Trans-Arterial Stem Cell Injection (TASI): The Role of Interventional Radiology in Regenerative Medicine
Abstract
1. Introduction
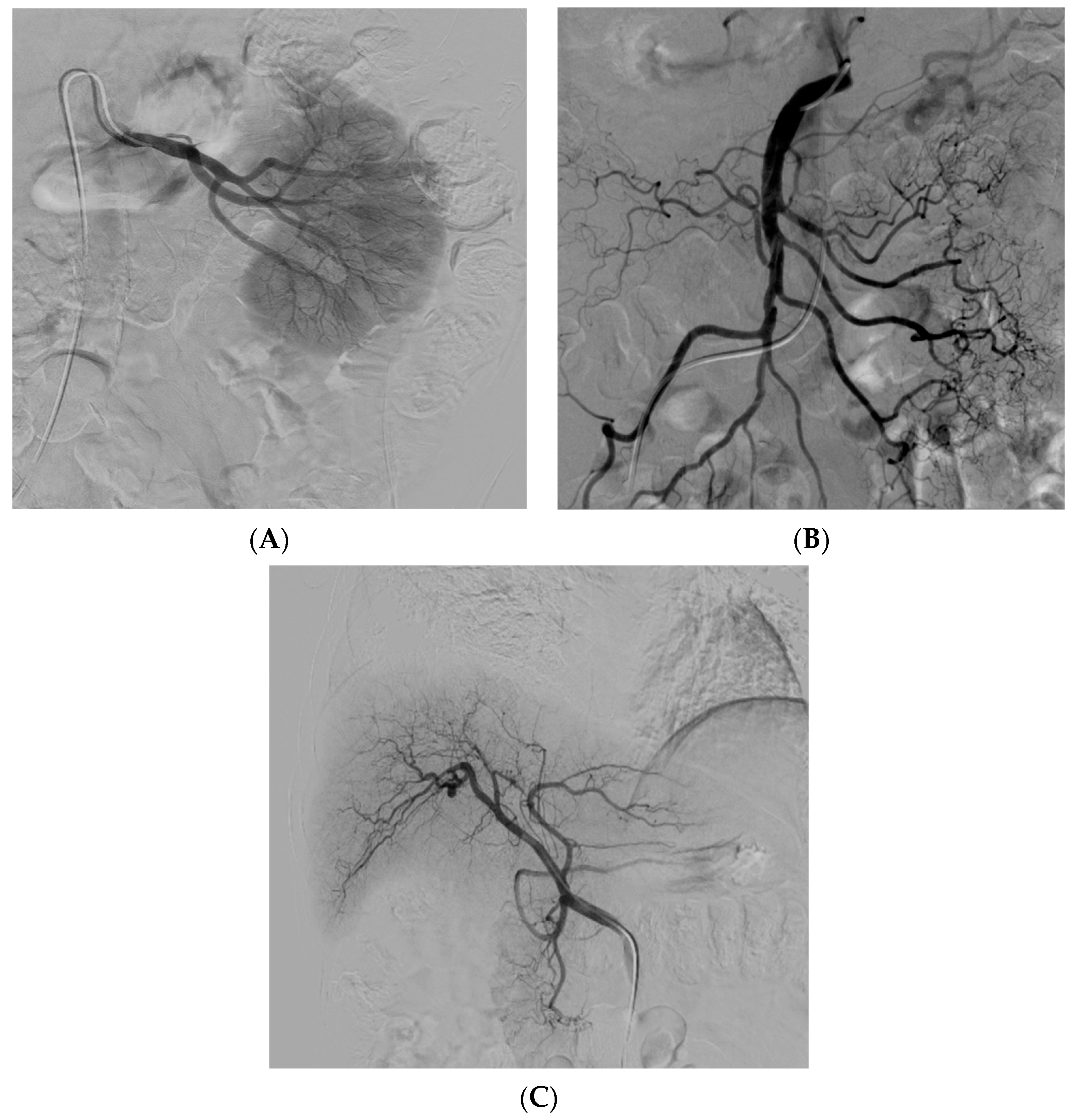
- (a)
- Minimally invasive: Interventional radiology procedures are minimally invasive, reducing the risks associated with open surgeries and promoting faster recovery for patients.
- (b)
- Precise and safe: Advanced imaging technologies allow interventional radiologists to visualize the delivery process in real time, ensuring accurate placement of stem cells and reducing the likelihood of complications.
- (c)
- Repeatable and scalable: Interventional radiology procedures are repeatable, enabling multiple stem cell injections over time. This scalability allows for personalized treatment plans based on the patient’s response and disease progression.
2. Cell and Tissue Grafts for Regenerative Medicine
2.1. Single Cells
2.2. Spheroids
2.3. Cell Sheets
2.4. Organoids
2.5. Extracellular Vesicles
3. Treatment of Specific Diseases with TASI
3.1. Liver Disease
3.2. Renal Disease
3.3. Diabetes
3.4. Osteonecrosis
3.5. Peripheral Arterial Disease
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajabzadeh, N.; Fathi, E.; Farahzadi, R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019, 6, 19. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tsuchiya, A.; Terai, S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin. Mol. Hepatol. 2021, 27, 70–80. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef]
- Boehler, R.M.; Graham, J.G.; Shea, L.D. Tissue engineering tools for modulation of the immune response. Biotechniques 2011, 51, 239–240, 242, 244. [Google Scholar] [CrossRef]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 16, 139–155. [Google Scholar] [CrossRef]
- Rosenthal, N.; Badylak, S. Regenerative medicine: Today’s discoveries informing the future of medical practice. NPJ Regen. Med. 2016, 1, 16007. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary Passage is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The Dynamic in vivo Distribution of Bone Marrow-Derived Mesenchymal Stem Cells after Infusion. Cells Tissues Organs 2001, 169, 12–20. [Google Scholar] [CrossRef]
- Prologo, J.D.; Hawkins, M.; Gilliland, C.; Chinnadurai, R.; Harkey, P.; Chadid, T.; Lee, Z.; Brewster, L. Interventional stem cell therapy. Clin. Radiol. 2016, 71, 307–311. [Google Scholar] [CrossRef]
- Thomson, K.R. Interventional radiology. Lancet 1997, 350, 354–358. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Ejtehadifar, M.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P.; Dehdilani, N.; Abbasi, P.; Molaeipour, Z.; Saleh, M. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv. Pharm. Bull. 2015, 5, 141–149. [Google Scholar] [CrossRef]
- Mohammadian, M.; Shamsasenjan, K.; Lotfi Nezhad, P.; Talebi, M.; Jahedi, M.; Nickkhah, H.; Minayi, N.; Movassagh Pour, A. Mesenchymal stem cells: New aspect in cell-based regenerative therapy. Adv. Pharm. Bull. 2013, 3, 433–437. [Google Scholar]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef]
- Gauvin, R.; Parenteau-Bareil, R.; Dokmeci, M.R.; Merryman, W.D.; Khademhosseini, A. Hydrogels and microtechnologies for engineering the cellular microenvironment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 235–246. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Wang, Y.; Gou, W.; Yuan, X.; Peng, J.; Guo, Q.; Lu, S. Past, present, and future of microcarrier-based tissue engineering. J. Orthop. Transl. 2015, 3, 51–57. [Google Scholar] [CrossRef]
- Chen, G.; Kawazoe, N. Porous Scaffolds for Regeneration of Cartilage, Bone and Osteochondral Tissue. Adv. Exp. Med. Biol. 2018, 1058, 171–191. [Google Scholar]
- Zhang, Z. Injectable biomaterials for stem cell delivery and tissue regeneration. Expert Opin. Biol. Ther. 2017, 17, 49–62. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Watanabe, K.; Kamiya, D.; Nishiyama, A.; Katayama, T.; Nozaki, S.; Kawasaki, H.; Watanabe, Y.; Mizuseki, K.; Sasai, Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 2005, 8, 288–296. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef]
- Subramanian, K.; Owens, D.J.; Raju, R.; Firpo, M.; O’Brien, T.D.; Verfaillie, C.M.; Hu, W.S. Spheroid culture for enhanced differentiation of human embryonic stem cells to hepatocyte-like cells. Stem Cells Dev. 2014, 23, 124–131. [Google Scholar] [CrossRef]
- Kusamori, K.; Nishikawa, M.; Mizuno, N.; Nishikawa, T.; Masuzawa, A.; Shimizu, K.; Konishi, S.; Takahashi, Y.; Takakura, Y. Transplantation of insulin-secreting multicellular spheroids for the treatment of type 1 diabetes in mice. J. Control. Release 2014, 173, 119–124. [Google Scholar] [CrossRef]
- Murphy, K.C.; Hoch, A.I.; Harvestine, J.N.; Zhou, D.; Leach, J.K. Mesenchymal Stem Cell Spheroids Retain Osteogenic Phenotype Through α2β1 Signaling. Stem Cells Transl. Med. 2016, 5, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Cho, S.W.; La, W.G.; Lee, T.J.; Yang, H.S.; Sun, A.Y.; Baek, S.H.; Rhie, J.W.; Kim, B.S. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 2011, 32, 2734–2747. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ge, J.; Zhou, Y.; Wang, S.; Zhao, R.C.; Wu, Y. Three-dimensional spheroid-cultured mesenchymal stem cells devoid of embolism attenuate brain stroke injury after intra-arterial injection. Stem Cells Dev. 2014, 23, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Kuruma, Y.; Sekine, H.; Dobashi, I.; Yamato, M.; Umezu, M.; Shimizu, T.; Okano, T. In vivo vascularization of cell sheets provided better long-term tissue survival than injection of cell suspension. J. Tissue Eng. Regen. Med. 2016, 10, 700–710. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Gao, Y.; Yang, L. Cell sheet technology: A promising strategy in regenerative medicine. Cytotherapy 2019, 21, 3–16. [Google Scholar] [CrossRef]
- Osada, H.; Ho, W.J.; Yamashita, H.; Yamazaki, K.; Ikeda, T.; Minatoya, K.; Masumoto, H. Novel device prototyping for endoscopic cell sheet transplantation using a three-dimensional printed simulator. Regen. Ther. 2020, 15, 258–264. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.K.; Huch, M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef]
- Noguchi, T.K.; Ninomiya, N.; Sekine, M.; Komazaki, S.; Wang, P.C.; Asashima, M.; Kurisaki, A. Generation of stomach tissue from mouse embryonic stem cells. Nat. Cell Biol. 2015, 17, 984–993. [Google Scholar] [CrossRef]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.; Tsai, Y.H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015, 4, e05098. [Google Scholar] [CrossRef]
- Jardé, T.; Lloyd-Lewis, B.; Thomas, M.; Kendrick, H.; Melchor, L.; Bougaret, L.; Watson, P.D.; Ewan, K.; Smalley, M.J.; Dale, T.C. Wnt and Neuregulin1/ErbB signalling extends 3D culture of hormone responsive mammary organoids. Nat. Commun. 2016, 7, 13207. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.W.; Yoo, Y.I.; Kim, J.Y.; Choi, B.; Park, S.B.; Park, W.; Rhim, W.K.; Han, D.K. Attenuation of Tumor Necrosis Factor-α Induced Inflammation by Umbilical Cord-Mesenchymal Stem Cell Derived Exosome-Mimetic Nanovesicles in Endothelial Cells. Tissue Eng. Regen. Med. 2020, 17, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, H.S. Extracellular Vesicles in Regenerative Medicine: Potentials and Challenges. Tissue Eng. Regen. Med. 2021, 18, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Moghadasi, S.; Elveny, M.; Rahman, H.S.; Suksatan, W.; Jalil, A.T.; Abdelbasset, W.K.; Yumashev, A.V.; Shariatzadeh, S.; Motavalli, R.; Behzad, F.; et al. A paradigm shift in cell-free approach: The emerging role of MSCs-derived exosomes in regenerative medicine. J. Transl. Med. 2021, 19, 302. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.W.; Witten, C.M.; Califf, R.M. Clarifying Stem-Cell Therapy’s Benefits and Risks. N. Engl. J. Med. 2017, 376, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, A.L.; Miller, M.B.; Mir, S.A.; Cagney, D.; Chavakula, V.; Guleria, I.; Aizer, A.; Ligon, K.L.; Chi, J.H. Glioproliferative Lesion of the Spinal Cord as a Complication of “Stem-Cell Tourism”. N. Engl. J. Med. 2016, 375, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.P.K.; Stevens, M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef]
- Kim, D.Y.; Choi, S.H.; Lee, J.S.; Kim, H.J.; Kim, H.N.; Lee, J.E.; Shin, J.Y.; Lee, P.H. Feasibility and Efficacy of Intra-Arterial Administration of Embryonic Stem Cell Derived-Mesenchymal Stem Cells in Animal Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 76, 1281–1296. [Google Scholar] [CrossRef]
- Staudacher, D.L.; Sela, Y.; Itskovitz-Eldor, J.; Flugelman, M.Y. Intra-arterial injection of human embryonic stem cells in athymic rat hind limb ischemia model leads to arteriogenesis. Cardiovasc. Revasc. Med. 2011, 12, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Behr, L.; Hekmati, M.; Fromont, G.; Borenstein, N.; Noel, L.H.; Lelievre-Pegorier, M.; Laborde, K. Intra Renal Arterial Injection of Autologous Mesenchymal Stem Cells in an Ovine Model in the Postischemic Kidney. Nephron. Physiol. 2007, 107, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, J.; Pei, X.; Zhao, W. Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology 2013, 18, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Komatsu, H.; Poku, E.K.; Olafsen, T.; Huang, K.X.; Huang, L.A.; Chea, J.; Bowles, N.; Chang, B.; Rawson, J.; et al. Biodistribution of Intra-Arterial and Intravenous Delivery of Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles in a Rat Model to Guide Delivery Strategies for Diabetes Therapies. Pharmaceuticals 2022, 15, 595. [Google Scholar] [CrossRef]
- Jadlowiec, C.C.; Taner, T. Liver transplantation: Current status and challenges. World J. Gastroenterol. 2016, 22, 4438–4445. [Google Scholar] [CrossRef]
- Sakai, Y.; Fukunishi, S.; Takamura, M.; Kawaguchi, K.; Inoue, O.; Usui, S.; Takashima, S.; Seki, A.; Asai, A.; Tsuchimoto, Y. Clinical trial of autologous adipose tissue-derived regenerative (stem) cells therapy for exploration of its safety and efficacy. Regen. Ther. 2021, 18, 97–101. [Google Scholar] [CrossRef]
- Suk, K.T.; Yoon, J.H.; Kim, M.Y.; Kim, C.W.; Kim, J.K.; Park, H.; Hwang, S.G.; Kim, D.J.; Lee, B.S.; Lee, S.H.; et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 2016, 64, 2185–2197. [Google Scholar] [CrossRef]
- Zhou, G.P.; Jiang, Y.Z.; Sun, L.Y.; Zhu, Z.J. Therapeutic effect and safety of stem cell therapy for chronic liver disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2020, 11, 419. [Google Scholar] [CrossRef]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef]
- Zhang, L.; Lai, X.; Guo, Y.; Ma, J.; Fang, J.; Li, G.; Xu, L.; Yin, W.; Chen, Z. Autologous bone marrow-derived mesenchymal stem cells for interstitial fibrosis and tubular atrophy: A pilot study. Ren. Fail. 2021, 43, 1266–1275. [Google Scholar] [CrossRef]
- Saad, A.; Dietz, A.B.; Herrmann, S.M.S.; Hickson, L.J.; Glockner, J.F.; McKusick, M.A.; Misra, S.; Bjarnason, H.; Armstrong, A.S.; Gastineau, D.A.; et al. Autologous Mesenchymal Stem Cells Increase Cortical Perfusion in Renovascular Disease. J. Am. Soc. Nephrol. 2017, 28, 2777–2785. [Google Scholar] [CrossRef]
- Maahs, D.M.; West, N.A.; Lawrence, J.M.; Mayer-Davis, E.J. Epidemiology of type 1 diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 481–497. [Google Scholar] [CrossRef]
- Madani, S.; Amanzadi, M.; Aghayan, H.R.; Setudeh, A.; Rezaei, N.; Rouhifard, M.; Larijani, B. Investigating the safety and efficacy of hematopoietic and mesenchymal stem cell transplantation for treatment of T1DM: A systematic review and meta-analysis. Syst. Rev. 2022, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, Z.; Xu, X.; Liao, L.; Chen, J.; Huang, L.; Wu, W.; Luo, F.; Wu, C.; Pugliese, A.; et al. Umbilical Cord Mesenchymal Stromal Cell with Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care 2016, 39, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Johannson, H.R.; Zywiel, M.G.; Marker, D.R.; Jones, L.C.; McGrath, M.S.; Mont, M.A. Osteonecrosis is not a predictor of poor outcomes in primary total hip arthroplasty: A systematic literature review. Int. Orthop. 2011, 35, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.M.; Angrisani, A.T.; Santiago, M.B. The use of extracorporeal shock waves in the treatment of osteonecrosis of the femoral head: A systematic review. Clin. Rheumatol. 2009, 28, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Andriolo, L.; Merli, G.; Tobar, C.; Altamura, S.A.; Kon, E.; Filardo, G. Regenerative therapies increase survivorship of avascular necrosis of the femoral head: A systematic review and meta-analysis. Int. Orthop. 2018, 42, 1689–1704. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Wang, W.; Xu, T.; Zhang, S.; Xiao, L.; Chen, D.; Jin, H.; Tong, P. Combination treatment of biomechanical support and targeted intra-arterial infusion of peripheral blood stem cells mobilized by granulocyte-colony stimulating factor for the osteonecrosis of the femoral head: A randomized controlled clinical trial. J. Bone Miner. Res. 2015, 30, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ding, Q.; Lv, S.; Xia, B.; Jin, H.; Chen, D.; Xiao, L.; Tong, P. Prognosis after autologous peripheral blood stem cell transplantation for osteonecrosis of the femoral head in the pre-collapse stage: A retrospective cohort study. Stem Cell Res. Ther. 2020, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S1), S5–S67. [Google Scholar] [CrossRef]
- Reinecke, H.; Unrath, M.; Freisinger, E.; Bunzemeier, H.; Meyborg, M.; Lüders, F.; Gebauer, K.; Roeder, N.; Berger, K.; Malyar, N.M. Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur. Heart J. 2015, 36, 932–938. [Google Scholar] [CrossRef]
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ. Res. 2017, 120, 1326–1340. [Google Scholar] [CrossRef]
- Klepanec, A.; Mistrik, M.; Altaner, C.; Valachovicova, M.; Olejarova, I.; Slysko, R.; Balazs, T.; Urlandova, T.; Hladikova, D.; Liska, B.; et al. No difference in intra-arterial and intramuscular delivery of autologous bone marrow cells in patients with advanced critical limb ischemia. Cell Transplant. 2012, 21, 1909–1918. [Google Scholar] [CrossRef]
- Gao, W.; Chen, D.; Liu, G.; Ran, X. Autologous stem cell therapy for peripheral arterial disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef] [PubMed]

| Culture Type | Image Guided Cell Delivery Method | TASI Animal Studies | TASI Clinical Studies | Pathologies Treated with TASI in Clinical Studies |
|---|---|---|---|---|
| Single cells |
| Yes | Yes |
|
| Spheroids |
| Not performed yet | Not performed yet | - |
| Cell sheets |
| - | - | - |
| Organoids |
| Not performed yet | Not performed yet | - |
| Extracellular vesicles |
| Yes | Yes |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taninokuchi Tomassoni, M.; Zhou, Y.; Braccischi, L.; Modestino, F.; Fukuda, J.; Mosconi, C. Trans-Arterial Stem Cell Injection (TASI): The Role of Interventional Radiology in Regenerative Medicine. J. Clin. Med. 2024, 13, 910. https://doi.org/10.3390/jcm13030910
Taninokuchi Tomassoni M, Zhou Y, Braccischi L, Modestino F, Fukuda J, Mosconi C. Trans-Arterial Stem Cell Injection (TASI): The Role of Interventional Radiology in Regenerative Medicine. Journal of Clinical Medicine. 2024; 13(3):910. https://doi.org/10.3390/jcm13030910
Chicago/Turabian StyleTaninokuchi Tomassoni, Makoto, Yinghui Zhou, Lorenzo Braccischi, Francesco Modestino, Junji Fukuda, and Cristina Mosconi. 2024. "Trans-Arterial Stem Cell Injection (TASI): The Role of Interventional Radiology in Regenerative Medicine" Journal of Clinical Medicine 13, no. 3: 910. https://doi.org/10.3390/jcm13030910
APA StyleTaninokuchi Tomassoni, M., Zhou, Y., Braccischi, L., Modestino, F., Fukuda, J., & Mosconi, C. (2024). Trans-Arterial Stem Cell Injection (TASI): The Role of Interventional Radiology in Regenerative Medicine. Journal of Clinical Medicine, 13(3), 910. https://doi.org/10.3390/jcm13030910








