Lack of Consensus on the Definition of Aseptic Loosening in Total Ankle Replacement: A Narrative Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Data Extraction and Data Items
3. Results
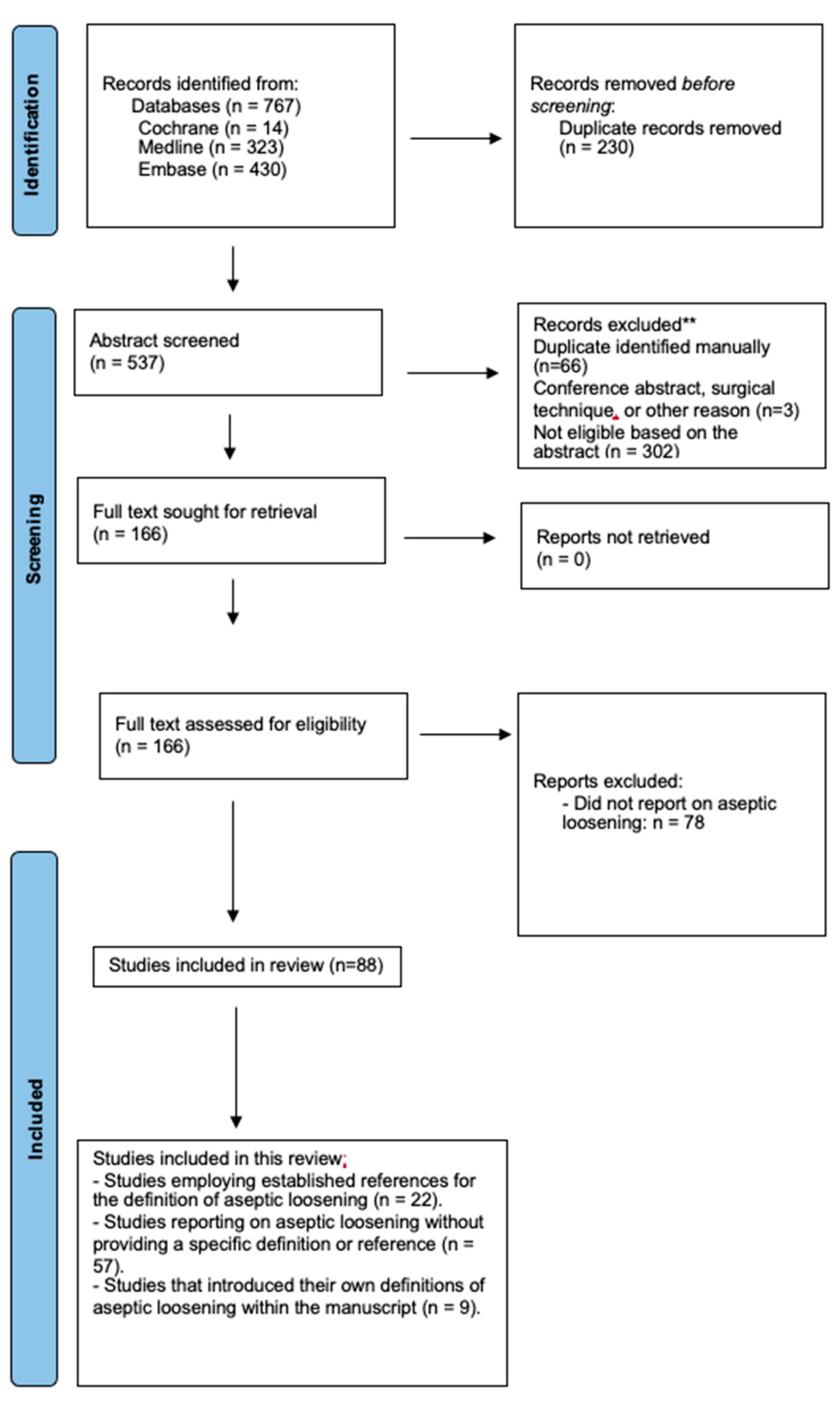
3.1. Study Selection
3.2. Study Characteristics
3.3. Applied Definitions in Included Studies
3.4. References Utilized for the Definition of Aseptic Loosening
3.5. Quality Assessment
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kvarda, P.; Peterhans, U.-S.; Susdorf, R.; Barg, A.; Ruiz, R.; Hintermann, B. Long-Term Survival of HINTEGRA Total Ankle Replacement in 683 Patients: A Concise 20-Year Follow-up of a Previous Report. J. Bone Jt. Surg. Am. 2022, 104, 881–888. [Google Scholar] [CrossRef]
- Haddad, S.L.; Coetzee, J.C.; Estok, R.; Fahrbach, K.; Banel, D.; Nalysnyk, L. Intermediate and long-term outcomes of total ankle arthroplasty and ankle arthrodesis. A systematic review of the literature. J. Bone Jt. Surg. Am. 2007, 89, 1899–1905. [Google Scholar] [CrossRef]
- Hintermann, B.; Zwicky, L.; Knupp, M.; Henninger, H.B.; Barg, A. HINTEGRA Revision Arthroplasty for Failed Total Ankle Prostheses: Surgical Technique. JBJS Essent. Surg. Tech. 2014, 3, e12. [Google Scholar] [CrossRef]
- Yoon, H.S.; Lee, J.W.J.; Choi, W.J. Periprosthetic osteolysis after total ankle arthroplasty. Foot Ankle Int. 2014, 35, 14–21. [Google Scholar] [CrossRef]
- Holt, G.; Murnaghan, C.; Reilly, J.; Meek, R.M.D. The biology of aseptic osteolysis. Clin. Orthop. Relat. Res. 2007, 460, 240–252. [Google Scholar] [CrossRef]
- Espinosa, N.; Klammer, G.; Wirth, S.H. Osteolysis in Total Ankle Replacement: How Does It Work? Foot Ankle Clin. 2017, 22, 267–275. [Google Scholar] [CrossRef]
- Espinosa, N.; Wirth, S.H. Revision of the Aseptic and Septic Total Ankle Replacement. Clin. Podiatr. Med. Surg. 2013, 30, 171–185. [Google Scholar] [CrossRef]
- Gaden, M.T.; Ollivere, B.J. Periprosthetic Aseptic Osteolysis in Total Ankle Replacement. Cause and Management. Clin. Podiatr. Med. Surg. 2013, 30, 145–155. [Google Scholar] [CrossRef]
- Mehta, N.; Serino, J.; Hur, E.S.; Smith, S.; Hamid, K.S.; Lee, S.; Bohl, D.D. Pathogenesis, Evaluation, and Management of Osteolysis Following Total Ankle Arthroplasty. Foot Ankle Int. 2021, 42, 230–242. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 372, n71. [Google Scholar] [CrossRef]
- Pieper, D.; Rombey, T. Where to prospectively register a systematic review. Syst. Rev. 2022, 11, 8. [Google Scholar] [CrossRef]
- INPLASY International Platform of Registered Systematic Review and Meta-Analysis Protocols. Available online: https://inplasy.com (accessed on 14 June 2023).
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Kerkhoff, Y.R.A.; Kosse, N.M.; Metsaars, W.P.; Louwerens, J.W.K. Long-term Functional and Radiographic Outcome of a Mobile Bearing Ankle Prosthesis. Foot Ankle Int. 2016, 37, 1292–1302. [Google Scholar] [CrossRef]
- Preis, M.; Bailey, T.; Marchand, L.S.; Barg, A. Can a Three-Component Prosthesis be Used for Conversion of Painful Ankle Arthrodesis to Total Ankle Replacement? Clin. Orthop. Relat. Res. 2017, 475, 2283–2294. [Google Scholar] [CrossRef]
- Omar, I.M.; Abboud, S.F.; Youngner, J.M. Imaging of Total Ankle Arthroplasty: Normal Imaging Findings and Hardware Complications. Semin. Musculoskelet. Radiol. 2019, 23, 177–194. [Google Scholar] [CrossRef]
- Marks, R.M. Mid-Term Prospective Clinical and Radiographic Outcomes of a Modern Fixed-Bearing Total Ankle Arthroplasty. J. Foot Ankle Surg. 2019, 58, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.J.; Kallemose, T.; Benyahia, M.; Ebskov, L.B.; Penny, J.Ø. 12-year survival analysis of 322 Hintegra total ankle arthroplasties from an independent center. Acta Orthop. 2020, 91, 444–449. [Google Scholar] [CrossRef]
- Gurbani, A.; Demetracopoulos, C.; O’Malley, M.; Deland, J.; Cody, E.; Sofka, C.; Scharf, S.; Ellis, S. Correlation of Single-Photon Emission Computed Tomography Results With Clinical and Intraoperative Findings in Painful Total Ankle Replacement. Foot Ankle Int. 2020, 41, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Albagli, A.; Ge, S.M.; Park, P.; Cohen, D.; Epure, L.; Chaytor, R.E.; Volesky, M. Total ankle arthroplasty results using fixed bearing CT-guided patient specific implants in posttraumatic versus nontraumatic arthritis. Foot Ankle Surg. 2022, 28, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Krahenbuhl, N.; Susdorf, R.; Barg, A.; Ruiz, R.; Hintermann, B. What Are the Indications for Implant Revision in Three-component Total Ankle Arthroplasty? Clin. Orthop. Relat. Res. 2021, 479, 601–609. [Google Scholar] [CrossRef]
- Baumfeld, D.; Baumfeld, T.; Rezende, R.F.; Lemos, A.V.; Nery, C. Corin ankle arthroplasty: Case-series. Foot Ankle Surg. 2022, 28, 745–749. [Google Scholar] [CrossRef]
- Nunley, J.A.; Adams, S.B.; Easley, M.E.; DeOrio, J.K. Prospective Randomized Trial Comparing Mobile-Bearing and Fixed-Bearing Total Ankle Replacement. Foot Ankle Int. 2019, 40, 1239–1248. [Google Scholar] [CrossRef]
- Kim, B.S.; Knupp, M.; Zwicky, L.; Lee, J.W.; Hintermann, B. Total ankle replacement in association with hindfoot fusion: Outcome and complications. J. Bone Jt. Surg. Br. 2010, 92, 1540–1547. [Google Scholar] [CrossRef]
- Barg, A.; Zwicky, L.; Knupp, M.; Henninger, H.B.; Hintermann, B. HINTEGRA total ankle replacement: Survivorship analysis in 684 patients. J. Bone Jt. Surg. Am. 2013, 95, 1175–1183. [Google Scholar] [CrossRef]
- Bianchi, A.; Martinelli, N.; Caboni, E.; Raggi, G.; Manfroni, F. Long-term follow-up of Bologna-Oxford (BOX) total ankle arthroplasty. Int. Orthop. 2021, 45, 1223–1231. [Google Scholar] [CrossRef]
- Brunner, S.; Barg, A.; Knupp, M.; Zwicky, L.; Kapron, A.L.; Valderrabano, V.; Hintermann, B. The Scandinavian total ankle replacement: Long-term, eleven to fifteen-year, survivorship analysis of the prosthesis in seventy-two consecutive patients. J. Bone Jt. Surg. Am. 2013, 95, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Escudero, M.I.; Symes, M.; Bemenderfer, T.B.; Barahona, M.; Anderson, R.; Davis, H.; Wing, K.J.; Penner, M.J. Does Patient-Specific Instrumentation Have a Higher Rate of Early Osteolysis Than Standard Referencing Techniques in Total Ankle Arthroplasty? A Radiographic Analysis. Foot Ankle Spec. 2020, 13, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Hintermann, B.; Zwicky, L.; Knupp, M.; Henninger, H.B.; Barg, A. HINTEGRA revision arthroplasty for failed total ankle prostheses. J. Bone Jt. Surg. 2013, 95, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Kvarda, P.; Toth, L.; Horn-Lang, T.; Susdorf, R.; Ruiz, R.; Hintermann, B. How Does a Novel In Situ Fixed-bearing Implant Design Perform in Revision Ankle Arthroplasty in the Short Term? A Survival, Clinical, and Radiologic Analysis. Clin. Orthop. Relat. Res. 2023, 481, 1360–1370. [Google Scholar] [CrossRef]
- Penner, M.; Davis, W.H.; Wing, K.; Bemenderfer, T.; Waly, F.; Anderson, R.B. The Infinity Total Ankle System: Early Clinical Results With 2- to 4-Year Follow-up. Foot Ankle Spec. 2019, 12, 159–166. [Google Scholar] [CrossRef]
- Hintermann, B.; Valderrabano, V.; Dereymaeker, G.; Dick, W. The HINTEGRA ankle: Rationale and short-term results of 122 consecutive ankles. Clin. Orthop. Relat. Res. 2004, 424, 57–68. [Google Scholar] [CrossRef]
- Rushing, C.J.; Mckenna, B.J.; Zulauf, E.A.; Hyer, C.F.; Berlet, G.C. Intermediate-Term Outcomes of a Third-Generation, 2-Component Total Ankle Prosthesis. Foot Ankle Int. 2021, 42, 935–943. [Google Scholar] [CrossRef]
- Pugely, A.J.; Lu, X.; Amendola, A.; Callaghan, J.J.; Martin, C.T.; Cram, P. Trends in the use of total ankle replacement and ankle arthrodesis in the United States Medicare population. Foot Ankle Int. 2014, 35, 207–215. [Google Scholar] [CrossRef]
- Rushing, C.J.; Kibbler, K.; Hyer, C.F.; Berlet, G.C. The INFINITY Total Ankle Prosthesis: Outcomes at Short-Term Follow-up. Foot Ankle Spec. 2020, 15, 119–126. [Google Scholar] [CrossRef]
- Saito, G.H.; Sanders, A.E.; de Cesar Netto, C.; O’Malley, M.J.; Ellis, S.J.; Demetracopoulos, C.A. Short-Term Complications, Reoperations, and Radiographic Outcomes of a New Fixed-Bearing Total Ankle Arthroplasty. Foot Ankle Int. 2018, 39, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, T.; Pinsker, E.; Cadden, A.; Daniels, T. Outcomes of ankle arthroplasty with preoperative coronal-plane varus deformity of 10degree or greater. J. Bone Jt. Surg. 2013, 95, 1382–1388. [Google Scholar] [CrossRef]
- Jamjoom, B.A.; Siddiqui, B.M.; Salem, H.; Raglan, M.; Dhar, S. Clinical and Radiographic Outcomes of Revision Total Ankle Arthroplasty Using the INBONE II Prosthesis. J. Bone Jt. Surg. Am. 2022, 104, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Behrens, S.B.; Irwin, T.A.; Bemenderfer, T.B.; Schipper, O.N.; Odum, S.M.; Anderson, R.B.; Davis, W.H. Clinical and Radiographic Outcomes of Revision Total Ankle Arthroplasty Using an Intramedullary-Referencing Implant. Foot Ankle Int. 2020, 41, 1510–1518. [Google Scholar] [CrossRef]
- Koh, D.T.S.; Chen, J.Y.; Tan, S.M.; Tay, K.S.; Singh, I.R.; Yeo, N.E.M. Mid-Term Functional and Radiological Outcomes of Total Ankle Replacement in an Asian Cohort. J. Foot Ankle Surg. 2022, 61, 363–368. [Google Scholar] [CrossRef]
- Kim, B.S.; Choi, W.J.; Kim, Y.S.; Lee, J.W. Total ankle replacement in moderate to severe varus deformity of the ankle. J. Bone Jt. Surg. Br. 2009, 91, 1183–1190. [Google Scholar] [CrossRef]
- Lee, G.-W.; Seon, J.-K.; Kim, N.-S.; Lee, K.-B. Comparison of Intermediate-Term Outcomes of Total Ankle Arthroplasty in Patients Younger and Older Than 55 Years. Foot Ankle Int. 2019, 40, 762–768. [Google Scholar] [CrossRef]
- Lee, G.-W.; Wang, S.-H.; Lee, K.-B. Comparison of Intermediate to Long-Term Outcomes of Total Ankle Arthroplasty in Ankles with Preoperative Varus, Valgus, and Neutral Alignment. J. Bone Jt. Surg. Am. 2018, 100, 835–842. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.-H. The HINTEGRA total ankle arthroplasty. Bone Jt. J. 2019, 101 B, 695–701. [Google Scholar] [CrossRef]
- Yano, K.; Ikari, K.; Okazaki, K. Radiographic Outcomes of Mobile-Bearing Total Ankle Arthroplasty for Patients With Rheumatoid Arthritis. Foot Ankle Int. 2019, 40, 1037–1042. [Google Scholar] [CrossRef]
- Lee, K.-B.; Cho, S.-G.; Hur, C.-I.; Yoon, T.-R. Perioperative complications of HINTEGRA total ankle replacement: Our initial 50 cases. Foot Ankle Int. 2008, 29, 978–984. [Google Scholar] [CrossRef]
- Bianchi, A.; Martinelli, N.; Hosseinzadeh, M.; Flore, J.; Minoli, C.; Malerba, F.; Galbusera, F. Early clinical and radiological evaluation in patients with total ankle replacement performed by lateral approach and peroneal osteotomy. BMC Musculoskelet. Disord. 2019, 20, 132. [Google Scholar] [CrossRef]
- Najefi, A.; Malhotra, K.; Chan, O.; Cullen, N.; Goldberg, A. The Bologna-Oxford ankle replacement: A case series of clinical and radiological outcomes. Int. Orthop. 2019, 43, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Frigg, A.; Germann, U.; Huber, M.; Horisberger, M. Survival of the Scandinavian total ankle replacement (STAR): Results of ten to nineteen years follow-up. Int. Orthop. 2017, 41, 2075–2082. [Google Scholar] [CrossRef]
- Wood, P.L.R.; Prem, H.; Sutton, C. Total ankle replacement: Medium-term results in 200 Scandinavian total ankle replacements. J. Bone Jt. Surg. Br. 2008, 90, 605–609. [Google Scholar] [CrossRef]
- Faber, F.W.M.; Mastboom, M.J.L.; Van Vliet-Koppert, S.T.; Bouman, I.C.E.; Van Kampen, P.M. Outcome after 52 Salto Ankle Prostheses Implanted by a Single Surgeon. Adv. Orthop. 2018, 2018, 2735634. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, J.J.P.; Walschot, L.H.B.; Louwerens, J.W.K. Comparison of the short-term results of the first and last 50 scandinavian total ankle replacements: Assessment of the learning curve in a consecutive series. Foot Ankle Int. 2014, 35, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Mushtaq, N.; To, K.; Gooding, C.; Khan, W. Radiological Imaging Evaluation of the Failing Total Hip Replacement. Front. Surg. 2019, 6, 35. [Google Scholar] [CrossRef]
- Brigido, S.A.; Wobst, G.M.; Galli, M.M.; Protzman, N.M. Evaluating Component Migration: Comparing Two Generations of the INBONE( R) Total Ankle Replacement. J. Foot Ankle Surg. 2015, 54, 892–895. [Google Scholar] [CrossRef]
- Christensen, J.C.; Schuberth, J.M.; Steck, J.K. Flawed Technique for Measuring Total Ankle Component Migration. J. foot ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2016, 55, 434–435. [Google Scholar] [CrossRef]
- Fong, J.W.-Y.; Veljkovic, A.; Dunbar, M.J.; Wilson, D.A.; Hennigar, A.W.; Glazebrook, M.A. Validation and precision of model-based radiostereometric analysis (MBRSA) for total ankle arthroplasty. Foot Ankle Int. 2011, 32, 1155–1163. [Google Scholar] [CrossRef]
- Dunbar, M.J.; Fong, J.W.; Wilson, D.A.; Hennigar, A.W.; Francis, P.A.; Glazebrook, M.A. Longitudinal migration and inducible displacement of the Mobility Total Ankle System. Acta Orthop. 2012, 83, 394–400. [Google Scholar] [CrossRef]
- Kärrholm, J.; Borssén, B.; Löwenhielm, G.; Snorrason, F. Does early micromotion of femoral stem prostheses matter? 4–7-year stereoradiographic follow-up of 84 cemented prostheses. J. Bone Jt. Surg. Br. 1994, 76, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Ryd, L.; Albrektsson, B.E.; Carlsson, L.; Dansgård, F.; Herberts, P.; Lindstrand, A.; Regnér, L.; Toksvig-Larsen, S. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J. Bone Jt. Surg. Br. 1995, 77, 377–383. [Google Scholar] [CrossRef]
- Endo, Y.; Burge, A.J.; Koff, M.F.; Lin, B.; Westrich, G.H.; Boettner, F.; Chiu, Y.-F.; Potter, H.G. Diagnostic Performance of MRI for Component Loosening in Total Knee Arthroplasty Compared with Radiography. Radiology 2022, 304, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Chew, C.G.; Lewis, P.; Middleton, F.; van den Wijngaard, R.; Deshaies, A. Radionuclide arthrogram with SPECT/CT for the evaluation of mechanical loosening of hip and knee prostheses. Ann. Nucl. Med. 2010, 24, 735–743. [Google Scholar] [CrossRef] [PubMed]
| Item | Specification |
|---|---|
| Population, or participants and conditions of interest | Patients with ankle OA |
| Interventions or exposures | Total ankle replacement |
| Comparisons or control groups | Not applicable |
| Outcomes of interest | Applied definition of aseptic loosening of TAR |
| Study designs | No restriction on study design. Only studies published in peer-reviewed journals. |
| Study | Year of Publication | Country of Study | Study Design | Publishing Journal | Sample Size | Applied Implant Design | Follow-Up Time | Imaging Modality | Outcome Measures Related to Aseptic Loosening |
|---|---|---|---|---|---|---|---|---|---|
| Kerkhoff et al. [15] | 2016 | The Netherlands | Retrospective case series | Foot and Ankle International | 124 | STAR | Minimum of 7.5 years | X-ray | n/a |
| Preis et al. [16] | 2016 | Germany | Retrospective case series | Clinical Orthopaedics and Related Research | 18 | HINTEGRA | mean 54 months | X-ray | n/a |
| Omar et al. [17] | 2019 | USA | Radiological descriptive | Seminars in Musculoskeletal Radiology | n/a | n/a | n/a | X-ray, CT | n/a |
| Marks et al. [18] | 2019 | USA | Prospective, multicenter, observational cohort | The Journal of Foot and Ankle Surgery | 46 | Salto Talaris | Mean 4.9 years ((range 0.9 to 8.6) | X-ray | n/a |
| Zafar et al. [19] | 2020 | Denmark | Retrospective case series | Acta Orthopaedica | 322 | HINTEGRA | Not described | X-ray | n/a |
| Gurbani et al. [20] | 2020 | USA | Retrospective case series | Foot and Ankle International | 37 | Not described | Mean 16.9 months (range 5–43) | SPECT-CT | n/a |
| Albagli et al. [21] | 2021 | Canada | Retrospective case series | Foot and Ankle Surgery | 41 | Infinity | mean 24 months | X-ray | n/a |
| Richter et al. [22] | 2021 | Switzerland | Retrospective case series | Clinical Orthopaedics and Related Research | 935 | HINTEGRA | Mean (range) 9 ± 4 years (2 to 17) | n/a (clinical definition) | n/a |
| Baumfeld et al. [23] | 2022 | Brazil | Retrospective case series | Foot and Ankle Surgery | 29 | Corin-Zennith | mean 5 years | X-ray | n/a |
| Study | Definition of Aseptic Loosening of TAR |
|---|---|
| Omar et al. [17] | Radiographically, loosening is determined when there is lucency around the prosthetic component at the prosthesis–bone interface that measures > 2 mm in width. |
| Preis et al. [16] | The criteria for radiographic loosening was defined as subsidence or migration of prosthesis components and/or a cystic lesion with a diameter at least 2 mm. |
| Baumfeld et al. [23] | Loosening of the tibial component was diagnosed when there were changes of more than two degrees in the alpha and beta angles, or when radiolucent lines of more than 2 mm in thickness appeared. Loosening of the talar component was diagnosed when deepening of the talus body greater than 5 mm occurred (distances “a” and “b”), or when the theta angle underwent changes greater than five degrees. |
| Albagli et al. [21] | Differences in alpha or beta angle of more than 2 degrees between the two timepoints and a radiolucent line of more than 2 mm around the component were indicative of undesired component motion. The talar component was assessed using the gamma angle as well as “a” and “b” distances measured on lateral radiographs. Differences in gamma angles of more than 5 degrees or a change in “a” or “b” values of more than 5 mm were indicative of undesired component motion and subsidence. |
| Richter et al. [22] | Aseptic loosening was defined as intra-operatively verified component loosening with or without periarticular cysts or avascular necrosis. |
| Zafar et al. [19] | Aseptic loosening was defined as the failure of the bond between an implant and bone, in the absence of infection, defined on radiographic findings of radiolucent lines around the implant and/or preoperatively as lack of bony ingrowth. |
| Gurbani et al. [20] | SPECT-CT images were interpreted using the following criteria. Strong activity that was evenly distributed beneath an implant was considered to be consistent with aseptic loosening. |
| Marks et al. [18] | Device loosening was defined as the presence of new or progressive radiolucency along the device–bone interface indicating a loss of fixation. |
| Kerkhoff et al. [15] | Radiographic loosening was defined as radiolucency of more than 2 mm which progressed over time. |
| Nr. of Studies | Used Reference |
|---|---|
| Overall n = 22 (25%) | |
| 1 [24] | Kim et al. [25] |
| 8 [1,26,27,28,29,30,31,32] | Hintermann et al. [33] |
| 1 [34] | Pugely et al. [35] |
| 2 [36,37] | Trajkovski et al. [38] |
| 1 [39] | Behrens et al. [40] |
| 1 [41] | Kim et al. [42] |
| 4 [43,44,45,46] | Lee et al. [47] |
| 3 [48,49,50] | Wood et al. [51] |
| 1 [52] | Schimmel et al. [53] |
| Study | Clearly Stated Aim | Inclusion of Consecutive Patients | Prospective Collection of Data | Endpoints Appropriate | Unbiased Assessment of Endpoints | Follow-Up Period Appropriate | Loss to Follow-Up Less Than 5% | Prospective Calculation Of Study Size | Overall Score |
|---|---|---|---|---|---|---|---|---|---|
| Omar et al. [17] | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Preis et al. [16] | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 0 | 12 |
| Baumfeld et al. [23] | 1 | 2 | 1 | 2 | 0 | 2 | 0 | 0 | 8 |
| Albagli et al. [21] | 2 | 2 | 1 | 2 | 1 | 2 | 0 | 0 | 10 |
| Richter et al. [22] | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 12 |
| Zafar et al. [19] | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 0 | 9 |
| Gurbani et al. [20] | 2 | 2 | 1 | 2 | 1 | 2 | 0 | 0 | 10 |
| Marks et al. [18] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 13 |
| Kerkhoff et al. [15] | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvarda, P.; Mills, A.; Shepherd, D.; Schneider, T. Lack of Consensus on the Definition of Aseptic Loosening in Total Ankle Replacement: A Narrative Systematic Review. J. Clin. Med. 2024, 13, 786. https://doi.org/10.3390/jcm13030786
Kvarda P, Mills A, Shepherd D, Schneider T. Lack of Consensus on the Definition of Aseptic Loosening in Total Ankle Replacement: A Narrative Systematic Review. Journal of Clinical Medicine. 2024; 13(3):786. https://doi.org/10.3390/jcm13030786
Chicago/Turabian StyleKvarda, Peter, Andreea Mills, David Shepherd, and Tim Schneider. 2024. "Lack of Consensus on the Definition of Aseptic Loosening in Total Ankle Replacement: A Narrative Systematic Review" Journal of Clinical Medicine 13, no. 3: 786. https://doi.org/10.3390/jcm13030786
APA StyleKvarda, P., Mills, A., Shepherd, D., & Schneider, T. (2024). Lack of Consensus on the Definition of Aseptic Loosening in Total Ankle Replacement: A Narrative Systematic Review. Journal of Clinical Medicine, 13(3), 786. https://doi.org/10.3390/jcm13030786






