Sonorheometry Device Thresholds in Liver Transplantation: An Observational Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients and LT Characteristics
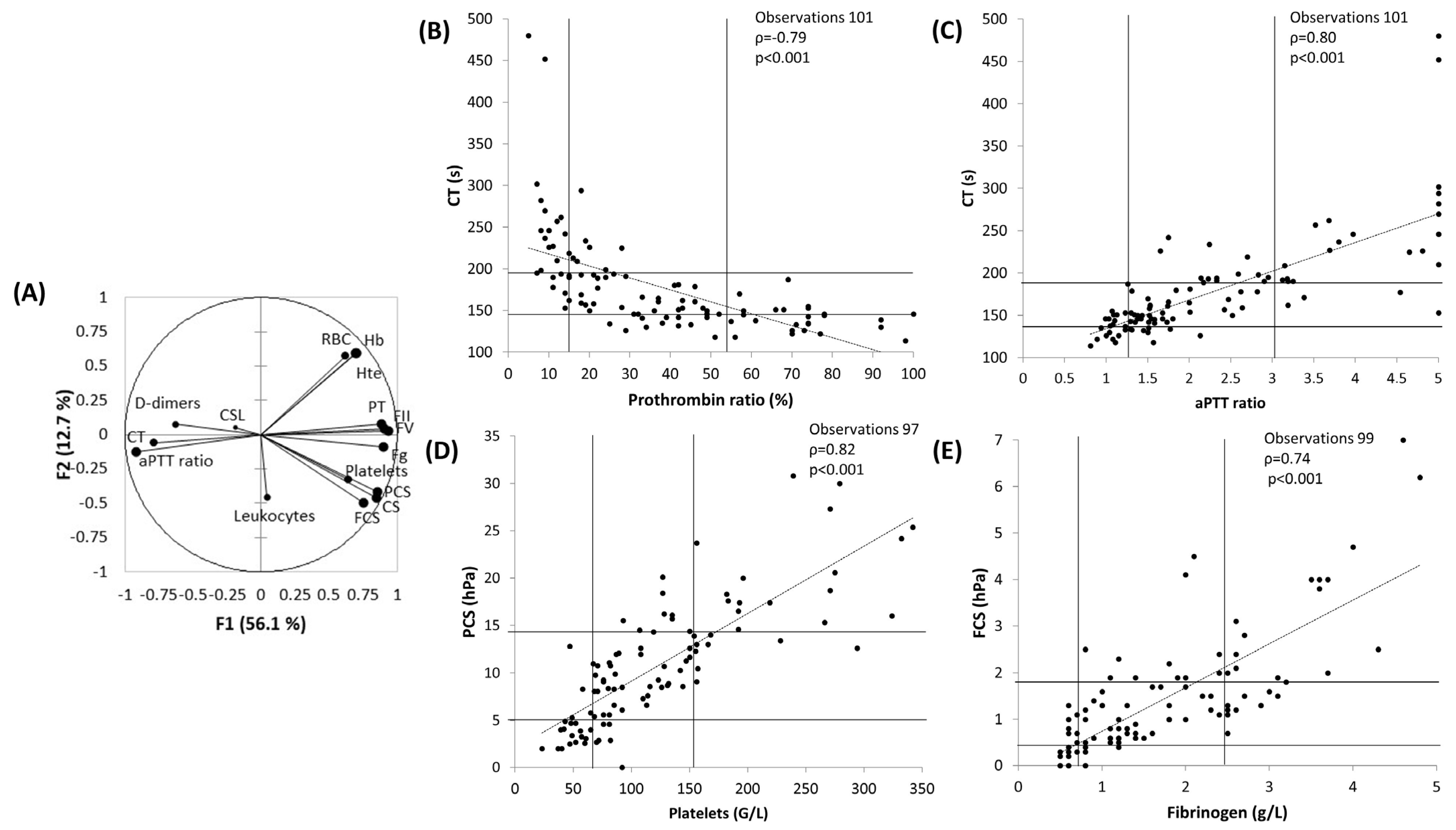
3.2. Principal Component Analysis
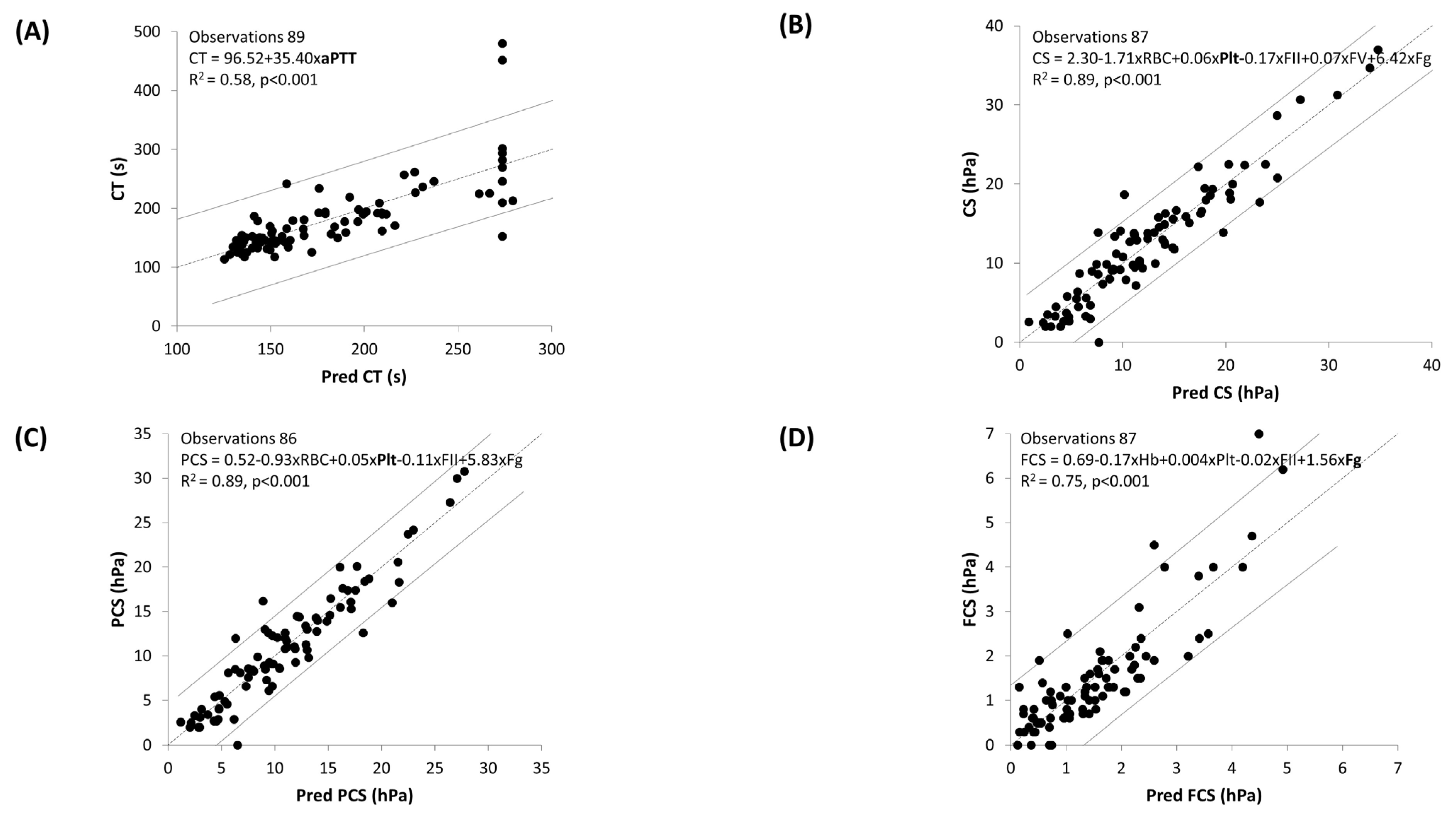
3.3. Multiple Linear Regression
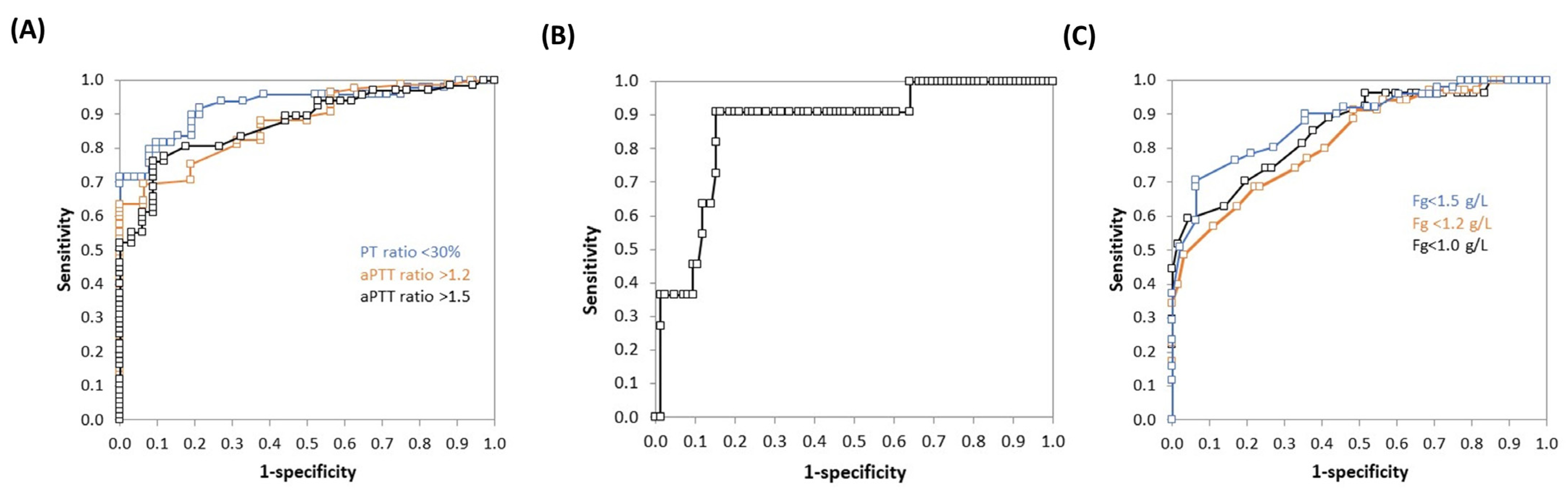
3.4. ROC Curves Analysis
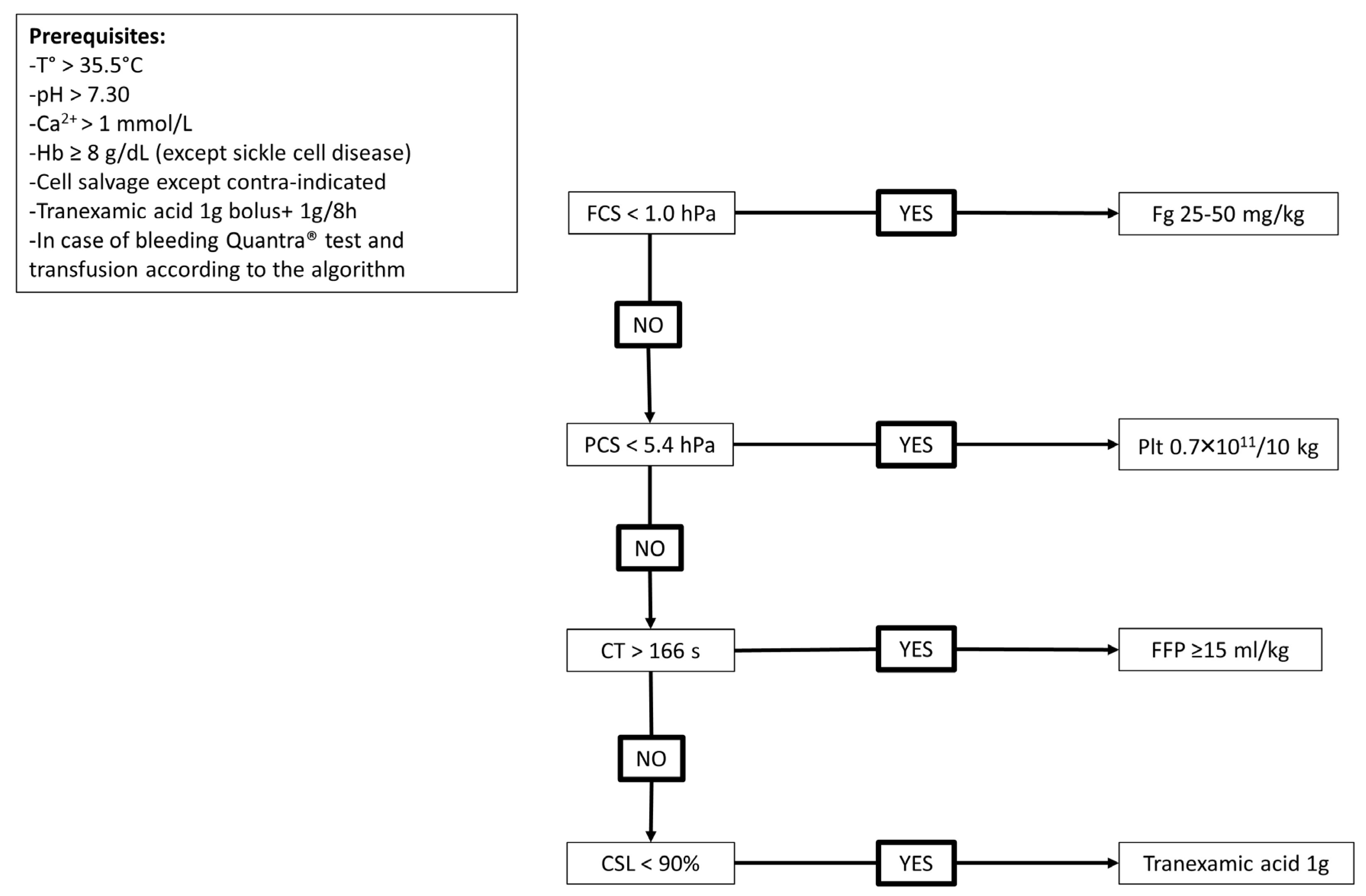
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villa, E.; Bianchini, M.; Blasi, A.; Denys, A.; Giannini, E.G.; de Gottardi, A.; Lisman, T.; de Raucourt, E.; Ripoll, C.; Rautou, P.-E. EASL Clinical Practice Guidelines on Prevention and Management of Bleeding and Thrombosis in Patients with Cirrhosis. J. Hepatol. 2022, 76, 1151–1184. [Google Scholar] [CrossRef]
- Yoon, U.; Bartoszko, J.; Bezinover, D.; Biancofiore, G.; Forkin, K.T.; Rahman, S.; Spiro, M.; Raptis, D.A.; Kang, Y.; the ERAS4OLT.org Working Group. Intraoperative Transfusion Management, Antifibrinolytic Therapy, Coagulation Monitoring and the Impact on Short-term Outcomes after Liver Transplantation—A Systematic Review of the Literature and Expert Panel Recommendations. Clin. Transplant. 2022, 36, e14637. [Google Scholar] [CrossRef] [PubMed]
- Kietaibl, S.; Ahmed, A.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of Severe Peri-Operative Bleeding: Guidelines from the European Society of Anaesthesiology and Intensive Care: Second Update 2022. Eur. J. Anaesthesiol. 2023, 40, 226–304. [Google Scholar] [CrossRef] [PubMed]
- Biancofiore, G.; Blasi, A.; De Boer, M.T.; Franchini, M.; Hartmann, M.; Lisman, T.; Liumbruno, G.M.; Porte, R.J.; Saner, F.; Senzolo, M.; et al. Perioperative Hemostatic Management in the Cirrhotic Patient: A Position Paper on Behalf of the Liver Intensive Care Group of Europe (LICAGE). Minerva Anestesiol. 2019, 85, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Aceto, P.; Punzo, G.; Di Franco, V.; Teofili, L.; Gaspari, R.; Avolio, A.W.; Del Tedesco, F.; Posa, D.; Lai, C.; Sollazzi, L. Viscoelastic versus Conventional Coagulation Tests to Reduce Blood Product Transfusion in Patients Undergoing Liver Transplantation: A Systematic Review and Meta-Analysis. Eur. J. Anaesthesiol. 2023, 40, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Hermelin, D.; Levy, J.H. Viscoelastic Testing. An Illustrated Review of Technology and Clinical Applications. Res. Pract. Thromb. Haemost. 2023, 7, e100031. [Google Scholar] [CrossRef] [PubMed]
- Viola, F.; Kramer, M.D.; Lawrence, M.B.; Oberhauser, J.P.; Walker, W.F. Sonorheometry: A Noncontact Method for the Dynamic Assessment of Thrombosis. Ann. Biomed. Eng. 2004, 32, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Corey, F.S.; Walker, W.F. Sonic Estimation of Elasticity via Resonance: A New Method of Assessing Hemostasis. Ann. Biomed. Eng. 2016, 44, 1405–1424. [Google Scholar] [CrossRef]
- Michelson, E.A.; Cripps, M.W.; Ray, B.; Winegar, D.A.; Viola, F. Initial Clinical Experience with the Quantra QStat System in Adult Trauma Patients. Trauma. Surg. Acute Care Open 2020, 5, e000581. [Google Scholar] [CrossRef]
- Rossetto, A.; Wohlgemut, J.M.; Brohi, K.; Davenport, R. Sonorheometry versus Rotational Thromboelastometry in Trauma: A Comparison of Diagnostic and Prognostic Performance. J. Thromb. Haemost. 2023, 21, 2114–2125. [Google Scholar] [CrossRef]
- Huffmyer, J.L.; Fernandez, L.G.; Haghighian, C.; Terkawi, A.S.; Groves, D.S. Comparison of SEER Sonorheometry With Rotational Thromboelastometry and Laboratory Parameters in Cardiac Surgery. Anesth. Analg. 2016, 123, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Groves, D.S.; Welsby, I.J.; Naik, B.I.; Tanaka, K.; Hauck, J.N.; Greenberg, C.S.; Winegar, D.A.; Viola, F. Multicenter Evaluation of the Quantra QPlus System in Adult Patients Undergoing Major Surgical Procedures. Anesth. Analg. 2020, 130, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Duclos, G.; Fleury, M.; Grosdidier, C.; Lakbar, I.; Antonini, F.; Lassale, B.; Arbelot, C.; Albaladejo, P.; Zieleskiewicz, L.; Leone, M. Blood Coagulation Test Abnormalities in Trauma Patients Detected by Sonorheometry: A Retrospective Cohort Study. Res. Pract. Thromb. Haemost. 2023, 7, e100163. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, D.; Abdallah, G.A.; Lang, E.; Boucebci, K.-J.; Gautier, C.-H.; François, A.; Gaussem, P.; Godier, A. Assessment of a Quantra-Guided Hemostatic Algorithm in High-Bleeding-Risk Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2023, 37, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.S.; Forkin, K.T.; Brennan, M.M.; Kumar, S.S.; Winegar, D.A.; Viola, F. Multicenter Evaluation of the Quantra with the QStat Cartridge in Adult Patients Undergoing Liver Transplantation. Liver Transpl. 2023, 29, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC 15189:2022; Medical Laboratories: Requirements for Quality and Competence. ISO: Geneva, Switzerland, 2022.
- Naik, B.I.; Tanaka, K.; Sudhagoni, R.G.; Viola, F. Prediction of Hypofibrinogenemia and Thrombocytopenia at the Point of Care with the Quantra® QPlus® System. Thromb. Res. 2021, 197, 88–93. [Google Scholar] [CrossRef]
- Demailly, Z.; Wurtz, V.; Barbay, V.; Surlemont, E.; Scherrer, V.; Compère, V.; Billoir, P.; Clavier, T.; Besnier, E. Point-of-Care Viscoelastic Hemostatic Assays in Cardiac Surgery Patients: Comparison of Thromboelastography 6S, Thromboelastometry Sigma, and Quantra. J. Cardiothorac. Vasc. Anesth. 2023, 37, 948–955. [Google Scholar] [CrossRef]
- Roullet, S.; Freyburger, G.; Cruc, M.; Quinart, A.; Stecken, L.; Audy, M.; Chiche, L.; Sztark, F. Management of Bleeding and Transfusion during Liver Transplantation before and after the Introduction of a Rotational Thromboelastometry-Based Algorithm. Liver Transpl. 2015, 21, 169–179. [Google Scholar] [CrossRef]
- Roullet, S.; Rivoire, T.; Houssin, C.; Labrouche, S.; Paquin, S.; Nouette-Gaulain, K.; Deneux-Tharaux, C.; Amiral, J.; James, C.; Sentilhes, L. Hemostatic Effects of Tranexamic Acid in Cesarean Delivery: An Ancillary Study of the TRAAP2 Study. Thromb. Haemost. 2022, 122, 1869–1878. [Google Scholar] [CrossRef]
- Roullet, S.; Labrouche, S.; Mouton, C.; Quinart, A.; Nouette-Gaulain, K.; Laurent, C.; Freyburger, G. Lysis Timer: A New Sensitive Tool to Diagnose Hyperfibrinolysis in Liver Transplantation. J. Clin. Pathol. 2019, 72, 58–65. [Google Scholar] [CrossRef]
- Costa, M.; Dalmau, A.; Sabate, A.; Koo, M.; Aparicio, I.; Contreras, L. Low Plasma Fibrinogen Levels and Blood Product Transfusion in Liver Transplantation. Minerva Anestesiol. 2014, 80, 568–573. [Google Scholar] [PubMed]
- Carrier, F.M.; Deshêtres, A.; Ferreira Guerra, S.; Rioux-Massé, B.; Zaouter, C.; Lee, N.; Amzallag, É.; Joosten, A.; Massicotte, L.; Chassé, M. Preoperative Fibrinogen Level and Bleeding in Liver Transplantation for End-Stage Liver Disease: A Cohort Study. Transplantation 2023, 107, 693–702. [Google Scholar] [CrossRef]
- Aster, R.H. Pooling of Platelets in the Spleen: Role in the Pathogenesis of “Hypersplenic” Thrombocytopenia. J. Clin. Investig. 1966, 45, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Primignani, M.; Chantarangkul, V.; Lemma, L.; Jovani, M.; Rebulla, P.; Mannucci, P.M. Global Hemostasis Tests in Patients with Cirrhosis before and after Prophylactic Platelet Transfusion. Liver Int. 2013, 33, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.; Cadamuro, J.; Ziegler, B.; Schöchl, H.; Varvenne, M.; Sørensen, B.; Hochleitner, G.; Rahe-Meyer, N. A Comparison of Fibrinogen Measurement Methods with Fibrin Clot Elasticity Assessed by Thromboelastometry, before and after Administration of Fibrinogen Concentrate in Cardiac Surgery Patients. Transfusion 2011, 51, 1695–1706. [Google Scholar] [CrossRef]
- Lisman, T. Interpreting Hemostatic Profiles Assessed with Viscoelastic Tests in Patients with Cirrhosis. J. Clin. Gastroenterol. 2020, 54, 389–391. [Google Scholar] [CrossRef]
| Characteristics | Values |
|---|---|
| Patients (n = 34) | |
| Gender M/F, n (%) | 27 (79.4)/7 (20.6) |
| Age, years | 60.5 [51.2–63.8] |
| Height, m | 1.71 [1.65–1.78] |
| Weight, kg | 86.0 [78.0–93.2] |
| BMI, kg/m2 | 29.4 [25.2–31.7] |
| Liver disease leading to LT, n (%) | |
| Alcoholic cirrhosis | 20 (58.8) |
| HCC | 7 (20.6) |
| Acute hepatitis | 5 (14.7) |
| Amyloid neuropathy | 1 (2.9) |
| Hepatic metastasis | 1 (2.9) |
| MELD score | 20 [12–32] |
| Child–Pugh score A/B/C/NA | 8 (23.5)/5 (14.7)/18 (52.9)/3 (8.8) |
| Donors (n = 34) | |
| Age (years) Type of donors: DBD/M3 DCD/LDD | 58 [48–71] 32 (94.2)/1 (2.9)/1(2.9) |
| Liver transplantations (n = 34) | |
| Surgical time, min | 424 [373–487] |
| Cold ischemia time, min | 359 [307–426] |
| Warm ischemia time, min | 30 [26–37] |
| Type of caval anastomosis: 3 veins piggyback technique/side to side/caval replacement | 27 (79.4)/2 (5.9)/5 (14.7) |
| Characteristics Number of Patients Concerned (%) | Values |
|---|---|
| Bleeding, mL | 1500 [1025–2525] |
| Re-transfused blood volume from cell salvage (mL) N = 20 (58.8%) | 262 [179–567] |
| Blood labile products transfusion, n (%) | 20 (58.8) |
| PRBC, n N = 18 (52.9%) | 4 [3–7] |
| FFP, n N = 11 (32.4%) | 3 [2–8] |
| FFP, mL/kg N = 11 (32.4%) | 10.6 [7.0–20.7] |
| Platelet units, n N = 5 (14.7%) | 1 [1–2] |
| Fg concentrate, g N = 7 (20.6%) | 3.0 [2.5–3.5] |
| Fg concentrate, mg/kg N = 7 (20.6%) | 33 [24–38] |
| Tranexamic acid, g N = 34 (100.0%) | 1.8 [1.7–2.0] |
| Tranexamic acid, mg/kg N = 34 (100.0%) | 21.8 [19.1–24.6] |
| Variables (Normal Values for Healthy Controls) | All | T1 | T2 | T3 |
|---|---|---|---|---|
| Laboratory results | ||||
| Leukocytes, G/L (4.0–10.0) | 7.3 [5.5–9.0] | 6.5 [4.7–8.2] | 6.4 [5.4–9.7] | 8.0 [6.5–9.7] |
| Red blood cells, T/L (3.8–5.0) | 3.0 [2.5–3.8] | 3.6 [2.5–4.2] | 3.0 [2.6–3.5] | 2.8 [2.3–3.2] |
| Haemoglobin, g/dL (12.0–16.0) | 9.2 [7.9–11.4] | 10.8 [7.9–12.8] | 9.2 [8.2–11.2] | 8.3 [7.5–10.1] |
| Haematocrit, % (34.0–45.0) | 27.0 [23.4–33.3] | 31.2 [23.2–37.0] | 27.0 [24.6–32.7] | 24.6 [22.2–29.5] |
| Platelets, G/L (150.0–400.0) | 107.5 [70.2–155.8] | 100.5 [67.5–156.8] | 116.0 [77.0–163.5] | 85.5 [70.5–140.2] |
| Prothrombin ratio, % (>70) | 32 [15–52] | 42 [20–70] | 32 [18–50] | 21 [13–40] |
| Factor II, % (60–140) | 27 [12–51] | 38 [25–69] | 28 [14–52] | 13 [10–34] |
| Factor V, % (60–140) | 28 [17–51] | 41 [27–73] | 29 [18–47] | 17 [11–33] |
| aPTT ratio (<1.20) | 1.76 [1.34–3.08] | 1.48 [1.17–1.97] | 1.74 [1.45–2.78] | 2.80 [1.66–4.95] |
| Fibrinogen, g/L (1.5–4.0) | 1.4 [0.8–2.5] | 2.1 [1.2–2.8] | 1.4 [0.9–2.4] | 1.1 [0.8–1.8] |
| D-dimers, ng FEU/mL (<500) | 2085 [1111–3474] | 2235 [669–7085] | 1840 [1128–3290] | 2443 [1436–3228] |
| Quantra® results | ||||
| CT, s (103–153) | 161 [144–194] | 150 [136–180] | 152 [138–164] | 194 [177–226] |
| CS, hPa (13.0–33.2) | 11.2 [6.2–16.2] | 13.6 [7.2–18.1] | 12.4 [8.0–16.7] | 9.0 [5.4–12.0] |
| PCS, hPa (11.9–29.8) | 9.9 [5.6–14.4] | 12.0 [8.6–16.1] | 11.3 [6.6–14.6] | 8.1 [4.6–10.7] |
| FCS, hPa (1.0–3.7) | 1.1 [0.6–1.9] | 1.6 [0.8–2.2] | 1.2 [0.7–1.7] | 1.0 [0.5–1.2] |
| CSL, % (>92) | 99 [98–100] | 100 [98–100] | 99 [98–100] | 100 [98–100] |
| Parameters | AUC | Best Cut-Off | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| Ability of CT to predict | ||||||
| PT ratio < 30% | 0.93 (0.87–0.98) * | 166 | 0.82 (0.68–0.90) | 0.90 (0.79–0.96) | 0.89 | 0.84 |
| aPTT ratio > 1.2 | 0.87 (0.80–0.95) * | 155 | 0.64 (0.53–0.73) | 1.00 (0.77–1.00) | 1.00 | 0.34 |
| aPTT ratio > 1.5 | 0.87 (0.81–0.94) * | 155 | 0.76 (0.65–0.85) | 0.92 (0.76–0.98) | 0.94 | 0.66 |
| Ability of PCS to predict | ||||||
| Plt count < 50 G/L | 0.87 (0.76–0.98) * | 5.4 | 0.91 (0.59–1.00) | 0.85 (0.76–0.91) | 0.44 | 0.99 |
| Ability of FCS to predict | ||||||
| Fg < 1.0 g/L | 0.86 (0.77–0.94) * | 0.6 | 0.59 (0.41–0.75) | 0.96 (0.88–0.99) | 0.84 | 0.86 |
| Fg < 1.2 g/L | 0.82 (0.74–0.91) * | 0.9 | 0.69 (0.52–0.81) | 0.78 (0.66–0.87) | 0.63 | 0.82 |
| Fg < 1.5 g/L | 0.88 (0.82–0.95) * | 1.0 | 0.71 (0.57–0.81) | 0.94 (0.82–0.98) | 0.92 | 0.75 |
| Parameters | AUC | Best Cut-Off | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| Ability of FCS to predict | ||||||
| Fg < 1.0 g/L | 0.81 (0.68–0.93) * | 1.3 | 0.87 (0.61–0.97) | 0.62 (0.50–0.73) | 0.35 | 0.95 |
| Fg < 1.2 g/L | 0.80 (0.70–0.91) * | 1.5 | 0.91 (0.71–0.98) | 0.55 (0.42–0.68) | 0.44 | 0.94 |
| Fg < 1.5 g/L | 0.85 (0.76–0.94) * | 1.0 | 0.61 (0.44–0.75) | 0.96 (0.84–0.99) | 0.91 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soucy-Proulx, M.; Kato, H.; Coeckelenbergh, S.; Naili Kortaia, S.; Herboulier, L.; Pittau, G.; Pham, P.; Lemoine, A.; Duranteau, J.; Roullet, S. Sonorheometry Device Thresholds in Liver Transplantation: An Observational Retrospective Study. J. Clin. Med. 2024, 13, 696. https://doi.org/10.3390/jcm13030696
Soucy-Proulx M, Kato H, Coeckelenbergh S, Naili Kortaia S, Herboulier L, Pittau G, Pham P, Lemoine A, Duranteau J, Roullet S. Sonorheometry Device Thresholds in Liver Transplantation: An Observational Retrospective Study. Journal of Clinical Medicine. 2024; 13(3):696. https://doi.org/10.3390/jcm13030696
Chicago/Turabian StyleSoucy-Proulx, Maxim, Hiromi Kato, Sean Coeckelenbergh, Salima Naili Kortaia, Laurence Herboulier, Gabriella Pittau, Patrick Pham, Antoinette Lemoine, Jacques Duranteau, and Stéphanie Roullet. 2024. "Sonorheometry Device Thresholds in Liver Transplantation: An Observational Retrospective Study" Journal of Clinical Medicine 13, no. 3: 696. https://doi.org/10.3390/jcm13030696
APA StyleSoucy-Proulx, M., Kato, H., Coeckelenbergh, S., Naili Kortaia, S., Herboulier, L., Pittau, G., Pham, P., Lemoine, A., Duranteau, J., & Roullet, S. (2024). Sonorheometry Device Thresholds in Liver Transplantation: An Observational Retrospective Study. Journal of Clinical Medicine, 13(3), 696. https://doi.org/10.3390/jcm13030696






