Programmatically Localizing Diabetic Retinopathy Features in 45-Degree Retinal Photographs Using Anatomical Colocation
Abstract
1. Introduction
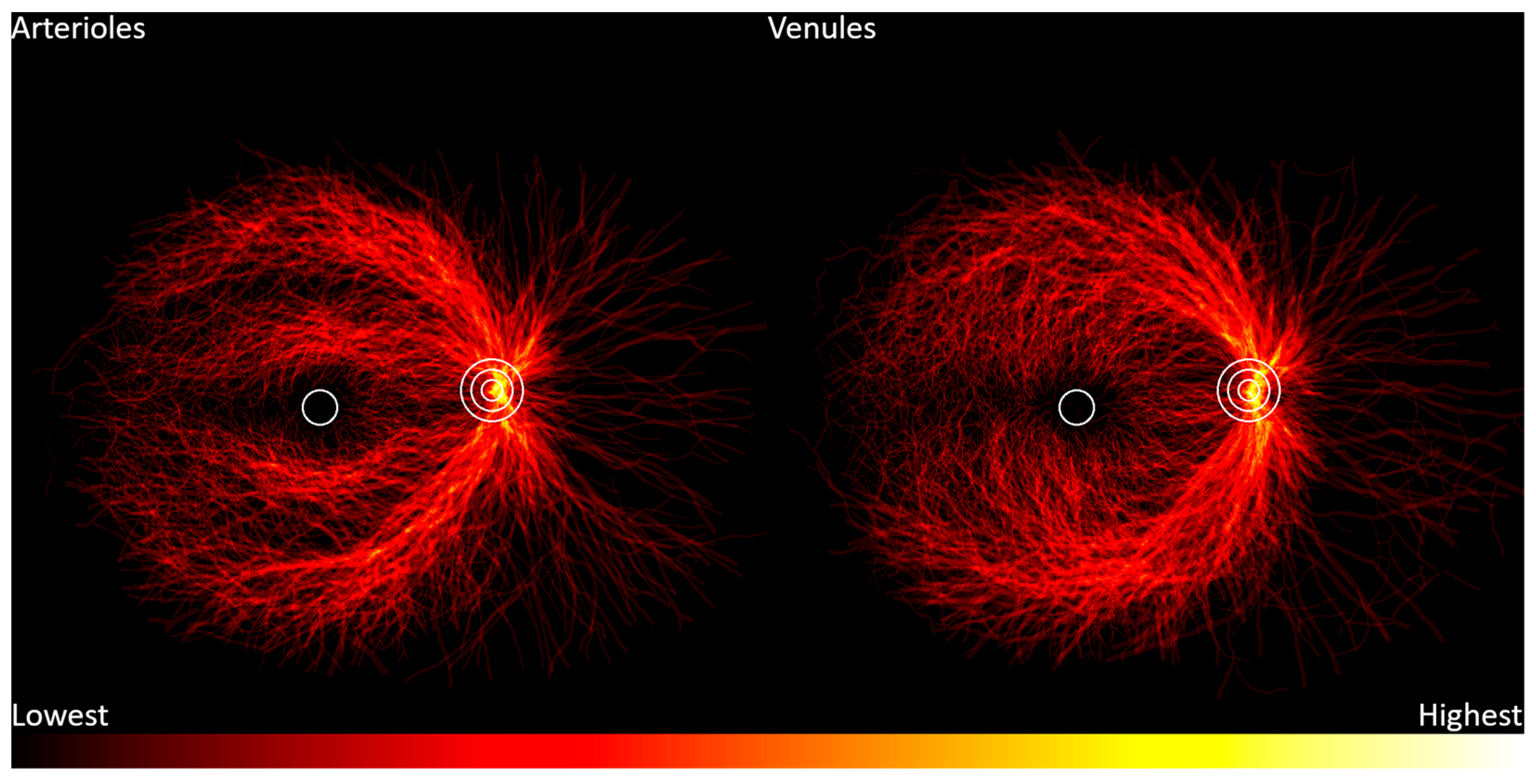
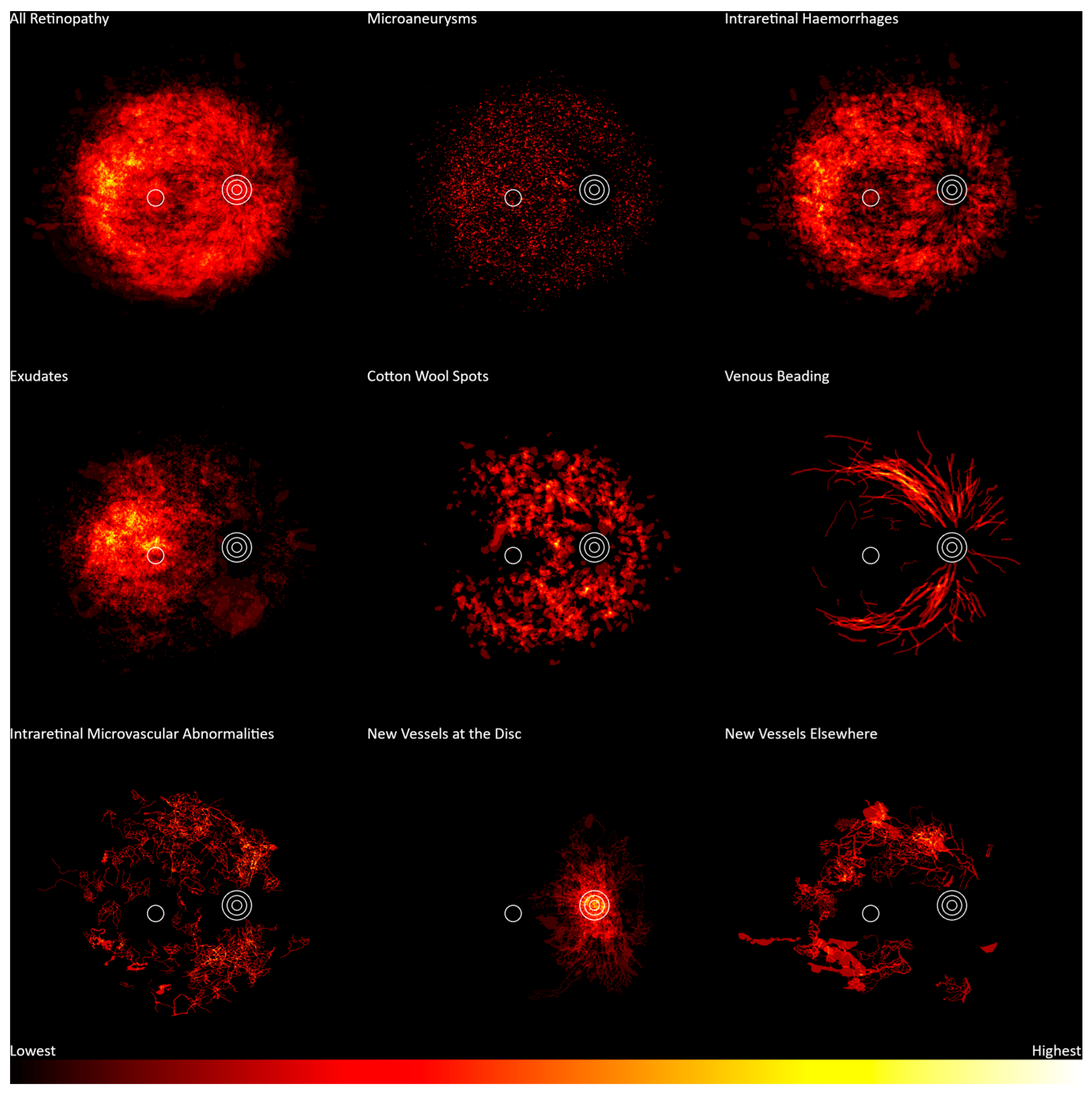
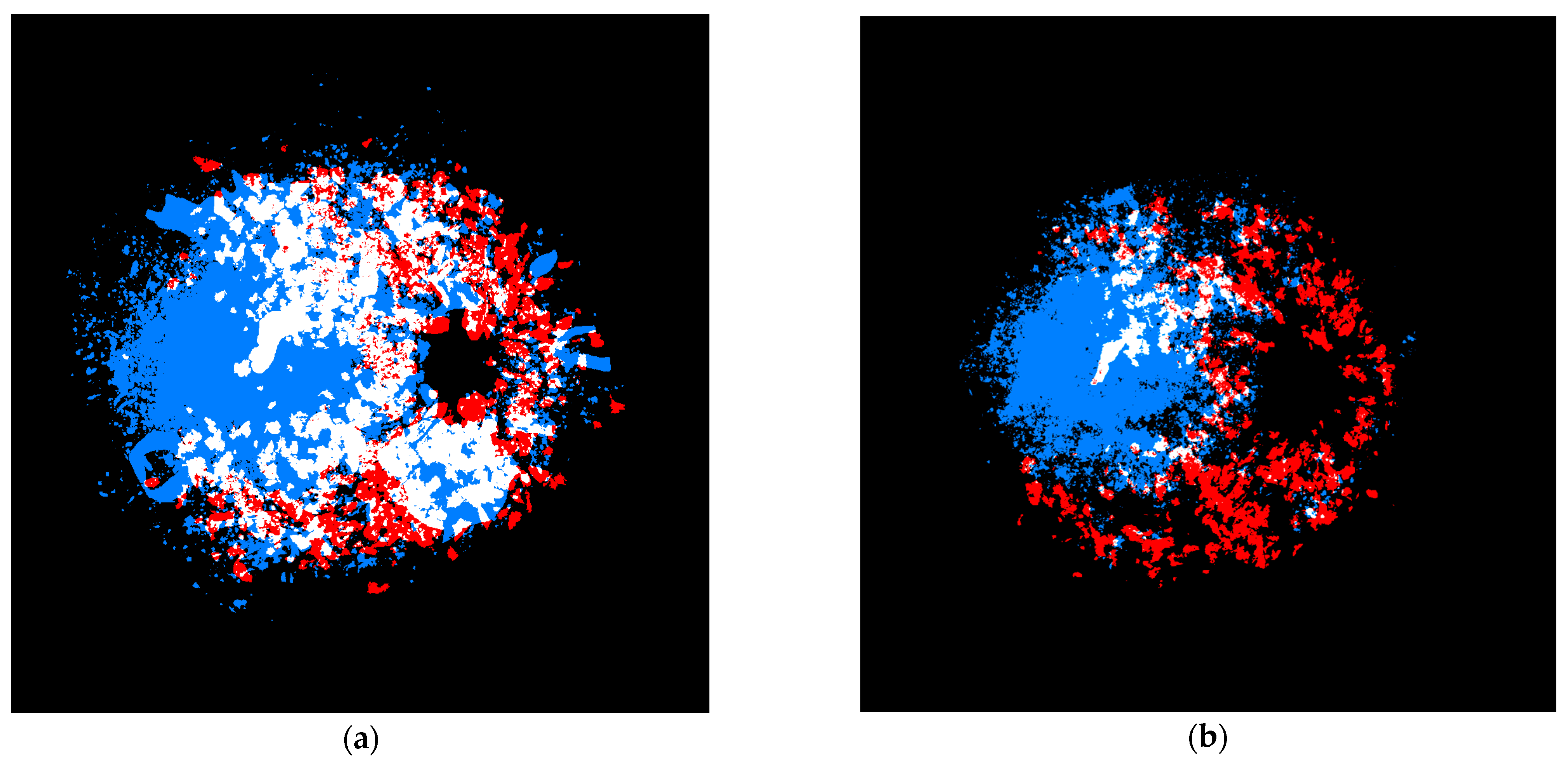
2. Materials and Methods
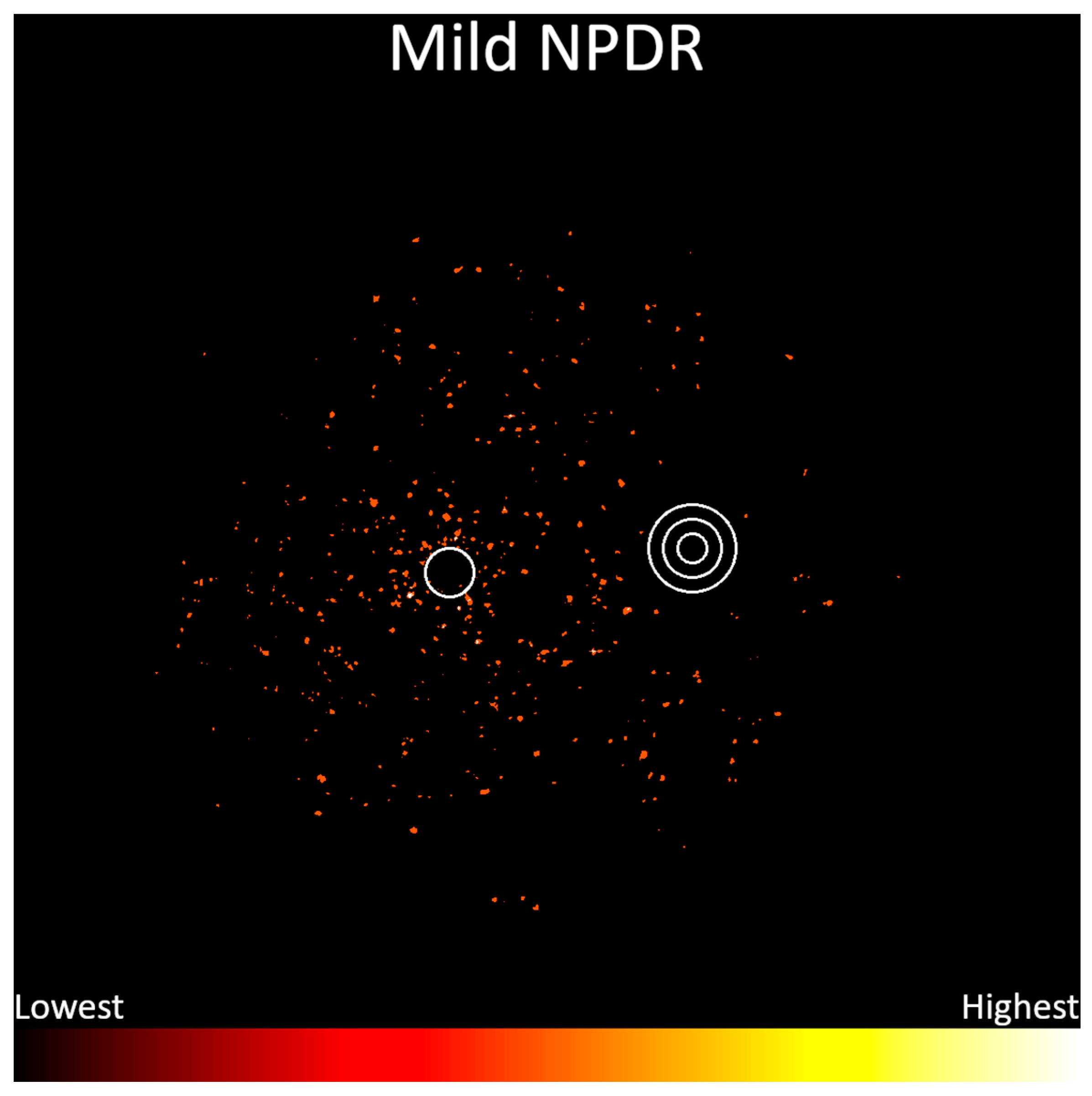
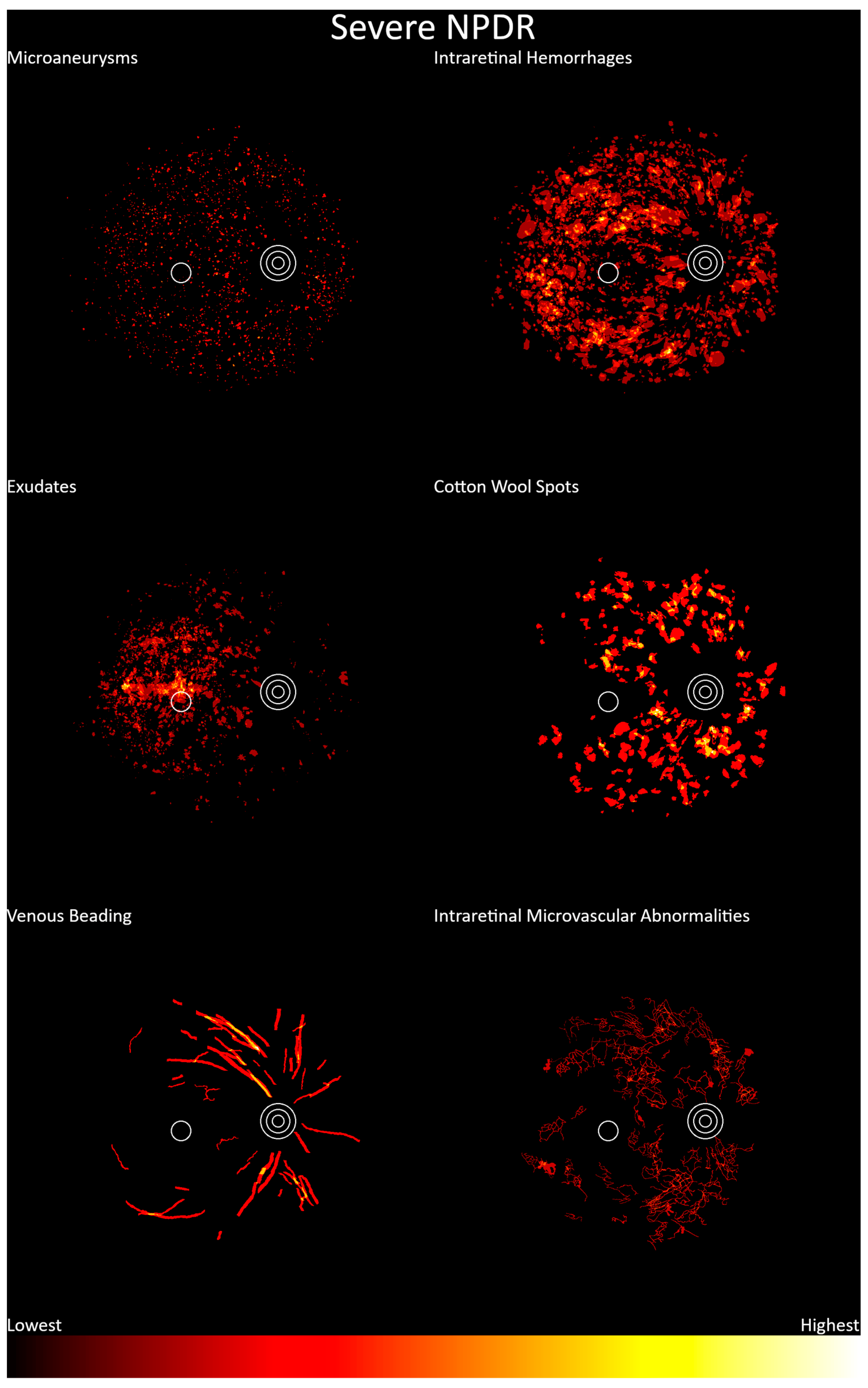
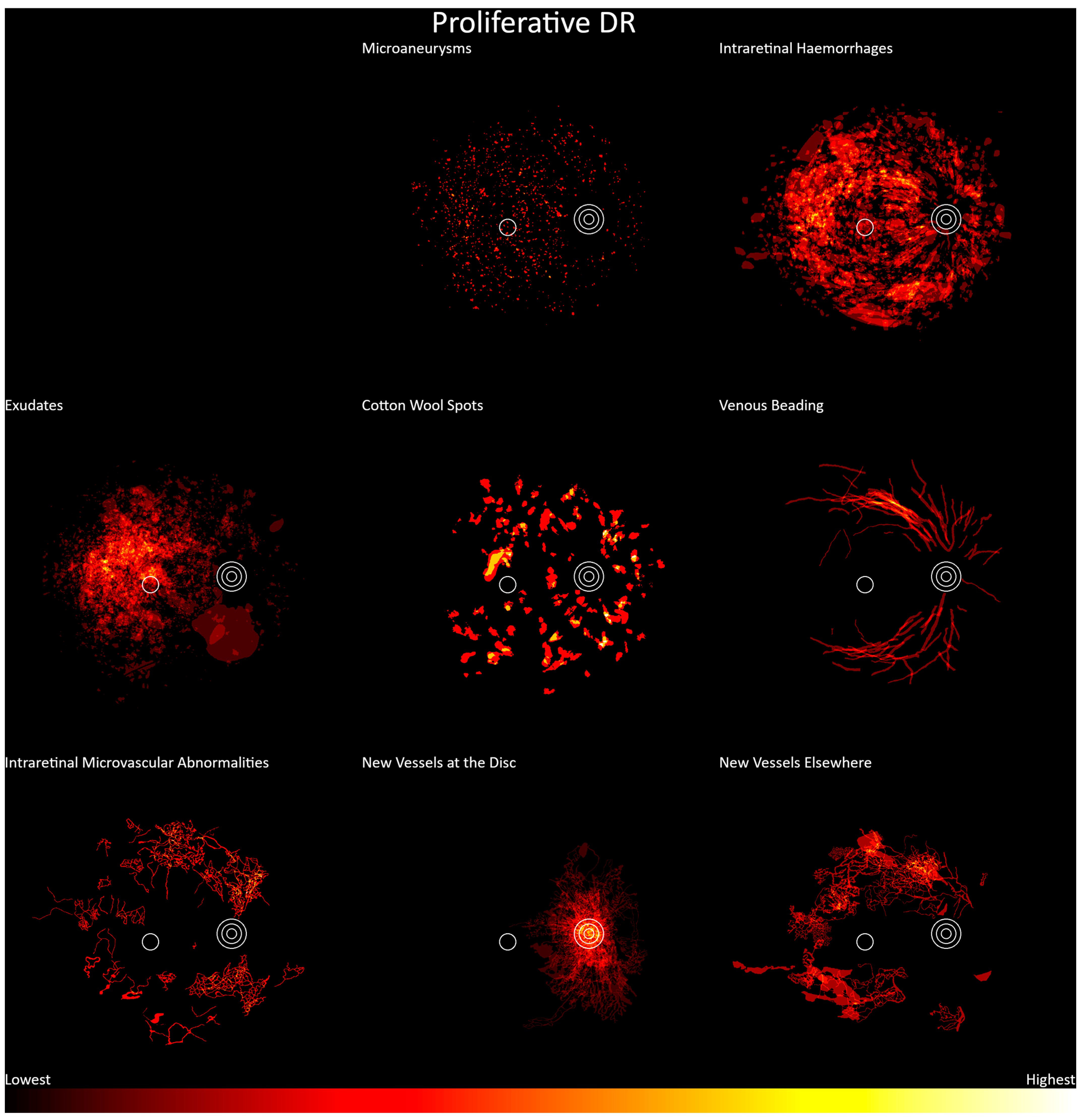
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 978-2-930229-98-0. [Google Scholar]
- McKay, R.; McCarty, C.A.; Taylor, H.R. Diabetic Retinopathy in Victoria, Australia: The Visual Impairment Project. Br. J. Ophthalmol. 2000, 84, 865. [Google Scholar] [CrossRef]
- Harrison, W.W.; Yevseyenkov, V. Early Interventions to Prevent Retinal Vasculopathy in Diabetes: A Review. Clin. Optom. 2015, 2015, 71–80. [Google Scholar] [CrossRef]
- Mitchell, P.; Foran, S.; Wong, T.Y.; Chua, B.; Patel, I.; Ojaimi, E.; Foran, J. Guidelines for the Management of Diabetic Retinopathy; National Health and Medical Research Council: Melbourne, Australia, 2008. [Google Scholar]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on Diabetic Eye Care; The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018, 125, 1608. [Google Scholar] [CrossRef]
- Public Health England Diabetic Eye Screening Standards Valid for Data Collected from 1 April 2019. Available online: https://www.gov.uk/government/publications/diabetic-eye-screening-programme-standards/diabetic-eye-screening-standards-valid-for-data-collected-from-1-april-2019 (accessed on 5 December 2023).
- Wilkinson, C.P.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Fundus Photographic Risk Factors for Progression of Diabetic Retinopathy: ETDRS Report Number 12. Ophthalmology 1991, 98, 823–833. [Google Scholar] [CrossRef]
- Tang, H.L.; Goh, J.; Peto, T.; Ling, B.W.-K.; Al Turk, L.I.; Hu, Y.; Wang, S.; Saleh, G.M. The Reading of Components of Diabetic Retinopathy: An Evolutionary Approach for Filtering Normal Digital Fundus Imaging in Screening and Population Based Studies. PLoS ONE 2013, 8, e66730. [Google Scholar] [CrossRef][Green Version]
- Sellahewa, L.; Simpson, C.; Maharajan, P.; Duffy, J.; Idris, I. Grader Agreement, and Sensitivity and Specificity of Digital Photography in a Community Optometry-Based Diabetic Eye Screening Program. Clin. Ophthalmol. 2014, 8, 1345. [Google Scholar] [CrossRef]
- An, D.; Chandrasekera, E.; Yu, D.-Y.; Balaratnasingam, C. Non-Proliferative Diabetic Retinopathy Is Characterized by Nonuniform Alterations of Peripapillary Capillary Networks. Investig. Ophthalmol. Vis. Sci. 2020, 61, 39. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Zhang, L.; Cui, Y.; Zhang, G.; Wang, J.; Zhang, A.; Chen, X.; Huang, T.; Meng, Q. Differential Distribution of Manifest Lesions in Diabetic Retinopathy by Fundus Fluorescein Angiography and Fundus Photography. BMC Ophthalmol. 2020, 20, 471. [Google Scholar] [CrossRef]
- McLeod, D. Why Cotton Wool Spots Should Not Be Regarded as Retinal Nerve Fibre Layer Infarcts. Br. J. Ophthalmol. 2005, 89, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N. Pathophysiology of retinal cotton-wool spots. Br. Med. Bull. 1970, 26, 143–150. [Google Scholar] [CrossRef]
- Silva, P.S.; Cavallerano, J.D.; Sun, J.K.; Soliman, A.Z.; Aiello, L.M.; Aiello, L.P. Peripheral Lesions Identified by Mydriatic Ultrawide Field Imaging: Distribution and Potential Impact on Diabetic Retinopathy Severity. Ophthalmology 2013, 120, 2587–2595. [Google Scholar] [CrossRef]
- Verma, A.; Alagorie, A.R.; Ramasamy, K.; van Hemert, J.; Yadav, N.K.; Pappuru, R.R.; Tufail, A.; Nittala, M.G.; Sadda, S.R.; Raman, R. Distribution of Peripheral Lesions Identified by Mydriatic Ultra-Wide Field Fundus Imaging in Diabetic Retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 725–733. [Google Scholar] [CrossRef]
- Agrawal, M.; Singhal, A.; Kumar, P.; Vats, S.; Kaushik, J.; Srujana, D.; Yadav, A. Pattern and Distribution of Neovascularization in Proliferative Diabetic Retinopathy on Fundus Fluorescein Angiography: A Growing Paradigm. Med. J. Armed Forces India 2023, 79, 207–212. [Google Scholar] [CrossRef]
- Munuera-Gifre, E.; Saez, M.; Rodríguez-Poncelas, A.; Barceló, M.A.; Coll-de-Tuero, G.; Juvinyà-Canals, D.; Barrot-de-la–Puente, J.-F.; Franch-Nadal, J.; Romero-Aroca, P. Analysis of the Location of Retinal Lesions in Central Retinographies of Patients with Type 2 Diabetes. Acta Ophthalmol. 2020, 98, e13–e21. [Google Scholar] [CrossRef]
- Li, T.; Gao, Y.; Wang, K.; Guo, S.; Liu, H.; Kang, H. Diagnostic Assessment of Deep Learning Algorithms for Diabetic Retinopathy Screening. Inf. Sci. 2019, 501, 511–522. [Google Scholar] [CrossRef]
- Hu, Q.; Abràmoff, M.D.; Garvin, M.K. Automated Separation of Binary Overlapping Trees in Low-Contrast Color Retinal Images. Med. Image Comput. Comput. Assist. Interv. 2013, 16, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Sureshjani, S.; Smit-Ockeloen, I.; Zhang, J.; Ter Haar Romeny, B. Biologically-Inspired Supervised Vasculature Segmentation in SLO Retinal Fundus Images BT—Image Analysis and Recognition; Kamel, M., Campilho, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 325–334. [Google Scholar]
- Vélez Escolà, L.; Galán Terraza, A.; Lagrèze, W.A.; Martín Begué, N.; Puig Galy, J.; Velázquez Villoria, D.; Arcos Algaba, G.; Mora Ramírez, D.; García-Arumí, J. Disc-Foveal Angle and Ocular Counterrolling as a Key in Its Interpretation. Arch. Soc. Española Oftalmol. 2019, 94, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; LabelImg. V. 1.8.5 Github 2021. Available online: https://github.com/HumanSignal/labelImg (accessed on 4 August 2021).
- Sørensen, T.J. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species Content and Its Application to Analyses of the Vegetation on Danish Commons; I kommission hos E. Munksgaard: Copenhagen, Denmark, 1948. [Google Scholar]
- Dice, L.R. Measures of the Amount of Ecologic Association between Species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Barbosa, M.; Maddess, T.; Ahn, S.; Chan-Ling, T. Novel Morphometric Analysis of Higher Order Structure of Human Radial Peri-Papillary Capillaries: Relevance to Retinal Perfusion Efficiency and Age. Sci. Rep. 2019, 9, 13464. [Google Scholar] [CrossRef]
- Garhofer, G.; Werkmeister, R.; Dragostinoff, N.; Schmetterer, L. Retinal Blood Flow in Healthy Young Subjects. Investig. Ophthalmol. Vis. Sci. 2012, 53, 698–703. [Google Scholar] [CrossRef]
- Gardiner, T.A.; Archer, D.B.; Curtis, T.M.; Stitt, A.W. Arteriolar Involvement in the Microvascular Lesions of Diabetic Retinopathy: Implications for Pathogenesis. Microcirculation 2007, 14, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Engerman, R.L. Vascular Lesions in Diabetes Are Distributed Non-Uniformly within the Retina. Exp. Eye Res. 1995, 60, 545–549. [Google Scholar] [CrossRef]
- Cunha-Vaz, J. Diabetic Retinopathy; World Scientific: Singapore, 2011; ISBN 9789814304436. [Google Scholar]
- Bhimavarapu, U.; Chintalapudi, N.; Battineni, G. Automatic Detection and Classification of Diabetic Retinopathy Using the Improved Pooling Function in the Convolution Neural Network. Diagnostics 2023, 13, 2606. [Google Scholar] [CrossRef]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. Int. J. Comput. Vis. 2020, 128, 336–359. [Google Scholar] [CrossRef]
- Zeiler, M.D.; Fergus, R. Visualizing and Understanding Convolutional Networks; Fleet, D., Pajdla, T., Schiele, B., Tuytelaars, T., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 818–833. [Google Scholar]
- Murphy, T.I.; Armitage, J.A.; van Wijngaarden, P.; Abel, L.A.; Douglass, A.G. A Guide to Optometrists for Appraising and Using Artificial Intelligence in Clinical Practice. Clin. Exp. Optom. 2023, 106, 569–579. [Google Scholar] [CrossRef]
- Niemeijer, M.; Staal, J.; van Ginneken, B.; Loog, M.; Abramoff, M.D. Comparative Study of Retinal Vessel Segmentation Methods on a New Publicly Available Database. In Proceedings of the SPIE, Medical Imaging 2004: Image Processing, San Diego, CA, USA, 12 May 2004; Volume 5370. [Google Scholar]
- Tillin, T.; Evans, R.M.; Witt, N.W.; Sharp, P.S.; McKeigue, P.M.; Chaturvedi, N.; Hughes, A.D. Ethnic Differences in Retinal Microvascular Structure. Diabetologia 2008, 51, 1719–1722. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Wong, W.L.; Cheung, C.Y.; Cheng, C.-Y.; Ikram, M.K.; Li, J.; Chia, K.S.; Wong, T.Y. Racial Differences in Retinal Vessel Geometric Characteristics: A Multiethnic Study in Healthy Asians. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3650–3656. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Gupta, B.; Crosby-Nwaobi, R.; Evans, J. Prevalence of Diabetic Retinopathy in Various Ethnic Groups: A Worldwide Perspective. Surv. Ophthalmol. 2012, 57, 347–370. [Google Scholar] [CrossRef]


| Image | Old Grade | New Grade | Comments |
|---|---|---|---|
| 007-2689-100.jpg | Mild NPDR | Moderate NPDR | Hemorrhages present |
| 007-2697-100.jpg | Mild NPDR | Moderate NPDR | Hemorrhages present |
| 007-2753-100.jpg | Moderate NPDR | Proliferative DR | NVD present |
| 007-2788-100.jpg | Mild NPDR | Severe NPDR | Venous beading and hemorrhages present |
| 007-3308-200.jpg | Moderate NPDR | Severe NPDR | Venous beading and hemorrhages present |
| 007-3527-200.jpg | Moderate NPDR | Severe NPDR | Venous beading in two quadrants |
| 007-3916-200.jpg | Moderate NPDR | Severe NPDR | Venous beading in two quadrants |
| 007-3933-200.jpg | Moderate NPDR | Proliferative DR | NVD present |
| 007-3975-200.jpg | Moderate NPDR | Severe NPDR | IRMA present |
| 007-4268-200.jpg | Moderate NPDR | Proliferative DR | NVD present |
| 007-4847-300.jpg | Severe NPDR | Proliferative DR | NVD present |
| 007-5106-300.jpg | Moderate NPDR | Severe NPDR | IRMA present |
| 007-5135-300.jpg | Moderate NPDR | Severe NPDR | IRMA present |
| 007-5434-300.jpg | Moderate NPDR | Severe NPDR | IRMA present |
| 007-5885-300.jpg | Severe NPDR | Proliferative DR | NVD present |
| 20170505094751770.jpg | Moderate NPDR | Severe NPDR | IRMA present |
| 20170518092011904.jpg | Moderate NPDR | Severe NPDR | IRMA present |
| 20170602155248106.jpg | Severe NPDR | Proliferative DR | NVD present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, T.I.; Douglass, A.G.; van Wijngaarden, P.; Armitage, J.A. Programmatically Localizing Diabetic Retinopathy Features in 45-Degree Retinal Photographs Using Anatomical Colocation. J. Clin. Med. 2024, 13, 807. https://doi.org/10.3390/jcm13030807
Murphy TI, Douglass AG, van Wijngaarden P, Armitage JA. Programmatically Localizing Diabetic Retinopathy Features in 45-Degree Retinal Photographs Using Anatomical Colocation. Journal of Clinical Medicine. 2024; 13(3):807. https://doi.org/10.3390/jcm13030807
Chicago/Turabian StyleMurphy, Timothy I., Amanda G. Douglass, Peter van Wijngaarden, and James A. Armitage. 2024. "Programmatically Localizing Diabetic Retinopathy Features in 45-Degree Retinal Photographs Using Anatomical Colocation" Journal of Clinical Medicine 13, no. 3: 807. https://doi.org/10.3390/jcm13030807
APA StyleMurphy, T. I., Douglass, A. G., van Wijngaarden, P., & Armitage, J. A. (2024). Programmatically Localizing Diabetic Retinopathy Features in 45-Degree Retinal Photographs Using Anatomical Colocation. Journal of Clinical Medicine, 13(3), 807. https://doi.org/10.3390/jcm13030807







