Direct Exposure to Outdoor Air Pollution Worsens the Functional Status of Stroke Patients Treated with Mechanical Thrombectomy
Abstract
1. Introduction
2. Methods
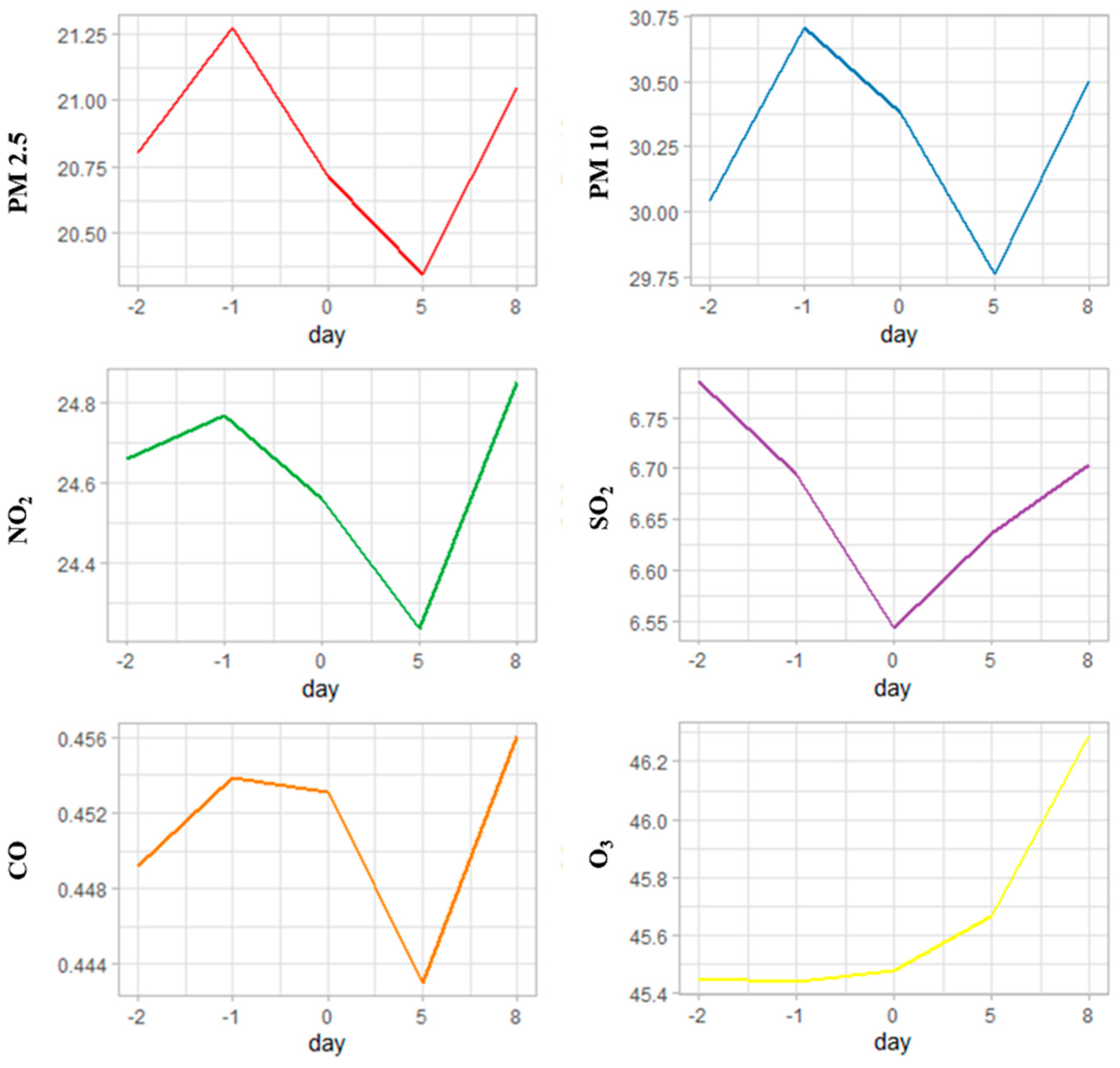
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Cheng, Z.; Li, M.; Luo, P.; Duan, Y.; Fan, J.; Xu, Y.; Pu, K.; Zhou, L. Ambient Air Pollution and Hospitalizations for Ischemic Stroke: A Time Series Analysis Using a Distributed Lag Nonlinear Model in Chongqing, China. Front. Public Health 2022, 9, 762597. [Google Scholar] [CrossRef]
- Liu, H.-X.; Li, D.; Li, B.; Xiong, J.; Chen, G. Devil or angel: Two roles of carbon monoxide in stroke. Med. Gas Res. 2022, 12, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Liu, F.; Yu, H.; Wu, S.; Xiang, H. Association between exposure to ambient air pollution and hospital admission, incidence, and mortality of stroke: An updated systematic review and meta-analysis of more than 23 million participants. Environ. Health Prev. Med. 2021, 26, 15. [Google Scholar] [CrossRef]
- Yeager, R.; Riggs, D.W.; DeJarnett, N.; Tollerud, D.J.; Wilson, J.; Conklin, D.J.; O’Toole, T.E.; McCracken, J.; Lorkiewicz, P.; Xie, Z.; et al. Association between Residential Greenness and Cardiovascular Disease Risk. J. Am. Heart Assoc. 2018, 7, e009117. [Google Scholar] [CrossRef]
- Eskandari, Z.; Maleki, H.; Neisi, A.; Riahi, A.; Hamid, V.; Goudarzi, G. Temporal fluctuations of PM2.5 and PM10, population exposure, and their health impacts in Dezful city, Iran. J. Environ. Health Sci. Eng. 2020, 18, 723–731. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Shi, M.-C.; Wang, Z.-X.; Li, C.; Sun, M.-Y.; Zhou, J.; Zhang, W.-B.; Huo, L.-W.; Wang, S.-C. Factors Associated with Poor Outcomes in Patients Undergoing Endovascular Therapy for Acute Ischemic Stroke due to Large-Vessel Occlusion in Acute Anterior Circulation: A Retrospective Study. World Neurosurg. 2021, 149, e128–e134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Liebeskind, D.S.; Jahan, R.; Menon, B.K.; Goyal, M.; Nogueira, R.G.; Pereira, V.M.; Gralla, J.; Saver, J.L. Impact of Hyperglycemia According to the Collateral Status on Outcomes in Mechanical Thrombectomy. Stroke 2018, 49, 2706–2714. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Tsivgoulis, G.; Pandhi, A.; Dillard, K.; Katsanos, A.H.; Magoufis, G.; Chang, J.J.; Zand, R.; Hoit, D.; Safouris, A.; et al. Admission hyperglycemia and outcomes in large vessel occlusion strokes treated with mechanical thrombectomy. J. Neurointerv. Surg. 2018, 10, 112–117. [Google Scholar] [CrossRef]
- Broocks, G.; Kemmling, A.; Aberle, J.; Kniep, H.; Bechstein, M.; Flottmann, F.; Leischner, H.; Faizy, T.D.; Nawabi, J.; Schön, G.; et al. Elevated blood glucose is associated with aggravated brain edema in acute stroke. J. Neurol. 2020, 267, 440–448. [Google Scholar] [CrossRef]
- Lasek-Bal, A.; Żak, A.; Binek, Ł.; Student, S.; Tomalski, W.; Krzan, A.; Puz, P.; Uchwat, U. The effect of atrial fibrillation on the safety and efficacy of mechanical thrombectomy in patients with stroke. Pol. Arch. Intern. Med. 2022, 132, 16148. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xie, X.; Lei, L.; Zhou, H.; Deng, S.; Xu, Y.; Liu, Z.; Bao, J.; Peng, J.; Huang, C. Short-term associations between ambient air pollution and stroke hospitalisations: Time-series study in Shenzhen, China. BMJ Open 2020, 10, e032974. [Google Scholar] [CrossRef]
- Chung, J.-W.; Bang, O.Y.; Ahn, K.; Park, S.-S.; Park, T.H.; Kim, J.G.; Ko, Y.; Lee, S.; Lee, K.B.; Lee, J.; et al. Air Pollution is associated with ischemic stroke via cardiogenic embolism. Stroke 2017, 48, 17–23. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Zhao, Z.; Xiang, X.; Li, M.; Juan, J.; Song, J.; Cao, Y.; Wang, X.; Chen, L.; et al. Association between ambient air pollution and daily hospital admissions for ischemic stroke: A nationwide time-series analysis. PLoS Med. 2018, 15, e1002668. [Google Scholar] [CrossRef]
- Sarnat, J.A.; Schwartz, J.; Suh, H.H. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N. Engl. J. Med. 2000, 343, 1742–1749. [Google Scholar] [CrossRef]
- Radmanesh, E.; Maleki, H.; Goudarzi, G.; Zahedi, A.; Kalkhajeh, S.G.; Hopke, P.K.; Mard, S.A.; Olad, S. Cerebral ischemic attack, epilepsy and hospital admitted patients with types of headaches attributed to PM10 mass concentration in Abadan, Iran. Aeolian Res. 2019, 41, 100541. [Google Scholar] [CrossRef]
- Briggs, D. Environmental pollution and the global burden of disease. Br. Med. Bull. 2003, 68, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease:an update to the scientific statement from the AmericanHeart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Buteau, S.; Goldberg, M.S. A structured review of panel studies used to investigate associations between ambient air pollution and heart rate variability. Environ. Res. 2016, 148, 207–247. [Google Scholar] [CrossRef]
- Münzel, T.; Gori, T.; Al-Kindi, S.; Deanfield, J.; Lelieveld, J.; Daiber, A.; Rajagopalan, S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 2018, 39, 3543–3550. [Google Scholar] [CrossRef]
- Hajipour, S.; Farbood, Y.; Gharib-Naseri, M.K.; Goudarzi, G.; Rashno, M.; Maleki, H.; Bakhtiari, N.; Nesari, A.; Khoshnam, S.E.; Dianat, M.; et al. Exposure to ambient dusty particulate matter impairs spatial memory and hippocampal LTP by increasing brain inflammation and oxidative stress in rats. Life Sci. 2020, 242, 117210. [Google Scholar] [CrossRef]
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American Symptomatic Carotid Endarterectomy Trial Surgical Stroke. Stroke 1999, 30, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Kozielska, B.; Mainka, A.; Żak, M.; Kaleta, D.; Mucha, W. Indoor air quality in residential buildings in Upper Silesia, Poland. Build. Environ. 2020, 177, 106914. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air pollution and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef] [PubMed]
- Young, F.B.; Weir, C.J.; Lees, K.R. Comparison of the National Institutes of Health Stroke Scale with Disability Outcome Measures in Acute Stroke Trials. Stroke 2005, 36, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Weisscher, N.; Vermeulen, M.; Roos, Y.B.; de Haan, R.J. What should be defined as good outcome in stroke trials; a modified Rankin score of 0–1 or 0–2? J. Neurol. 2008, 255, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://powietrze.gios.gov.pl/pjp/current (accessed on 15 October 2022).
- Larrue, V.; von Kummer, R.R.; Müller, A.; Bluhmki, E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001, 32, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Variable selection with stepwise and best subset approaches. Ann. Transl. Med. 2016, 4, 136. [Google Scholar] [CrossRef]
- Hastie, T.J.; Pregibon, D. Generalized linear models. In Statistical Models S; Chapter 6; Chambers, J.M., Hastie, T.J., Eds.; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1992. [Google Scholar]
- Konduracka, E. A link between environmental pollution and civilization disorders: A mini review. Rev. Environ. Health 2019, 34, 227–233. [Google Scholar] [CrossRef]
- Liu, L.; Yan, L.L.; Lv, Y.; Zhang, Y.; Li, T.; Huang, C.; Kan, H.; Zhang, J.; Zeng, Y.; Shi, X.; et al. Air pollution, residential greenness, and metabolic dysfunction biomarkers: Analyses in the Chinese Longitudinal Healthy Longevity Survey. BMC Public Health 2022, 22, 885. [Google Scholar] [CrossRef]
- Konduracka, E.; Rostoff, P. Links between chronic exposure to outdoor air pollution and cardiovascular diseases: A review. Environ. Chem. Lett. 2022, 20, 2971–2988. [Google Scholar] [CrossRef]
- Sangani, R.G.; Soukup, J.M.; Ghio, A.J. Metals in air pollution particles decrease whole-blood coagulation time. Inhal. Toxicol. 2010, 22, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Pun, V.C.; Kabrhel, C.; Camargo CAJr Baccarelli, A.A.; Laden, F. Prospective study of ambient particulate matter exposure and risk of pulmonary embolism in the nurses’ health study cohort. Environ. Health Perspect. 2015, 123, 1265–1270. [Google Scholar] [CrossRef]
- Gray, D.L.; Wallace, L.A.; Brinkman, M.C.; Buehler, S.S.; La Londe, C. Respiratory and cardiovascular effects of metals in ambient particulate matter: A critical review. Rev. Environ. Contam. Toxicol. 2015, 234, 135–203. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Skalnaya, M.G.; Aaseth, J.; Bjørklund, G.; Gatiatulina, E.R.; Popova, E.V.; Nemereshina, O.N.; Huang, P.-T.; et al. Cadmium and atherosclerosis: A review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ. Res. 2018, 162, 240–260. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Oliveri Conti, G.; Zanobetti, A.; Baccarelli, A.; Fiore, M.; Ferrante, M. Effect of particulate matter-bound metalsexposure on prothrombotic biomarkers: A systematic review. Environ. Res. 2019, 177, 108573. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Mutlu, G.M. Particulate matter air pollution: Effects on the cardiovascular system. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef]
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015, 36, 83–93. [Google Scholar] [CrossRef]
- Rich, D.Q.; Kipen, H.M.; Huang, W.; Wang, G.; Wang, Y.; Zhu, P.; Ohman-Strickland, P.; Hu, M.; Philipp, C.; Diehl, S.R.; et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA 2012, 307, 2068–2078. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Lee, K.K.; McAllister, D.A.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N.L. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef]
- Kettunen, J.; Lanki, T.; Tiittanen, P.; Aalto, P.P.; Koskentalo, T.; Kulmala, M.; Salomaa, V.; Pekkanen, J. Associations of fine and ultrafine particulate air pollution with stroke mortality in an area of low air pollution levels. Stroke 2007, 38, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Lee, J.T.; Kim, H.; Kwon, H.J. Air pollution: A new risk factor in ischemic stroke mortality. Stroke 2002, 33, 2165–2169. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, W.-K.; Hung, D.-Z.; Chen, Y.-W.; Lin, C.-L.; Sung, F.-C.; Kao, C.-H. Association between ischemic stroke and carbon monoxide poisoning: A population-based retrospective cohort analysis. Eur. J. Intern. Med. 2016, 29, 65–70. [Google Scholar] [CrossRef]
- Maleki, H.; Goudarzi, G.; Baboli, Z.; Khodadadi, R.; Yazdani, M.; Babaei, A.A.; Mohammadi, M.J. Temporal profiles of ambient air pollutants and associated health outcomes in two polluted cities of the Middle East. J. Environ. Health Sci. Eng. 2022, 20, 347–361. [Google Scholar] [CrossRef]
- Tian, L.; Qiu, H.; Pun, V.C.; Ho, K.-F.; Chan, C.S.; Yu, I.T. Carbon monoxide and stroke: A time series study of ambient air pollution and emergency hospitalizations. Int. J. Cardiol. 2015, 201, 4–9. [Google Scholar] [CrossRef]
- Paital, B.; Agrawal, P.K. Air pollution by NO2 and PM2.5 explains COVID-19 infection severity by overexpression of angiotensinconverting enzyme 2 in respiratory cells: A review. Environ. Chem. Lett. 2020, 2020, 25–42. [Google Scholar] [CrossRef]
- Coogan, P.F.; White, L.F.; Yu, J.; Brook, R.D.; Burnett, R.T.; Marshall, J.D.; Bethea, T.N.; Rosenberg, L.; Jerrett, M. Long-term exposure to NO2 and ozone and hypertension incidence in the black women’s health study. Am. J. Hypertens. 2017, 30, 367–372. [Google Scholar] [CrossRef]
- Cakmak, S.; Hebbern, C.; Pinault, L.; Lavigne, E.; Vanos, J.; Crouse, D.L.; Tjepkema, M. Associations between long-term PM2.5 and ozone exposure and mortality in the Canadian Census Health and Environment Cohort (CANCHEC), by spatial synoptic classification zone. Environ. Int. 2018, 111, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Air Pollution. Available online: www.who.int/health-topics/air-pollution (accessed on 15 October 2022).
- Babatola, S.S. Global burden of diseases attributable to air pollution. J. Public Health Afr. 2018, 9, 813. [Google Scholar] [CrossRef]
- Avellaneda-Gómez, C.; Vivanco-Hidalgo, R.; Olmos, S.; Lazcano, U.; Valentin, A.; Milà, C.; Ambrós, A.; Roquer, J.; Tonne, C. Air pollution and surrounding greenness in relation to ischemic stroke: A population-based cohort study. Environ. Int. 2022, 161, 107147. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value |
|---|---|
| Age, mean, med., QR | 67.53, 70, 16 |
| Female, n (%) | 239 (47.19) |
| NIHSS_1, med., IQR | 13, 8 |
| NIHSS_ 2, med., IQR | 12, 8 |
| mRS 8d, med., IQR | 4, 2 |
| mRS 90d, med., IQR | 5, 4 |
| rtPA iv (actylise), n (%) | 309 (61.24) |
| MT time *, mean, SD | 108.4, 42.24 |
| TICI 2B-3, n (%) | 339 (67.87) |
| ICB, n (%) | 115 (22.7) |
| sICB, n (%) | 25 (5) |
| Atrial fibrillation, n (%) | 259 (51.67) |
| Arterial hypertension, n (%) | 379 (76.12) |
| Diabetes mellitus, n (%) | 122 (24.34) |
| Peripheral artery disease, n (%) | 235 (47.14) |
| Nicotinism, n (%) | 182 (36.48) |
| Lipid disorders, n (%) | 209 (40.83) |
| Carotid artery stenosis, n (%) | 53 (10.72) |
| Parameter | OR | 95% CI | p |
|---|---|---|---|
| NIHSS_2 | 1.324 | (1.204–1.472) | 0.000 |
| TICI | 0.890 | (0.811–0.967) | 0.009 |
| MT time | 1.010 | (1.001–1.021) | 0.042 |
| DM | 4.341 | (1.691–12.367) | 0.004 |
| Smoking | 2.212 | (1.064–4.712) | 0.035 |
| LD | 0.458 | (0.208–0.977) | 0.046 |
| CO_0 day | 0.014 | (0–0.908) | 0.048 |
| Parameter | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.065 | (1.018–1.123) | 0.011 |
| NIHSS_2 | 1.633 | (1.328–2.125) | 0.000 |
| sICB | 0.232 | (0.047–0.992) | 0.056 |
| Parameter | OR | 95% CI | p |
|---|---|---|---|
| NIHSS | 1.271 | (1.153–1.422) | <0.001 |
| sICB | 4.080 | (1.752–9.763) | 0.001 |
| SO2_2 | 1.212 | 1.022–1.474 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasek-Bal, A.; Rybicki, W.; Student, S.; Puz, P.; Krzan, A.; Derra, A. Direct Exposure to Outdoor Air Pollution Worsens the Functional Status of Stroke Patients Treated with Mechanical Thrombectomy. J. Clin. Med. 2024, 13, 746. https://doi.org/10.3390/jcm13030746
Lasek-Bal A, Rybicki W, Student S, Puz P, Krzan A, Derra A. Direct Exposure to Outdoor Air Pollution Worsens the Functional Status of Stroke Patients Treated with Mechanical Thrombectomy. Journal of Clinical Medicine. 2024; 13(3):746. https://doi.org/10.3390/jcm13030746
Chicago/Turabian StyleLasek-Bal, Anetta, Wiktor Rybicki, Sebastian Student, Przemysław Puz, Aleksandra Krzan, and Aleksandra Derra. 2024. "Direct Exposure to Outdoor Air Pollution Worsens the Functional Status of Stroke Patients Treated with Mechanical Thrombectomy" Journal of Clinical Medicine 13, no. 3: 746. https://doi.org/10.3390/jcm13030746
APA StyleLasek-Bal, A., Rybicki, W., Student, S., Puz, P., Krzan, A., & Derra, A. (2024). Direct Exposure to Outdoor Air Pollution Worsens the Functional Status of Stroke Patients Treated with Mechanical Thrombectomy. Journal of Clinical Medicine, 13(3), 746. https://doi.org/10.3390/jcm13030746









