Delayed Diagnosis of Spinal Dural Arteriovenous Fistula: A Case Report and Scoping Review
Abstract
1. Scoping Review Introduction
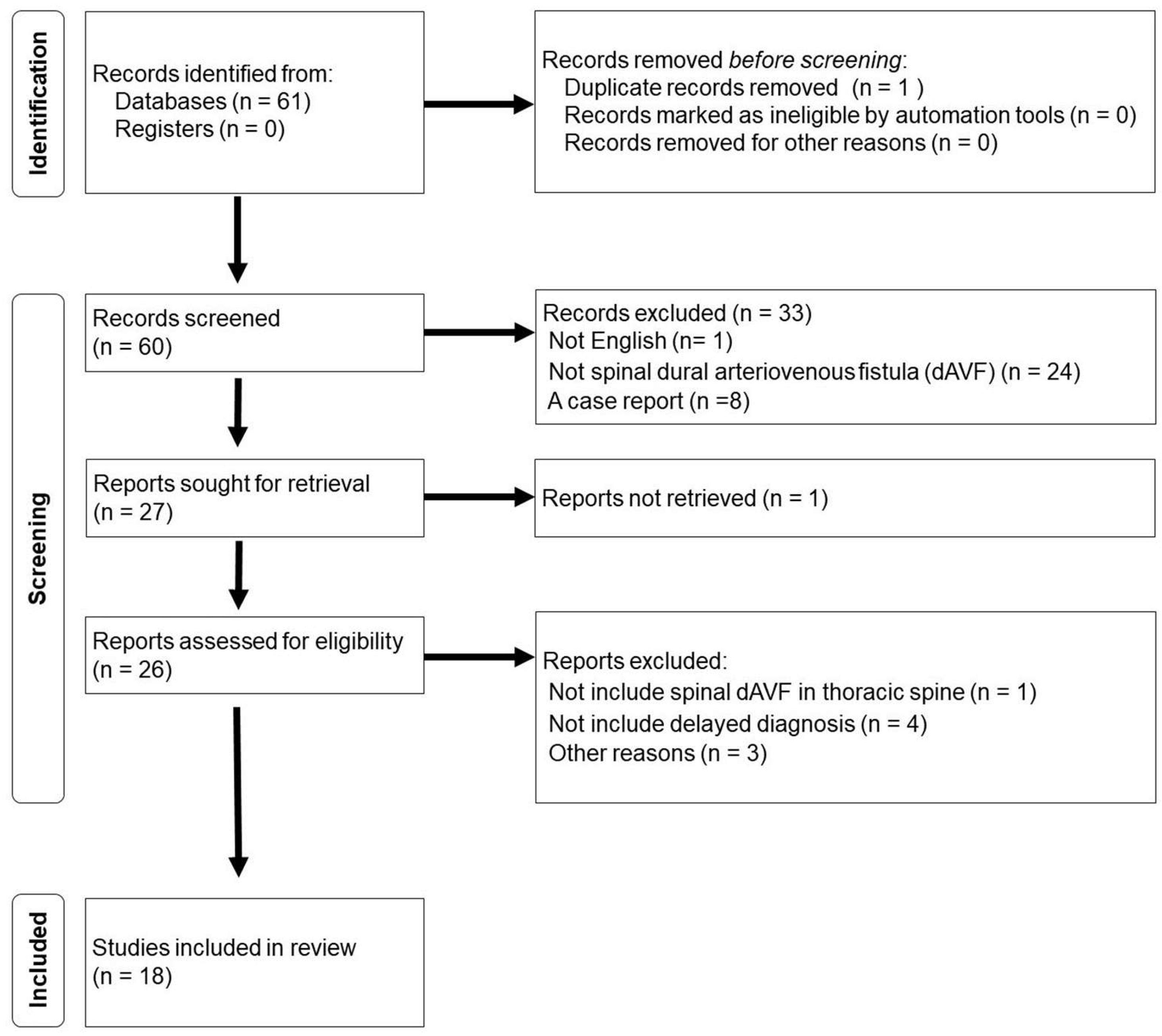
2. Scoping Review
- Prospective or retrospective cohort studies, case–control studies, and case series.
- Patients of both sexes aged 19 years or older.
- SDAVF of thoracic spine (with the arterial blood supply at the thoracic level), including idiopathic, iatrogenic, traumatic, and syndromic types.
- Patients treated with open and endovascular procedures were included, as were those who underwent repeated surgery.
- The exclusion criteria were as follows:
- Reports not including SDAVF cases of the thoracic spine.
- Spinal arteriovenous shunt diseases other than SDAVF (intramedullary AVM, peripheral AVF, and perimedullary AVF) were excluded from each article.
- Inadequate reporting of demographic characteristics, imaging features, or intraoperative procedures.
3. Results
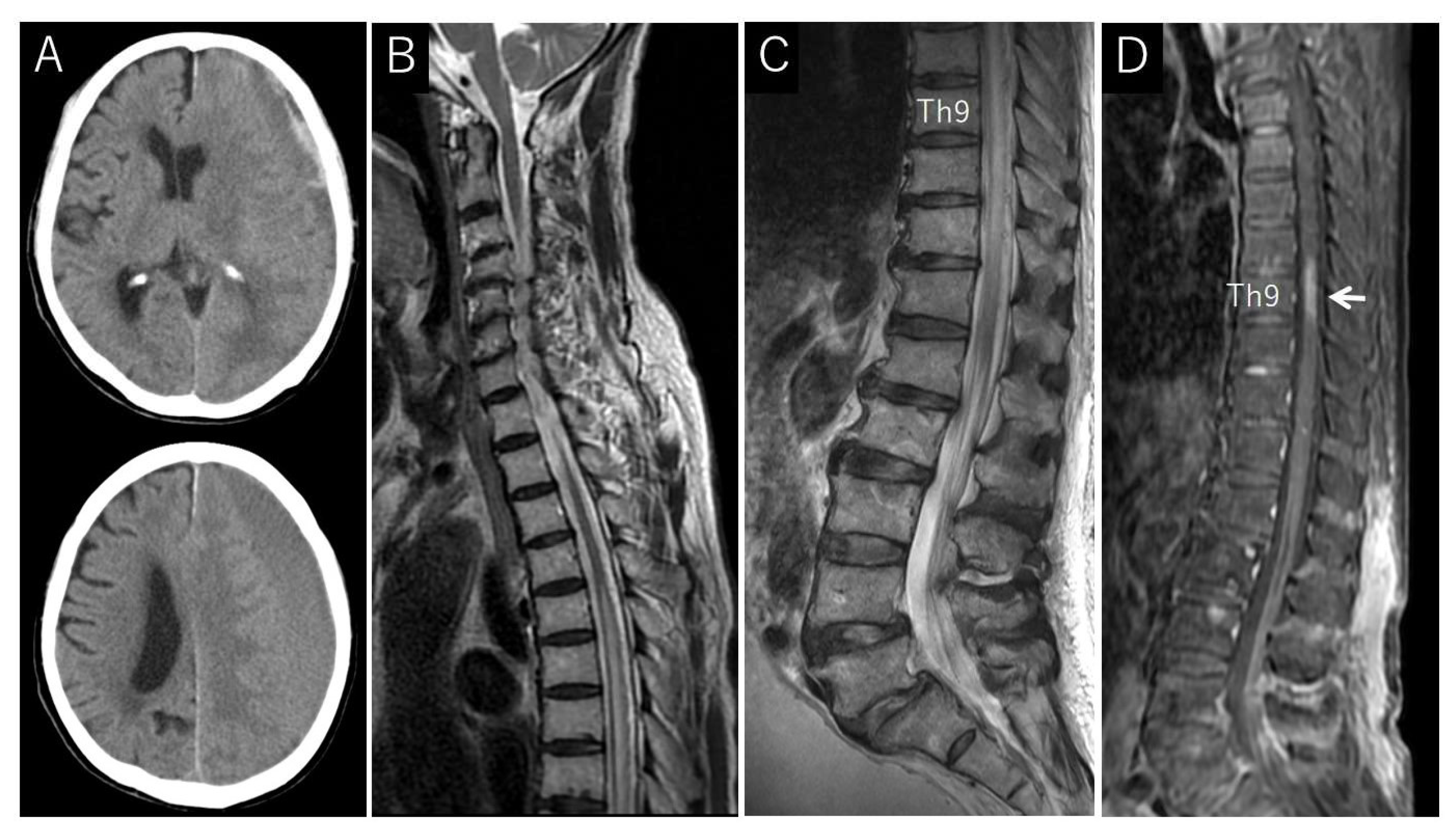
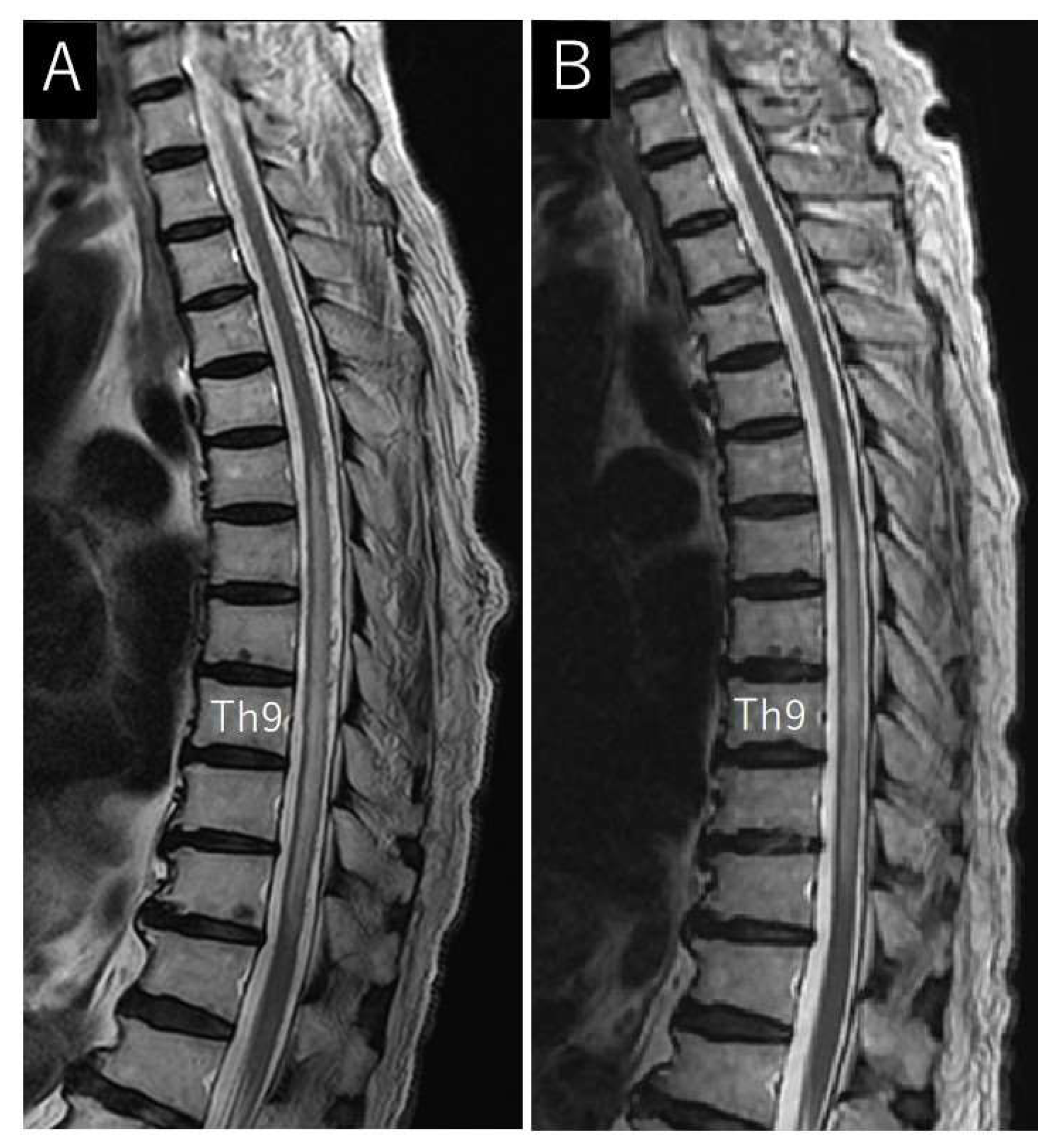
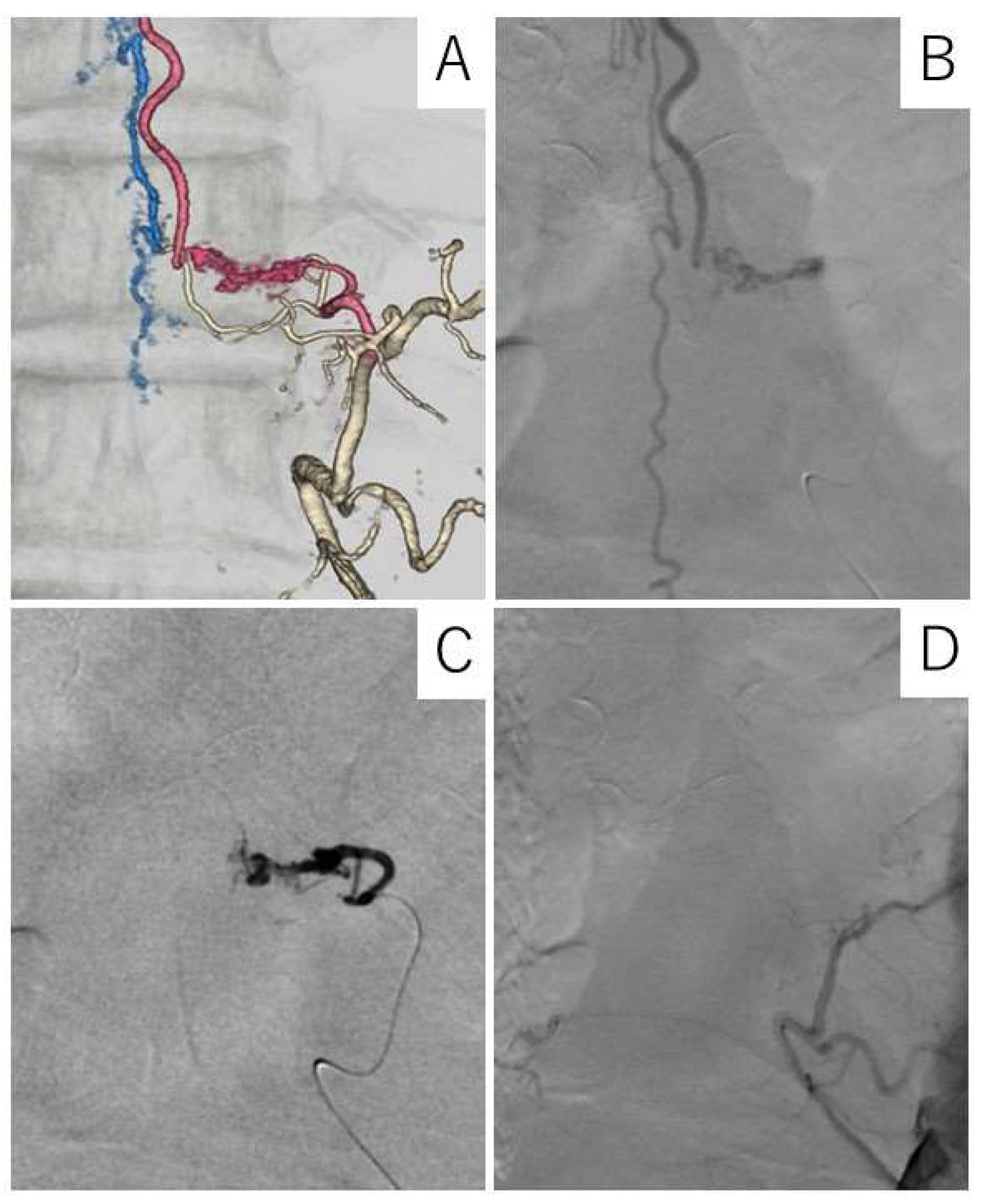
Case Description
4. Discussion
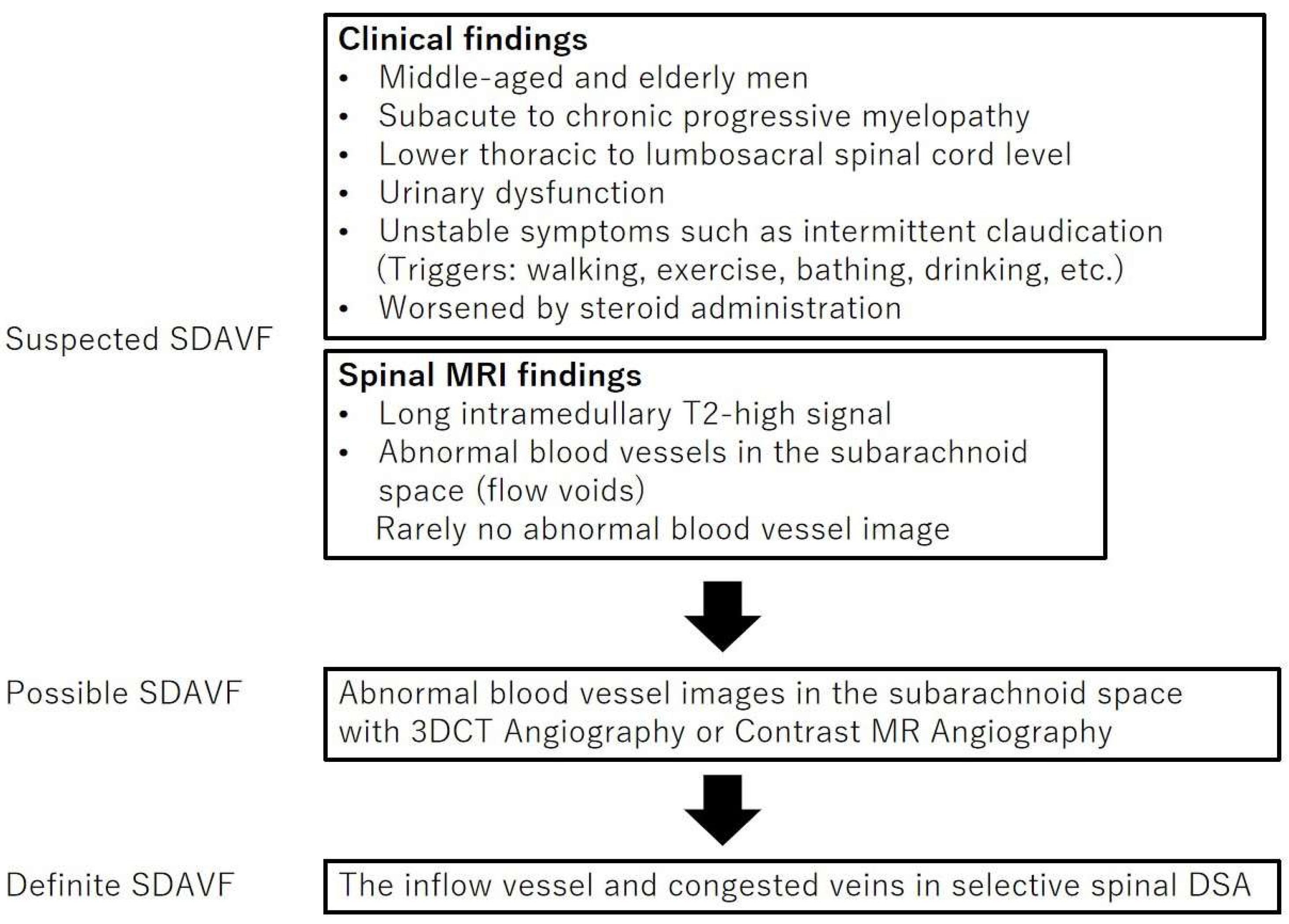
4.1. Symptoms
4.2. Neuroimaging Findings
4.3. Misdiagnosed Diseases
4.3.1. Spinal Degenerative Disease
4.3.2. Myelitis
4.4. Diagnostic Procedure
4.5. Treatment and Outcomes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takai, K.; Taniguchi, M. Clinical and neuroimaging findings of spinal dural arteriovenous fistulas: How to avoid misdiagnosis of this disease. J. Orthop. Sci. 2019, 24, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiao, G.; Shang, A.; Yu, X. Long-term surgical outcomes of patients with delayed diagnosis of spinal dural arteriovenous fistula. J. Clin. Neurosci. 2020, 77, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ronald, A.A.; Yao, B.; Winkelman, R.D.; Piraino, D.; Masaryk, T.J.; Krishnaney, A.A. Spinal dural arteriovenous fistula: Diagnosis, outcomes, and prognostic factors. World Neurosurg. 2020, 144, e306–e315. [Google Scholar] [CrossRef] [PubMed]
- Jablawi, F.; Schubert, G.A.; Dafotakis, M.; Pons-Kühnemann, J.; Hans, F.J.; Mull, M. Long-term outcome of patients with spinal dural arteriovenous fistula: The dilemma of delayed diagnosis. AJNR Am. J. Neuroradiol. 2020, 41, 357–363. [Google Scholar] [CrossRef]
- Hunt, R.; Roberts, R.M.; Mortimer, A.M. Spinal dural arteriovenous fistula: Delay to radiological diagnosis and sources of radiological error. Clin. Radiol. 2018, 73, 835.e11–835.e16. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Suero, G.; Porta Etessam, J.; Moreu Gamazo, M.; Rodríguez-Boto, G. Spinal arteriovenous fistulas in adults: Management of a series of patients treated at a neurology department. Neurologia 2018, 33, 438–448. [Google Scholar] [CrossRef]
- Barreras, P.; Heck, D.; Greenberg, B.; Wolinsky, J.P.; Pardo, C.A.; Gailloud, P. Analysis of 30 spinal angiograms falsely reported as normal in 18 patients with subsequently documented spinal vascular malformations. AJNR Am. J. Neuroradiol. 2017, 38, 1814–1819. [Google Scholar] [CrossRef]
- Lee, J.; Lim, Y.M.; Suh, D.C.; Rhim, S.C.; Kim, S.J.; Kim, K.K. Clinical presentation, imaging findings, and prognosis of spinal dural arteriovenous fistula. J. Clin. Neurosci. 2016, 26, 105–109. [Google Scholar] [CrossRef]
- Brinjikji, W.; Nasr, D.M.; Morris, J.M.; Rabinstein, A.A.; Lanzino, G. Clinical outcomes of patients with delayed diagnosis of spinal dural arteriovenous fistulas. AJNR Am. J. Neuroradiol. 2016, 37, 380–386. [Google Scholar] [CrossRef]
- Zogopoulos, P.; Nakamura, H.; Ozaki, T.; Asai, K.; Ima, H.; Kidani, T.; Kadono, Y.; Murakami, T.; Fujinaka, T.; Yoshimine, T. Endovascular and surgical treatment of spinal dural arteriovenous fistulas: Assessment of post-treatment clinical outcome. Neurol. Med. Chir. 2016, 56, 27–32. [Google Scholar] [CrossRef][Green Version]
- Iovtchev, I.; Hiller, N.; Ofran, Y.; Schwartz, I.; Cohen, J.; Rubin, S.A.; Meiner, Z. Late diagnosis of spinal dural arteriovenous fistulas resulting in severe lower-extremity weakness: A case series. Spine J. 2015, 15, e39–e44. [Google Scholar] [CrossRef]
- Donghai, W.; Ning, Y.; Peng, Z.; Shuo, X.; Xueen, L.; Peng, Z.; Bin, H.; Xingang, L. The diagnosis of spinal dural arteriovenous fistulas. Spine 2013, 38, E546–E553. [Google Scholar] [CrossRef]
- Cecchi, P.C.; Musumeci, A.; Rizzo, P.; Faccioli, F.; Bricolo, A. Late deterioration of neurologic function in patients surgically treated for spinal dural arteriovenous fistulas. Surg. Neurol. 2009, 72, 257–261, discussion 261–262. [Google Scholar] [CrossRef]
- Aghakhani, N.; Parker, F.; David, P.; Lasjaunias, P.; Tadie, M. Curable cause of paraplegia: Spinal dural arteriovenous fistulae. Stroke 2008, 39, 2756–2759. [Google Scholar] [CrossRef] [PubMed]
- Jellema, K.; Canta, L.R.; Tijssen, C.C.; van Rooij, W.J.; Koudstaal, P.J.; van Gijn, J. Spinal dural arteriovenous fistulas: Clinical features in 80 patients. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1438–1440. [Google Scholar] [CrossRef]
- Schick, U.; Hassler, W. Treatment and outcome of spinal dural arteriovenous fistulas. Eur. Spine J. 2003, 12, 350–355. [Google Scholar] [CrossRef]
- Atkinson, J.L.; Miller, G.M.; Krauss, W.E.; Marsh, W.R.; Piepgras, D.G.; Atkinson, P.P.; Brown, R.D., Jr.; Lane, J.I. Clinical and radiographic features of dural arteriovenous fistula, a treatable cause of myelopathy. Mayo Clin. Proc. 2001, 76, 1120–1130. [Google Scholar] [CrossRef]
- Huffmann, B.C.; Gilsbach, J.M.; Thron, A. Spinal dural arteriovenous fistulas: A plea for neurosurgical treatment. Acta Neurochir. 1995, 135, 44–51. [Google Scholar] [CrossRef]
- Khurana, V.G.; Perez-Terzic, C.M.; Petersen, R.C.; Krauss, W.E. Singing paraplegia: A distinctive manifestation of a spinal dural arteriovenous fistula. Neurology 2002, 58, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Toossi, S.; Josephson, S.A.; Hetts, S.W.; Chin, C.T.; Kralik, S.; Jun, P.; Douglas, V.C. Utility of MRI in spinal arteriovenous fistula. Neurology 2012, 79, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.W.; Grossman, R.I. Peripheral spinal cord hypointensity on T2-weighted MR images: A reliable imaging sign of venous hypertensive myelopathy. AJNR Am. J. Neuroradiol. 2000, 21, 781–786. [Google Scholar] [PubMed]
- Yen, P.P.; Ritchie, K.C.; Shankar, J.J. Spinal dural arteriovenous fistula: Correlation between radiological and clinical findings. J. Neurosurg. Spine 2014, 21, 837–842. [Google Scholar] [CrossRef] [PubMed]
- McKeon, A.; Lindell, E.P.; Atkinson, J.L.D.; Weinshenker, B.G.; Piepgras, D.G.; Pittock, S.J. Pearls & oysters: Clues for spinal dural arteriovenous fistulae. Neurology 2011, 76, e10–e12. [Google Scholar]
- Strom, R.G.; Derdeyn, C.P.; Moran, C.J.; Cross, D.T.; Esper, G.J.; Mazumdar, A.; Al-Lozi, M.; Lopate, G.; Pestronk, A. Frequency of spinal arteriovenous malformations in patients with unexplained myelopathy. Neurology 2006, 66, 928–931. [Google Scholar] [CrossRef]
- El Mekabaty, A.; Pardo, C.A.; Gailloud, P. The yield of initial conventional MRI in 115 cases of angiographically confirmed spinal vascular malformations. J. Neurol. 2017, 264, 733–739. [Google Scholar] [CrossRef]
- Miller, T.R.; Eskey, C.J.; Mamourian, A.C. Absence of abnormal vessels in the subarachnoid space on conventional magnetic resonance imaging in patients with spinal dural arteriovenous fistulas. Neurosurg. Focus. 2012, 32, E15. [Google Scholar] [CrossRef]
- Saraf-Lavi, E.; Bowen, B.C.; Quencer, R.M.; Sklar, E.M.L.; Holz, A.; Falcone, S.; Latchaw, R.E.; Duncan, R.; Wakhloo, A. Detection of spinal dural arteriovenous fistulae with MR imaging and contrast-enhanced MR angiography: Sensitivity, specificity, and prediction of vertebral level. AJNR Am. J. Neuroradiol. 2002, 23, 858–867. [Google Scholar]
- Takai, K.; Kin, T.; Oyama, H.; Shojima, M.; Saito, N. Three-dimensional angioarchitecture of spinal dural arteriovenous fistulas, with special reference to the intradural retrograde venous drainage system. J. Neurosurg. Spine 2013, 18, 398–408. [Google Scholar] [CrossRef]
- Broekx, S.; Houben, R.; Stockx, L.; Boulanger, T.; Gelin, G.; Weyns, F.; De Beule, T. The external carotid artery as a rare feeder of a spinal dural arteriovenous fistula causing cervical myelopathy: A review of the literature. Brain Spine 2021, 1, 100299. [Google Scholar] [CrossRef]
- Aoki, R.; Srivatanakul, K.; Osada, T.; Sorimachi, T.; Matsumae, M. A case of spinal dural arteriovenous fistula presenting with unusually rapid progression of symptoms. J. Neuroendovasc. Ther. 2018, 12, 181–185. [Google Scholar] [CrossRef]
- Ma, Y.; Hong, T.; Chen, S.; Peng, C.; Wang, C.; Yang, K.; Yu, J.; Ren, J.; Bian, L.; Liu, J.; et al. Steroid-associated acute clinical worsening and poor outcome in patients with spinal dural arteriovenous fistulas: A prospective cohort study. Spine 2020, 45, E656–E662. [Google Scholar] [CrossRef] [PubMed]
- Kudze, T.; Ono, S.; Fereydooni, A.; Gonzalez, L.; Isaji, T.; Hu, H.; Yatsula, B.; Taniguchi, R.; Koizumi, J.; Nishibe, T.; et al. Altered hemodynamics during arteriovenous fistula remodeling leads to reduced fistula patency in female mice. JVS Vasc. Sci. 2020, 1, 42–56. [Google Scholar] [CrossRef] [PubMed]
| Author and the Year | Study Population (n = Number of Patients) | Patient Age, Mean, Median (Range) | Males, n (%) | Spinal dAVF in Thoracic Spine, n (%) | Misdiagnosis, n (%) | Misdiagnosed Disease, n | Duration from Symptom Onset to Diagnosis | Duration from the First Radiologic Imaging Conduction That Showing Features of dAVF to Diagnosis | Duration from Symptom Onset to Treatment | Outcome Evaluation Method | Outcome of All | Outcome of Gait | Outcome of Micturition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ronald AA et al., 2020 [3] | 46 | median 63 (57–71) | 35 (76.0) | 28 (60.9) | 16 (34.8) | spondylosis/degenerative disease 6, idiopathic autoimmune inflammatory myelitis 2, Guillain-Barré syndrome 1, disc herniation 1, peripheral vascular disease 1, tumor 1, multiple sclerosis 1, neuromyelitis optica 1, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome 1,peripheral neuropathy 1 | median 2.3 (misdiagnosis) vs. 0.9 years (no-misdiagnosis) | NA | median 1.2 years | Aminoff-Logue Score | NA | improved 57% stable 30% worsened 14% | improved 35% stable 54% worsened 11% |
| Zhang L et al., 2020 [2] | 65 | mean 53.5 (25–81) | 52 (80.0) | 45 (66.2) | 65 (100) | spinal canal stenosis 12, demyelination 10, lumbar disc herniation 9, myelopathy 8, peripheral neuropathy 7, tumors 4, cervical spondylosis 2, other disease 13 | NA | mean 20.7 months (1–168) | NA | Aminoff-Logue Score | NA | improved 59.5% stable 32.3% worsened 4.6% | improved 32.3% stable 46.7% worsened 4.4% |
| Jablawi F et al., 2020 [4] | 40 | mean 69.27, median 71 (53–84) | 32 (80.0) | NA | NA | mean 20.2 months, median 10 months (1–120) | NA | NA | Aminoff-Logue Score | NA | improved 53% stable 28% worsened 20% | ||
| Takai K et al., 2019 [1] | 40 | mean 67 | 33 (82.5) | 29 (72.5) | 31 (77.5) | lumbar spinal stenosis 14, no abnormality 5, spinal lesion of unknown etiology 3, prostatomegaly 3, intramedullary tumor 2, cervical spinal stenosis 1, multiple sclerosis 1, polyneuritis 1, spinal cord infarction 1 | NA | NA | median 11 (misdiagnosis) vs. 4 months (no-misdiagnosis) | Aminoff-Logue Score | NA | NA | NA |
| Hunt R et al., 2018 [5] | 37 | median 62 | 28 (71.8) | 26 (70.3) | 22 (59.5) | ischaemia 4, Inflammatory 4, neoplasia 3, syrinx 3 | NA | mean 22 (include arteriovenous aetiology in the differential diagnosis) vs. 281 days (exclude arteriovenous aetiology) | NA | NA | NA | NA | NA |
| Ortega-Suero G et al., 2017 [6] | 7 | mean 66.9 (50–80) | 6 (85.7) | 4 (57.1) | 5 (71.4) | lumbar spinal stenosis 2, Restless legs syndrome 1, mixed headache 1, post-polio syndrome and syringomyelia 1 | mean 31 months, median 24 months. mean 41.4 months (misdiagnosis) vs. 5 months (no-misdiagnosis) | NA | NA | Aminoff-Logue Score | improved 57% stable 14% worsened 29% | improved 57% stable 14% worsened 29% | improved 0% stable 86% worsened 14% |
| Barreras P et al., 2017 [7] | 8 | mean 63.9 (49–75) | 7 (87.5) | 4 (50.0) | 8 (100) | transverse myelitis 7, neuromyelitis optica 1 | NA | NA | NA | Aminoff-Logue Score | improved 85% stable 12.5% worsened 12.5% | NA | NA |
| Lee J et al., 2016 [8] | 40 | mean 58.2, median 58 (21–82) | 27 (67.5) | 24 (60.0) | 7 (17.5) | myelitis 5, meningitis and arachnoiditis 1, spinal cord tumor 1 | mean 10.15 months | NA | mean 12.16 months | Aminoff-Logue Score | improved 48.7% stable 35.8% worsened 15.3% | NA | NA |
| Brinjikji W et al., 2016 [9] | 100 | mean 65.0 | 43 of the misdiagnosed patients (81.1) | NA | 53 (40.8) | spinal stenosis 13, myelopathy 10, transverse myelitis 9, ischemic myelopathy 4, peripheral neuropathy 3, myopathy 2, neuromyelitis optica 2, chronic inflammatory demyelinating polyneuropathy 2, Other 8 | NA | mean 9.2 months, median 6 months | NA | Aminoff-Logue Score | NA | improved 32% stable 62% worsened 6% | NA |
| Zogopoulos P et al., 2016 [10] | 14 | mean 62.1 (42–74) | 12 (71.4) | 10 (71.4) | NA | NA | mean 13.5 months (1–36) | NA | NA | modified Rankin Scale | NA | improved 50% stable 50% worsened 0% | NA |
| Iovtchev I et al., 2015 [11] | 7 | mean 60.3 (30–72) | 7 (100) | 6 (85.7) | NA | spinal stenosis, myeloradiculitis, peripheral neuropathy and more. | mean 302.8 days (60–730) | NA | NA | Aminoff-Logue Score | NA | Aminoff-Logue Score of gait 5 n = 4, 4 n = 2, 3 n = 1 | NA |
| Donghai W et al., 2013 [12] | 326 | mean 53.9 | 282 (86.5) | 234 (71.8) | 265 (81.3) | degenerative disc disease 120, myelitis 69, prostatic hyperplasia 17, spinal stenosis 13, intramedullary tumor 12, idiopathic scoliosis 9, syringomyelia 8, spinal cord injury 6, multiple sclerosis 5, tendinopathy 3, Guillan-Barre syndrome 3 | mean 19.9 months | NA | NA | Aminoff-Logue Score | Improved 42.3% stable 53.1% worsened 4.5% | NA | NA |
| Cecchi PC et al., 2009 [13] | 16 | mean 58.4 (23–72) | 14 (62.5) | 12 (75.0) | NA | NA | NA | NA | mean 18.8 months (7–48) | Aminoff-Logue Score | improved 56.25% stable 37.5% worsened 6.255% | improved 50% stable 43.75% worsened 6.25% | improved 31.25% stable 62.5% worsened 6.25% |
| Aghakhani N et al., 2008 [14] | 6 (paraplegic cases) | mean 53.3 (58–75) | 4 (66.7) | 3 (50.0) | NA | NA | NA | NA | mean 39 months (11–144) | American Spinal Injury Association Impairment Scale | NA | improved 100% stable 0 % worsened 0 % | NA |
| Jellema K et al., 2003 [15] | 80 | median 60.2 (34–79) | 66 (82.5) | 65 (85.0) | NA | NA | median 15 months (7 days–197 months) | NA | NA | NA | NA | NA | NA |
| Schick U et al., 2003 [16] | 18 | mean 60 (32–84) | 16 (88.9) | 10 (55.6) | 7 (38.9) | tumor, polyneuropathy, Guillain-Barré syndrome, syringomyelia, and knee disease | mean 15 months (4–45) | NA | NA | MMT | NA | improved 38.9% stable 38.9 % worsened 22.2 % | NA |
| Atkinson JL et al., 2001 [17] | 94 | mean 63 (31–83) | 75 (79.8) | 54 (57.4) | NA | NA | mean 23 months (2–120) | NA | NA | Aminoff-Logue Score | NA | improved 98.9% stable 0% worsened 1.1% | NA |
| Huffmann BC et al., 1995 [18] | 21 | median58 (38–78) | 19 (90.5) | 14 (66.7) | 9 (42.8) | tumor2, lumbar disc prolapse 2, prostate hypertrophy 1, polyneuropathy 1, funicular myelosis 1, knee disease 1 | NA | NA | NA | Aminoff-Logue Score | NA | improved 95.2% stable 4.8% worsened 0% | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Yamane, F.; Sashida, R.; Hirokawa, Y.; Wakamiya, T.; Michiwaki, Y.; Shimoji, K.; Suehiro, E.; Onoda, K.; Matsuno, A.; et al. Delayed Diagnosis of Spinal Dural Arteriovenous Fistula: A Case Report and Scoping Review. J. Clin. Med. 2024, 13, 711. https://doi.org/10.3390/jcm13030711
Tanaka T, Yamane F, Sashida R, Hirokawa Y, Wakamiya T, Michiwaki Y, Shimoji K, Suehiro E, Onoda K, Matsuno A, et al. Delayed Diagnosis of Spinal Dural Arteriovenous Fistula: A Case Report and Scoping Review. Journal of Clinical Medicine. 2024; 13(3):711. https://doi.org/10.3390/jcm13030711
Chicago/Turabian StyleTanaka, Tatsuya, Fumitaka Yamane, Ryohei Sashida, Yu Hirokawa, Tomihiro Wakamiya, Yuhei Michiwaki, Kazuaki Shimoji, Eiichi Suehiro, Keisuke Onoda, Akira Matsuno, and et al. 2024. "Delayed Diagnosis of Spinal Dural Arteriovenous Fistula: A Case Report and Scoping Review" Journal of Clinical Medicine 13, no. 3: 711. https://doi.org/10.3390/jcm13030711
APA StyleTanaka, T., Yamane, F., Sashida, R., Hirokawa, Y., Wakamiya, T., Michiwaki, Y., Shimoji, K., Suehiro, E., Onoda, K., Matsuno, A., & Morimoto, T. (2024). Delayed Diagnosis of Spinal Dural Arteriovenous Fistula: A Case Report and Scoping Review. Journal of Clinical Medicine, 13(3), 711. https://doi.org/10.3390/jcm13030711






