515 |
| Fluid amount (mL) | 550 (450–650) | 550 (450–650) | 0.939 |
| Remifentanil use (mcg) | 448 (388–576) | 442 (371–579) | 0.473 |
| Norepinephrine (mcg) | 256 (117–336) | 208 (124–293) | 0.416 |
| The number of patients who required intraoperative atropine | 0 | 3 (7) | 0.242 |
| Minimum SBP | 81 (78–83) | 83 (78–85) | 0.256 |
| Minimum DBP | 36 ± 6 | 38 ± 6 | 0.067 |
| Minimum MBP | 52 (49–57) | 55 (51–59) | 0.088 |
| Minimum heart rate | 52 (49–57) | 49 (46–53) | 0.007 |
| CSTB Group (n = 45) | SSTB + DEX Group (n = 45) | p Value | |
|---|---|---|---|
| Time to first pain report (h) | 14 (10–15) | 11 (10–13) | 0.014 |
| Patients experiencing rebound pain (n) | 9 (20) | 25 (56) | 0.001 |
| Patients requiring rescue analgesics (n) | |||
| 0–12 h | 9 (20) | 9 (20) | >0.999 |
| 12–24 h | 23 (51) | 36 (80) | 0.008 |
| Opioid consumption (morphine equivalents) | |||
| 0–12 h | 0 (0–0) | 0 (0–0) | >0.999 |
| 12–24 h | 10 (0–10) | 20 (10–20) | <0.001 |
| Postoperative anesthesia care unit (PACU) stay (min) | 38 (30–55) | 48 (35–63) | 0.070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.; Jang, J.; Lim, J.-R.; Kim, E.J.; Kim, D.; Chun, Y.-M.; Choi, Y.S. Continuous Superior Trunk Block versus Single-Shot Superior Trunk Block with Intravenous Dexmedetomidine for Postoperative Analgesia in Arthroscopic Shoulder Surgery: A Prospective Randomized Controlled Trial. J. Clin. Med. 2024, 13, 1845. https://doi.org/10.3390/jcm13071845
Lee B, Jang J, Lim J-R, Kim EJ, Kim D, Chun Y-M, Choi YS. Continuous Superior Trunk Block versus Single-Shot Superior Trunk Block with Intravenous Dexmedetomidine for Postoperative Analgesia in Arthroscopic Shoulder Surgery: A Prospective Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(7):1845. https://doi.org/10.3390/jcm13071845
Chicago/Turabian StyleLee, Bora, Jaewon Jang, Joon-Ryul Lim, Eun Jung Kim, Donghu Kim, Yong-Min Chun, and Yong Seon Choi. 2024. "Continuous Superior Trunk Block versus Single-Shot Superior Trunk Block with Intravenous Dexmedetomidine for Postoperative Analgesia in Arthroscopic Shoulder Surgery: A Prospective Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 7: 1845. https://doi.org/10.3390/jcm13071845
APA StyleLee, B., Jang, J., Lim, J.-R., Kim, E. J., Kim, D., Chun, Y.-M., & Choi, Y. S. (2024). Continuous Superior Trunk Block versus Single-Shot Superior Trunk Block with Intravenous Dexmedetomidine for Postoperative Analgesia in Arthroscopic Shoulder Surgery: A Prospective Randomized Controlled Trial. Journal of Clinical Medicine, 13(7), 1845. https://doi.org/10.3390/jcm13071845






