Abstract
The present review discusses restrictive perioperative fluid protocols within enhanced recovery after surgery (ERAS) pathways. Standardized definitions of a restrictive or liberal fluid regimen are lacking since they depend on conflicting evidence, institutional protocols, and personal preferences. Challenges related to restrictive fluid protocols are related to proper patient selection within standardized ERAS protocols. On the other hand, invasive goal-directed fluid therapy (GDFT) is reserved for more challenging disease presentations and polymorbid and frail patients. While the perfusion rate (mL/kg/h) appears less predictive for postoperative outcomes, the authors identified critical thresholds related to total intravenous fluids and weight gain. These thresholds are discussed within the available evidence. The authors aim to introduce their institutional approach to standardized practice.
1. Introduction
Over the last 20 years, fluid management has been increasingly recognized as a sensitive and modifiable parameter of perioperative care, directly affecting postoperative outcomes [1,2,3]. However, the optimal amount of perioperative fluid administration is controversial, and standardized definitions of a restrictive or liberal regimen are lacking due to conflicting evidence, institutional protocols, and personal preferences [4,5]. In line with these findings, a recent meta-analysis revealed various intra- and postoperative fluid volumes [6].
On the one hand, peri- and postoperative fluids are essential to maintain adequate organ perfusion and tissue fluid homeostasis [7]. An overly restrictive approach may lead to hypotension and decreased organ perfusion, ultimately associated with acute kidney injury (AKI) [4]. Furthermore, perioperative organ injury due to both inflammation and ischemia (due to a demand–supply mismatch) represents a potential hazard, thus needing preventive measures and close perioperative monitoring [8]. Enhanced recovery after surgery (ERAS) pathways aim to decrease the physiological surgical stress response represented by a state of insulin resistance [9]. Several measures, including preoperative carbohydrate loading, perioperative feeding strategies, minimally invasive surgery, and early resumption of a normal diet help to modulate the stress response, promote insulin sensitivity, and attenuate the breakdown of protein. Further consequences related to decreased organ perfusion due to an overly restrictive approach may be cardiovascular dysfunction (perioperative myocardial ischemia due to tachycardia, hypotension, hypoxia, or anemia), neurological complications (including confusional states or delirium), and intestinal dysfunction (including splanchnic or anastomotic hypoperfusion), which may be exacerbated by an excessive use of vasopressors [10,11].
On the other hand, fluid overload may result in harmful “third space” weight gain, associated with higher rates of pulmonary complications, postoperative ileus, altered mental status, and edema-related anastomotic complications, thus impeding postoperative recovery [12,13,14,15,16]. Furthermore, an excessive extracellular fluid volume may lead to abdominal compartment syndrome, which by itself may trigger adverse physiologic effects such as respiratory failure and renal failure [17]. In light of these findings, definitions must be set to guide clinical practice.
In the setting of established ERAS pathways, the authors’ institutions attempted to identify “safety” fluid thresholds for colorectal resections [13,18,19]. The present review aims to define optimal fluid management, provide an overview of suggested thresholds, and discuss this institutional practice in the light of available evidence.
2. What Is Optimal Fluid Management?
Optimal fluid management implies a normovolemic state during and beyond the surgical procedure without fluid management-related complications due to overly restrictive or generous fluid administration, least possible postoperative weight gain, and prompt functional recovery. Whether a specific patient can be managed by noninvasive monitoring and according to a “zero fluid” approach as suggested by the ERAS guidelines mainly depends on the disease presentation, physiological state at the time of surgery, comorbidities, and patient frailty [2]. A euvolemic, otherwise healthy patient without significant comorbidities warranting close surveillance going into elective, minimally invasive surgery is thus eligible for a standardized, restrictive fluid strategy, considering the physiologic principles of euvolemia [5]. On the other hand, patients at risk presenting with an impaired physical condition and distress due to a more acute or emergent disease presentation should benefit from invasive monitoring techniques and be treated within a more liberal strategy according to their physiologic reactions to surgery in a non-elective, acute setting [6]. This is even more important given the fact that these fragile patients are prone to postoperative morbidity and are not eligible for a simplified restrictive approach. On the contrary, management of these patients implies several critical perioperative assessments, including an evaluation of fluid responsiveness triggering, if appropriate, the administration of fluid boluses to increase stroke volume [20]. Of note, such a protocol does not necessarily need hemodynamic monitoring devices for reliable prediction but can also be carried out using echography after a passive leg raising test or by inferior vena cava evaluation, both in mechanically ventilated and spontaneously breathing patients [21,22,23]. In line with these basic principles, both authors’ institutions aimed to standardize fluid management over the last years to implement preset thresholds related to IV fluids and weight gain as red flags for guidance in clinical practice.
Definition of a Restrictive versus Liberal Approach
To date, there is no standardized definition of restrictive fluid therapy. The Enhanced Recovery After Surgery (ERAS) guidelines recommend aiming for a “zero fluid” balance and euvolemia intraoperatively and during the first postoperative days in patients undergoing elective colorectal resections [24,25]. Pre-operatively, carbohydrate loading and unrestricted access to clear fluids until 2 h before anesthesia induction help maintain fluid homeostasis and initiate surgery in a euvolemic, physiological state. Intraoperatively, a basal rate of crystalloid solution of <4 mL/kg/h is recommended [24,26]. This approach has been considered “restrictive”; however, its interpretation and application in clinical practice remain vague and subjective. Patients requiring goal-directed fluid therapy (GDFT) should receive boluses to maintain the cardiac stroke volume and, hence, central normovolemia [6]. However, recent guidance reserves a GDFT approach for high-risk patients (e.g., frailty and cardiopulmonary dysfunction) and high-risk procedures (e.g., emergent setting and disease-related distress) with large intravascular fluid loss [25,27,28]. Postoperatively, both early IV fluid lock and resumption of liquids and solids allow for adherence to the natural process of fluid homeostasis according to individual needs [29].
In a recent meta-analysis including 18 randomized controlled trials, the median intraoperative fluid administrated in the restrictive group was 1930 mL (interquartile range (IQR): 1480–2470 mL) compared to 3880 mL (IQR: 3000–4400 mL) in the liberal group [30]. On postoperative day 1, the median volume of intravenous fluids was 2340 mL (IQR 1640–3530 mL) versus 4350 mL (3100–5330 mL), respectively. However, important differences were observed among individual trials regarding total fluid volumes in the restrictive and liberal groups [30,31]. Consequently, a liberal approach in a specific trial could be equivalent to a restrictive approach in another trial [30,32]. While the concept of fluid restriction outside high-risk patients and procedures is widely accepted, “safety” thresholds may be valuable adjuncts and serve as red flags for clinical guidance during anesthesia and postoperative surveillance. Several randomized controlled trials compared both approaches (restrictive vs. liberal) and reported on fluid-related thresholds and postoperative complications, as summarized in Table 1.

Table 1.
Randomized controlled trials comparing restrictive and liberal fluid regimens.
Table 1 provides an overview of published RCTs comparing restrictive and liberal groups.
In a former meta-analysis, Varadhan et al. suggested stratifying fluid regimens of the perioperative day into restrictive (<1750 mL/d), balanced (1750–2750 mL/d), and liberal (>2750 mL/d) [32]. The balanced fluid range was calculated to compensate for the daily physiological water loss for an average human in a homeostatic state, estimated between 25–35 mL/kg [46,47]. This volume is supposed to replace the perioperative body water loss to approach a zero fluid balance. Interestingly, this upper cut-off of 2.7 L was independently confirmed by an institutional series of the Mayo Clinic [13].
3. Impact of Fluid Overload on Postoperative Complications
A considerable weight gain of >6 kg after elective colorectal surgery has been observed in several studies, requiring close postoperative surveillance to prevent associated complications, especially in fragile patients prone to pulmonary complications [33]. However, fluid management in these fragile patients represents a particular challenge given that they are at increased risk of experiencing postoperative morbidity. This impedes uncritical assumptions of cause (fluid overload) and effect (complications) patterns. While some of the data suggest a modest correlation between total perioperative IV fluid administration and weight gain [48,49], a dose–response correlation with consequent increased complication rates was observed by others [33,50]. Despite the seemingly easy-to-perform weight measurements in the postoperative period, postoperative weight is reported in only 50% of randomized controlled studies [30], Table 1.
Fluid overload induces prolonged gastric emptying [5], which, together with bowel edema and interstitial third space fluids, causes postoperative ileus (POI). The series of both our institutions confirmed an independent effect of fluid overload and weight gain on POI occurrence [18,51]. These findings were confirmed by others and independently validated [52,53,54]. Furthermore, similar associations were observed in the setting of ostomy procedures [55,56].
Pulmonary complications after surgery are a major concern, with an occurrence of up to 23% [12,57]. Fluid overload of the interstitial space triggers pulmonary edema, especially in patients with impaired cardiac function [57,58]. A significant decrease in mean blood saturation on the second night after surgery was observed in patients within the liberal fluid administration group; however, there was no increased morbidity in this study [37]. However, the results are conflicting, and cause–effect patterns are hard to establish in fragile patients with cardiopulmonary impairment. Several studies, including an institutional series, revealed that fluid overload and weight gain are associated with an increased risk of pulmonary complications [12,50,59].
Impact of Fluid Management on Renal Function
While perioperative hypotension may impact on several organs, a major concern of overly restrictive perioperative fluid administration is the development of AKI. The evidence is conflicting. A meta-analysis revealed a higher AKI rate in the restrictive group [30]. Further data suggest that even a minor increase in creatinine levels could increase in-hospital mortality in non-cardiac surgical patients [60]. However, no cause–effect patterns could be established due to its retrospective design. Myles et al. published a large multicentric randomized controlled landmark trial comparing restrictive versus liberal fluid administration in major abdominal surgery [4]. In their study, the restrictive approach had no impact on disability-free survival but was associated with a statistically significant AKI increase (8.6% vs. 5% in the restrictive and liberal groups, respectively). Notably, around 50% of patients in this trial were not treated according to the ERAS principles, impeding uncritical extrapolation of the results to the setting of our institutions offering care within longstanding, established, and standardized ERAS pathways [61,62]. A sizeable institutional series of elective patients revealed a low AKI rate of 2.5% according to loss of kidney function and end-stage kidney disease (RIFLE) criteria [63]. In another series of our group, an intraoperative fluid range defined as “balanced” (300 mL–2700 mL) was associated with the lowest rate of POI and a prolonged length of stay but not AKI [13]. Restrictive fluid management during elective colorectal resections appears safe if carried out within standardized pathways and it is supported by respective societies [24,25,64].
4. Fluid Management in the Perioperative Period: Which Indicators
Intraoperative oliguria occurring in isolation should not trigger fluid boluses since the predictive value for postoperative AKI appears low [65]. An institutional series of the Mayo Clinic revealed that a certain degree of postoperative hypotension in up to 10% of patients may persist for less than 20 h without negatively impacting AKI occurrence, which affected <3% [66]. There is a broad consensus that a permissive attitude to physiologic oliguria due to renal vasoconstriction can be adopted in the elective ERAS setting, providing no established cause exists [25]. Based on the available information, intraoperative fluid management should be protocolized to determine an underlying physiologic problem requiring reversal [67]. Standard monitoring integrating clinical data is thus likely sufficient in low-risk procedures, combining maintenance fluids at a low rate of < 4 ml/kg/h in the intraoperative and early postoperative period in the post-anesthesia care unit. Outside this low-risk setting and depending on the surgical risk, GDFT, including advanced hemodynamic monitoring devices, should be used as valuable adjuncts in higher-risk patients or procedures, triggering fluid administration if a decreased cardiac output or surrogates are suspected [68,69].
5. Summary of Institutional Thresholds and Practice Guidance
Based on the above discussed evidence and considering a 7-year experience in ERAS care in both authors’ institutions at that time, our groups aimed not only to focus on established, evidence-based perioperative ERAS care but also to standardize fluid management [19]. The need to improve perioperative fluid management standards in our institutions was motivated by the rather low compliance with guidelines, despite growing ERAS experience [19]. Importantly, the aim was not to set inflexible, dogmatic thresholds but to help with guidance in clinical practice. Restrictive fluid management through a zero-balance practice in elective surgery represents one puzzle piece in a comprehensive care pathway aiming to maintain a physiologic state throughout the perioperative period, significantly impacting postoperative recovery [5].
In total, 11 cohort studies of the authors’ institutions described fluid management-related thresholds, as summarized in Table 2.

Table 2.
Fluid thresholds and related outcomes within the authors’ institutions.
The thresholds are displayed with their respective impact on specific outcomes or clinical consequences. Three papers from the Lausanne group tried to identify thresholds through receiver operating characteristics (ROC) curves in different surgical settings: minimally invasive surgery [50], open surgery [73], and lastly, surgery for urgent indications [72]. Interestingly, the thresholds did not differ significantly across the different settings. The Mayo group analyzed an independent large dataset of elective colorectal surgeries with a focus on POI, prolonged LOS, and AKI, which were plotted against the rate of intraoperative Ringer lactate (RL) infusion (mL/kg/h) and total intraoperative volume [13]. Total intraoperative RL ≥2.7 L was independently associated with POI and prolonged LOS, but not AKI. Of note, the infusion rate (ml/kg/h) was not retained as a superior predictive tool. Further work focused on patients undergoing major surgery and needing postoperative surveillance in an intermediate care unit [48]. In this particularly vulnerable subgroup of patients, the fluid balance and weight course showed only a modest correlation. Both institutions further focused on POI in their analyses and found comparable results, with a strong correlation of fluid overload and POI in patients undergoing major surgery [18] and in patients undergoing loop ileostomy closure [56]. In the largest dataset of the Mayo group with over 7000 patients, early AKI was very uncommon within the institutional ERP (2.5%), and long-term sequelae were exceptionally low [63]. Interestingly, AKI patients received higher amounts of POD 0 fluids and had increased postoperative weight gain at POD 2. A further study of the Lausanne group revealed a protective effect of high compliance with the ERAS protocol to prevent postoperative pulmonary complications [12]. A threshold of 4 kg at POD 2 appeared to be critical in this setting. Finally, both author groups showed increasing interest in short stay processes in recent years, and excess intraoperative fluids of >3 L turned out to impede early discharge and thus an outpatient strategy [70,71].
Taking the above summarized evidence together, a threshold of 3000 mL intraoperatively serves presently as a red flag in daily clinical practice in both authors’ institutions. In addition to the mere focus on IV fluids, weight gain at postoperative day 2 turned out to be a valid surrogate for fluid overload [18].
Besides IV fluid management, several further ERAS care items help to maintain tissue homeostasis and an euvolemic state [24]. Preoperative carbohydrate loading helps to attenuate the catabolic response through a reduction of insulin resistance in response to surgery [74]. Clear fluids can be safely ingested until 2 h before surgery, whereas 6 h fasting for solid food is sufficient [75]. While there is growing evidence in favor of combined mechanical and oral antibiotic bowel preparation, mechanical bowel preparation alone may lead to preoperative dehydration and electrolyte imbalances and should thus be avoided [76]. Postoperatively, early oral nutrition is advocated and has proven its benefits by several meta-analyses and has been endorsed by different nutritional societies [64]. Finally, early mobilization of at least 6 h per day is of utmost importance and helps to prevent muscle loss and to promote functional recovery due to a direct prokinetic effect on the intestines [77]. Figure 1 summarizes the pre, intra, and postoperative measures within the institutions’ standardized ERAS protocol.
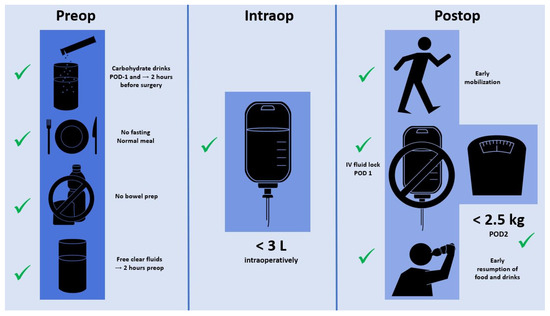
Figure 1.
Schematic representation of fluid management-related recommendations within the authors’ institutional ERAS pathways.
6. Implications in Daily Clinical Practice
The fast track concept that eventually led to standardized ERAS pathways was introduced 25 years ago by Henrik Kehlet and helped to simplify patient management by targeting the quality and speed of postoperative recovery [78]. Standardization of care is a way to facilitate patient management and improve a multidisciplinary team approach [79]. This holds true for surgical technique, but also intraoperative management and patient care in the ward. Postoperative care protocols with predefined care maps simplify the workflow, especially for frequently performed procedures. Perioperative fluid management represents a key element of ERAS care.
ERAS guidelines suggest aiming for a zero fluid balance for elective colorectal resections, while GDFT should be reserved for high-risk patients and procedures [24,25]. The use of vasopressors is advocated when fluid boluses fail to improve the stroke volume in order to prevent fluid overload [80]. The thresholds described in the present study and used in the authors’ institutions cannot replace careful individual risk-stratification in every patient before surgery. However, in the authors’ experience, they help with raising awareness among both surgeons and anesthesiologists to discuss fluid management during and after the procedure. Furthermore, a weight gain threshold of 2.5 kg at POD 2 serves as a useful point of reference in the surgical ward. Postoperative body weight is easy to assess and helps to timely launch counterregulatory measures [48,81]. In patients who exceed the threshold, subsequent fluid restriction, diuretics, and the promotion of mobilization can be initiated [50].
7. Conclusions
In conclusion, our practice of restrictive fluid management is based on institutional thresholds to help guide clinical practice, aiming to prevent deleterious fluid overload-related adverse outcomes.
Author Contributions
Conceptualization, P.D., J.J, M.H. and F.G.; methodology, P.D. and F.G.; validation, D.W.L. and C.B.; writing—original draft preparation, P.D., J.J. and F.G.; writing—review and editing, D.W.L., M.H. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Please contact the authors for specific data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prowle, J.R.; Kirwan, C.J.; Bellomo, R. Fluid management for the prevention and attenuation of acute kidney injury. Nat. Rev. Nephrol. 2014, 10, 37–47. [Google Scholar] [CrossRef]
- Voldby, A.W.; Brandstrup, B. Fluid therapy in the perioperative setting-a clinical review. J. Intensive Care 2016, 4, 27. [Google Scholar] [CrossRef]
- Aga, Z.; Machina, M.; McCluskey, S.A. Greater intravenous fluid volumes are associated with prolonged recovery after colorectal surgery: A retrospective cohort study. Br. J. Anaesth. 2016, 116, 804–810. [Google Scholar] [CrossRef]
- Myles, P.S.; Bellomo, R.; Corcoran, T.; Forbes, A.; Peyton, P.; Story, D.; Christophi, C.; Leslie, K.; McGuinness, S.; Parke, R.; et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N. Engl. J. Med. 2018, 378, 2263–2274. [Google Scholar] [CrossRef]
- Lobo, D.N.; Bostock, K.A.; Neal, K.R.; Perkins, A.C.; Rowlands, B.J.; Allison, S.P. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: A randomised controlled trial. Lancet 2002, 359, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Mathias, N.C.; Lobo, D.N. Meta-analysis of goal-directed fluid therapy using transoesophageal Doppler monitoring in patients undergoing elective colorectal surgery. BJS Open 2019, 3, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Buggy, D.J. Intraoperative fluids: How much is too much? Br. J. Anaesth. 2012, 109, 69–79. [Google Scholar] [CrossRef]
- Conrad, C.; Eltzschig, H.K. Disease Mechanisms of Perioperative Organ Injury. Anesth. Analg. 2020, 131, 1730–1750. [Google Scholar] [CrossRef]
- Carli, F. Physiologic considerations of Enhanced Recovery After Surgery (ERAS) programs: Implications of the stress response. Can. J. Anaesth. 2015, 62, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Botto, F.; Alonso-Coello, P.; Chan, M.T.; Villar, J.C.; Xavier, D.; Srinathan, S.; Guyatt, G.; Cruz, P.; Graham, M.; Wang, C.Y.; et al. Myocardial injury after noncardiac surgery: A large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014, 120, 564–578. [Google Scholar] [CrossRef]
- Klingensmith, N.J.; Coopersmith, C.M. The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness. Crit. Care Clin. 2016, 32, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Jurt, J.; Hubner, M.; Pache, B.; Hahnloser, D.; Demartines, N.; Grass, F. Respiratory Complications After Colorectal Surgery: Avoidable or Fate? World J. Surg. 2018, 42, 2708–2714. [Google Scholar] [CrossRef]
- Abd El Aziz, M.A.; Grass, F.; Calini, G.; Lovely, J.K.; Jacob, A.K.; Behm, K.T.; D’Angelo, A.D.; Shawki, S.F.; Mathis, K.L.; Larson, D.W. Intraoperative Fluid Management a Modifiable Risk Factor for Surgical Quality—Improving Standardized Practice. Ann. Surg. 2022, 275, 891–896. [Google Scholar] [CrossRef]
- Huisman, D.E.; Bootsma, B.T.; Ingwersen, E.W.; Reudink, M.; Slooter, G.D.; Stens, J.; Daams, F.; LekCheck Study, g. Fluid management and vasopressor use during colorectal surgery: The search for the optimal balance. Surg. Endosc. 2023, 37, 6062–6070. [Google Scholar] [CrossRef]
- Ouchi, A.; Sakuramoto, H.; Hoshino, H.; Matsuishi, Y.; Sakaguchi, T.; Enomoto, Y.; Hoshino, T.; Shimojo, N.; Inoue, Y. Association between fluid overload and delirium/coma in mechanically ventilated patients. Acute Med. Surg. 2020, 7, e508. [Google Scholar] [CrossRef]
- Boland, M.R.; Reynolds, I.; McCawley, N.; Galvin, E.; El-Masry, S.; Deasy, J.; McNamara, D.A. Liberal perioperative fluid administration is an independent risk factor for morbidity and is associated with longer hospital stay after rectal cancer surgery. Ann. R. Coll. Surg. Engl. 2017, 99, 113–116. [Google Scholar] [CrossRef][Green Version]
- Holte, K.; Sharrock, N.E.; Kehlet, H. Pathophysiology and clinical implications of perioperative fluid excess. Br. J. Anaesth. 2002, 89, 622–632. [Google Scholar] [CrossRef]
- Grass, F.; Lovely, J.K.; Crippa, J.; Hubner, M.; Mathis, K.L.; Larson, D.W. Potential Association Between Perioperative Fluid Management and Occurrence of Postoperative Ileus. Dis. Colon. Rectum 2020, 63, 68–74. [Google Scholar] [CrossRef]
- Grass, F.; Hubner, M.; Mathis, K.L.; Hahnloser, D.; Dozois, E.J.; Kelley, S.R.; Demartines, N.; Larson, D.W. Challenges to accomplish stringent fluid management standards 7 years after enhanced recovery after surgery implementation-The surgeon’s perspective. Surgery 2020, 168, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Malbrain, M.; Pinsky, M.R. The prediction of fluid responsiveness. Intensive Care Med. 2023, 49, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Marik, P.; Teboul, J.L. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; La Via, L.; Dezio, V.; Amelio, P.; Genoese, G.; Franchi, F.; Messina, A.; Robba, C.; Noto, A. Inferior vena cava distensibility from subcostal and trans-hepatic imaging using both M-mode or artificial intelligence: A prospective study on mechanically ventilated patients. Intensive Care Med. Exp. 2023, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; La Via, L.; Dezio, V.; Santonocito, C.; Amelio, P.; Genoese, G.; Astuto, M.; Noto, A. Assessment of the inferior vena cava collapsibility from subcostal and trans-hepatic imaging using both M-mode or artificial intelligence: A prospective study on healthy volunteers. Intensive Care Med. Exp. 2023, 11, 15. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef]
- Thiele, R.H.; Raghunathan, K.; Brudney, C.S.; Lobo, D.N.; Martin, D.; Senagore, A.; Cannesson, M.; Gan, T.J.; Mythen, M.M.; Shaw, A.D.; et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on perioperative fluid management within an enhanced recovery pathway for colorectal surgery. Perioper. Med. 2016, 5, 24. [Google Scholar] [CrossRef]
- Feldheiser, A.; Aziz, O.; Baldini, G.; Cox, B.P.; Fearon, K.C.; Feldman, L.S.; Gan, T.J.; Kennedy, R.H.; Ljungqvist, O.; Lobo, D.N.; et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: Consensus statement for anaesthesia practice. Acta Anaesthesiol. Scand. 2016, 60, 289–334. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.; Lemanu, D.P.; Singh, P.P.; Taylor, M.H.; Hill, A.G. Systematic review and meta-analysis of oesophageal Doppler-guided fluid management in colorectal surgery. Br. J. Surg. 2013, 100, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.; Taylor, M.H.; Singh, P.P.; Yu, T.C.; Soop, M.; Hill, A.G. Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br. J. Surg. 2013, 100, 66–74. [Google Scholar] [CrossRef]
- Braga, M.; Scatizzi, M.; Borghi, F.; Missana, G.; Radrizzani, D.; Gemma, M.; Perioperative Italian, S. Identification of core items in the enhanced recovery pathway. Clin. Nutr. ESPEN 2018, 25, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Robba, C.; Calabro, L.; Zambelli, D.; Iannuzzi, F.; Molinari, E.; Scarano, S.; Battaglini, D.; Baggiani, M.; De Mattei, G.; et al. Perioperative liberal versus restrictive fluid strategies and postoperative outcomes: A systematic review and metanalysis on randomised-controlled trials in major abdominal elective surgery. Crit. Care 2021, 25, 205. [Google Scholar] [CrossRef]
- Messina, A.; Robba, C.; Calabro, L.; Zambelli, D.; Iannuzzi, F.; Molinari, E.; Scarano, S.; Battaglini, D.; Baggiani, M.; De Mattei, G.; et al. Association between perioperative fluid administration and postoperative outcomes: A 20-year systematic review and a meta-analysis of randomized goal-directed trials in major visceral/noncardiac surgery. Crit. Care 2021, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Varadhan, K.K.; Lobo, D.N. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: Getting the balance right. Proc. Nutr. Soc. 2010, 69, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Brandstrup, B.; Tonnesen, H.; Beier-Holgersen, R.; Hjortso, E.; Ording, H.; Lindorff-Larsen, K.; Rasmussen, M.S.; Lanng, C.; Wallin, L.; Iversen, L.H.; et al. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann. Surg. 2003, 238, 641–648. [Google Scholar] [CrossRef]
- Nisanevich, V.; Felsenstein, I.; Almogy, G.; Weissman, C.; Einav, S.; Matot, I. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005, 103, 25–32. [Google Scholar] [CrossRef]
- Kabon, B.; Akca, O.; Taguchi, A.; Nagele, A.; Jebadurai, R.; Arkilic, C.F.; Sharma, N.; Ahluwalia, A.; Galandiuk, S.; Fleshman, J.; et al. Supplemental intravenous crystalloid administration does not reduce the risk of surgical wound infection. Anesth. Analg. 2005, 101, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- MacKay, G.; Fearon, K.; McConnachie, A.; Serpell, M.G.; Molloy, R.G.; O’Dwyer, P.J. Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. Br. J. Surg. 2006, 93, 1469–1474. [Google Scholar] [CrossRef]
- Holte, K.; Foss, N.B.; Andersen, J.; Valentiner, L.; Lund, C.; Bie, P.; Kehlet, H. Liberal or restrictive fluid administration in fast-track colonic surgery: A randomized, double-blind study. Br. J. Anaesth. 2007, 99, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Zalunardo, M.P.; Hubner, M.; Clavien, P.A.; Demartines, N.; Zurich Fast Track Study, G. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology 2009, 136, 842–847. [Google Scholar] [CrossRef] [PubMed]
- de Aguilar-Nascimento, J.E.; Diniz, B.N.; do Carmo, A.V.; Silveira, E.A.; Silva, R.M. Clinical benefits after the implementation of a protocol of restricted perioperative intravenous crystalloid fluids in major abdominal operations. World J. Surg. 2009, 33, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Constantin, J.M.; Petit, A.; Chanques, G.; Kwiatkowski, F.; Flamein, R.; Slim, K.; Sapin, V.; Jaber, S.; Bazin, J.E. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: A prospective randomized trial. Arch. Surg. 2010, 145, 1193–1200. [Google Scholar] [CrossRef]
- Abraham-Nordling, M.; Hjern, F.; Pollack, J.; Prytz, M.; Borg, T.; Kressner, U. Randomized clinical trial of fluid restriction in colorectal surgery. Br. J. Surg. 2012, 99, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kalyan, J.P.; Rosbergen, M.; Pal, N.; Sargen, K.; Fletcher, S.J.; Nunn, D.L.; Clark, A.; Williams, M.R.; Lewis, M.P. Randomized clinical trial of fluid and salt restriction compared with a controlled liberal regimen in elective gastrointestinal surgery. Br. J. Surg. 2013, 100, 1739–1746. [Google Scholar] [CrossRef]
- Jie, H.Y.; Ye, J.L.; Zhou, H.H.; Li, Y.X. Perioperative restricted fluid therapy preserves immunological function in patients with colorectal cancer. World J. Gastroenterol. 2014, 20, 15852–15859. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.D.; D’Souza, B.; Rattray, M.J.; Johnston, M.J.; Cowie, B.S. A randomised controlled trial of fluid restriction compared to oesophageal Doppler-guided goal-directed fluid therapy in elective major colorectal surgery within an Enhanced Recovery After Surgery program. Anaesth. Intensive Care 2014, 42, 752–760. [Google Scholar] [CrossRef]
- Gomez-Izquierdo, J.C.; Trainito, A.; Mirzakandov, D.; Stein, B.L.; Liberman, S.; Charlebois, P.; Pecorelli, N.; Feldman, L.S.; Carli, F.; Baldini, G. Goal-directed Fluid Therapy Does Not Reduce Primary Postoperative Ileus after Elective Laparoscopic Colorectal Surgery: A Randomized Controlled Trial. Anesthesiology 2017, 127, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.N. Fluid, electrolytes and nutrition: Physiological and clinical aspects. Proc. Nutr. Soc. 2004, 63, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.; Langer, T.; Annane, D.; Gattinoni, L.; Elbers, P.; Hahn, R.G.; De Laet, I.; Minini, A.; Wong, A.; Ince, C.; et al. Intravenous fluid therapy in the perioperative and critical care setting: Executive summary of the International Fluid Academy (IFA). Ann. Intensive Care 2020, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Butti, F.; Pache, B.; Winiker, M.; Grass, F.; Demartines, N.; Hubner, M. Correlation of postoperative fluid balance and weight and their impact on outcomes. Langenbecks Arch. Surg. 2020, 405, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Tolstrup, J.; Brandstrup, B. Clinical Assessment of Fluid Balance is Incomplete for Colorectal Surgical Patients. Scand. J. Surg. 2015, 104, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Hubner, M.; Pache, B.; Sola, J.; Blanc, C.; Hahnloser, D.; Demartines, N.; Grass, F. Thresholds for optimal fluid administration and weight gain after laparoscopic colorectal surgery. BJS Open 2019, 3, 532–538. [Google Scholar] [CrossRef]
- Grass, F.; Slieker, J.; Jurt, J.; Kummer, A.; Sola, J.; Hahnloser, D.; Demartines, N.; Hubner, M. Postoperative ileus in an enhanced recovery pathway-a retrospective cohort study. Int. J. Color. Dis. 2017, 32, 675–681. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Wu, J.; Zheng, H.; Yang, C. Nomogram for prediction of prolonged postoperative ileus after colorectal resection. BMC Cancer 2022, 22, 1273. [Google Scholar] [CrossRef] [PubMed]
- Wolthuis, A.M.; Bislenghi, G.; Fieuws, S.; de Buck van Overstraeten, A.; Boeckxstaens, G.; D’Hoore, A. Incidence of prolonged postoperative ileus after colorectal surgery: A systematic review and meta-analysis. Color. Dis. 2016, 18, O1–O9. [Google Scholar] [CrossRef]
- Shereef, A.; Raftery, D.; Sneddon, F.; Emslie, K.; Mair, L.; Mackay, C.; Ramsay, G.; Forget, P. Prolonged Ileus after Colorectal Surgery, a Systematic Review. J. Clin. Med. 2023, 12, 5769. [Google Scholar] [CrossRef]
- Greenberg, A.L.; Kelly, Y.M.; McKay, R.E.; Varma, M.G.; Sarin, A. Risk factors and outcomes associated with postoperative ileus following ileostomy formation: A retrospective study. Perioper. Med. 2021, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Grass, F.; Pache, B.; Butti, F.; Sola, J.; Hahnloser, D.; Demartines, N.; Hubner, M. Stringent fluid management might help to prevent postoperative ileus after loop ileostomy closure. Langenbecks Arch. Surg. 2019, 404, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef]
- Danziger, J.; Hoenig, M.P. The Role of the Kidney in Disorders of Volume: Core Curriculum 2016. Am. J. Kidney Dis. 2016, 68, 808–816. [Google Scholar] [CrossRef]
- Brandstrup, B.; Beier-Holgersen, R.; Iversen, L.H.; Starup, C.B.; Wentzel, L.N.; Lindorff-Larsen, K.; Petersen, T.C.; Tonnesen, H. The Influence of Perioperative Fluid Therapy on N-terminal-pro-brain Natriuretic Peptide and the Association With Heart and Lung Complications in Patients Undergoing Colorectal Surgery: Secondary Results of a Clinical Randomized Assessor-blinded Multicenter Trial. Ann. Surg. 2020, 272, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Kork, F.; Balzer, F.; Spies, C.D.; Wernecke, K.D.; Ginde, A.A.; Jankowski, J.; Eltzschig, H.K. Minor Postoperative Increases of Creatinine Are Associated with Higher Mortality and Longer Hospital Length of Stay in Surgical Patients. Anesthesiology 2015, 123, 1301–1311. [Google Scholar] [CrossRef]
- Lovely, J.K.; Maxson, P.M.; Jacob, A.K.; Cima, R.R.; Horlocker, T.T.; Hebl, J.R.; Harmsen, W.S.; Huebner, M.; Larson, D.W. Case-matched series of enhanced versus standard recovery pathway in minimally invasive colorectal surgery. Br. J. Surg. 2012, 99, 120–126. [Google Scholar] [CrossRef]
- Roulin, D.; Donadini, A.; Gander, S.; Griesser, A.C.; Blanc, C.; Hubner, M.; Schafer, M.; Demartines, N. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br. J. Surg. 2013, 100, 1108–1114. [Google Scholar] [CrossRef]
- Grass, F.; Lovely, J.K.; Crippa, J.; Mathis, K.L.; Hubner, M.; Larson, D.W. Early Acute Kidney Injury Within an Established Enhanced Recovery Pathway: Uncommon and Transitory. World J. Surg. 2019, 43, 1207–1215. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hubner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef] [PubMed]
- Tallarico, R.T.; McCoy, I.E.; Depret, F.; Legrand, M. Meaning and Management of Perioperative Oliguria. Anesthesiology 2023, 140, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Hubner, M.; Lovely, J.K.; Huebner, M.; Slettedahl, S.W.; Jacob, A.K.; Larson, D.W. Intrathecal analgesia and restrictive perioperative fluid management within enhanced recovery pathway: Hemodynamic implications. J. Am. Coll. Surg. 2013, 216, 1124–1134. [Google Scholar] [CrossRef]
- Romagnoli, S.; Ricci, Z.; Ronco, C. Perioperative Acute Kidney Injury: Prevention, Early Recognition, and Supportive Measures. Nephron 2018, 140, 105–110. [Google Scholar] [CrossRef] [PubMed]
- du Toit, L.; Biccard, B.M. The relationship between intraoperative oliguria and acute kidney injury. Br. J. Anaesth. 2019, 122, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Howitt, S.H.; Oakley, J.; Caiado, C.; Goldstein, M.; Malagon, I.; McCollum, C.; Grant, S.W. A Novel Patient-Specific Model for Predicting Severe Oliguria; Development and Comparison With Kidney Disease: Improving Global Outcomes Acute Kidney Injury Classification. Crit. Care Med. 2020, 48, e18–e25. [Google Scholar] [CrossRef] [PubMed]
- Grass, F.; Hubner, M.; Behm, K.T.; Mathis, K.L.; Hahnloser, D.; Day, C.N.; Harmsen, W.S.; Demartines, N.; Larson, D.W. Development and validation of a prediction score for safe outpatient colorectal resections. Surgery 2022, 171, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Grass, F.; Hubner, M.; Mathis, K.L.; Hahnloser, D.; Dozois, E.J.; Kelley, S.R.; Demartines, N.; Larson, D.W. Identification of patients eligible for discharge within 48 h of colorectal resection. Br. J. Surg. 2020, 107, 546–551. [Google Scholar] [CrossRef]
- Grass, F.; Pache, B.; Butti, F.; Sola, J.; Hahnloser, D.; Demartines, N.; Hubner, M. Fluid management for critical patients undergoing urgent colectomy. J. Eval. Clin. Pract. 2020, 26, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Pache, B.; Hubner, M.; Sola, J.; Hahnloser, D.; Demartines, N.; Grass, F. Receiver operating characteristic analysis to determine optimal fluid management during open colorectal surgery. Color. Dis. 2019, 21, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, L.; Biffi, R.; Sandini, M.; Marrelli, D.; Vignali, A.; Caccialanza, R.; Vigano, J.; Sabbatini, A.; Di Mare, G.; Alessiani, M.; et al. Preoperative Oral Carbohydrate Load Versus Placebo in Major Elective Abdominal Surgery (PROCY): A Randomized, Placebo-controlled, Multicenter, Phase III Trial. Ann. Surg. 2018, 267, 623–630. [Google Scholar] [CrossRef]
- Brady, M.; Kinn, S.; Stuart, P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst. Rev. 2003, 4, CD004423. [Google Scholar] [CrossRef]
- Rollins, K.E.; Javanmard-Emamghissi, H.; Lobo, D.N. Impact of mechanical bowel preparation in elective colorectal surgery: A meta-analysis. World J. Gastroenterol. 2018, 24, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Killewich, L.A. Strategies to minimize postoperative deconditioning in elderly surgical patients. J. Am. Coll. Surg. 2006, 203, 735–745. [Google Scholar] [CrossRef]
- Golder, H.J.; Papalois, V. Enhanced Recovery after Surgery: History, Key Advancements and Developments in Transplant Surgery. J. Clin. Med. 2021, 10, 1634. [Google Scholar] [CrossRef]
- Page, A.J.; Gani, F.; Crowley, K.T.; Lee, K.H.; Grant, M.C.; Zavadsky, T.L.; Hobson, D.; Wu, C.; Wick, E.C.; Pawlik, T.M. Patient outcomes and provider perceptions following implementation of a standardized perioperative care pathway for open liver resection. Br. J. Surg. 2016, 103, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Bijker, J.B.; van Klei, W.A.; Vergouwe, Y.; Eleveld, D.J.; van Wolfswinkel, L.; Moons, K.G.; Kalkman, C.J. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology 2009, 111, 1217–1226. [Google Scholar] [CrossRef]
- Lobo, D.N.; Macafee, D.A.; Allison, S.P. How perioperative fluid balance influences postoperative outcomes. Best. Pract. Res. Clin. Anaesthesiol. 2006, 20, 439–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).