Ultrasound and Sialendoscopy Findings in Radioactive Iodine–Induced Sialadenitis: Comparative Analysis and Possible Impact on Management
Abstract
1. Introduction
2. Methods
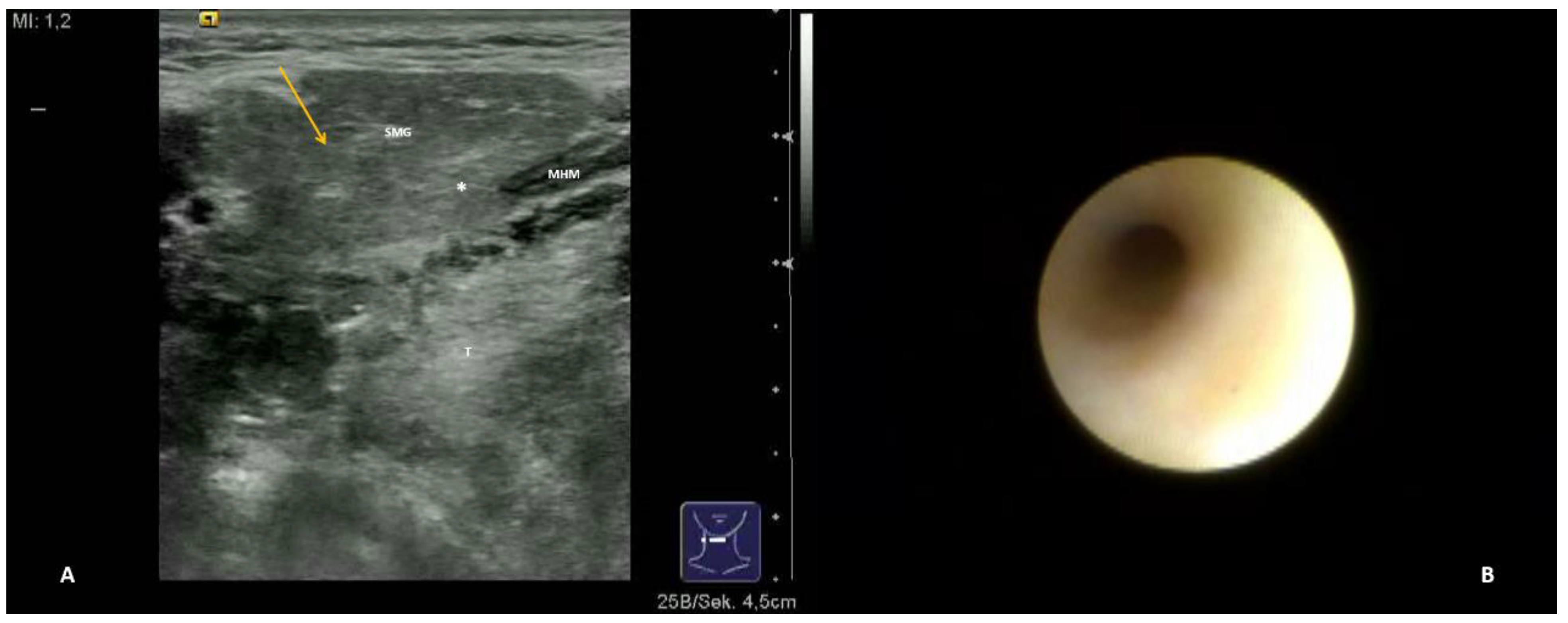
- Hypoechoic and homogeneous (no relevant loss of gland parenchyma; Figure 1A)
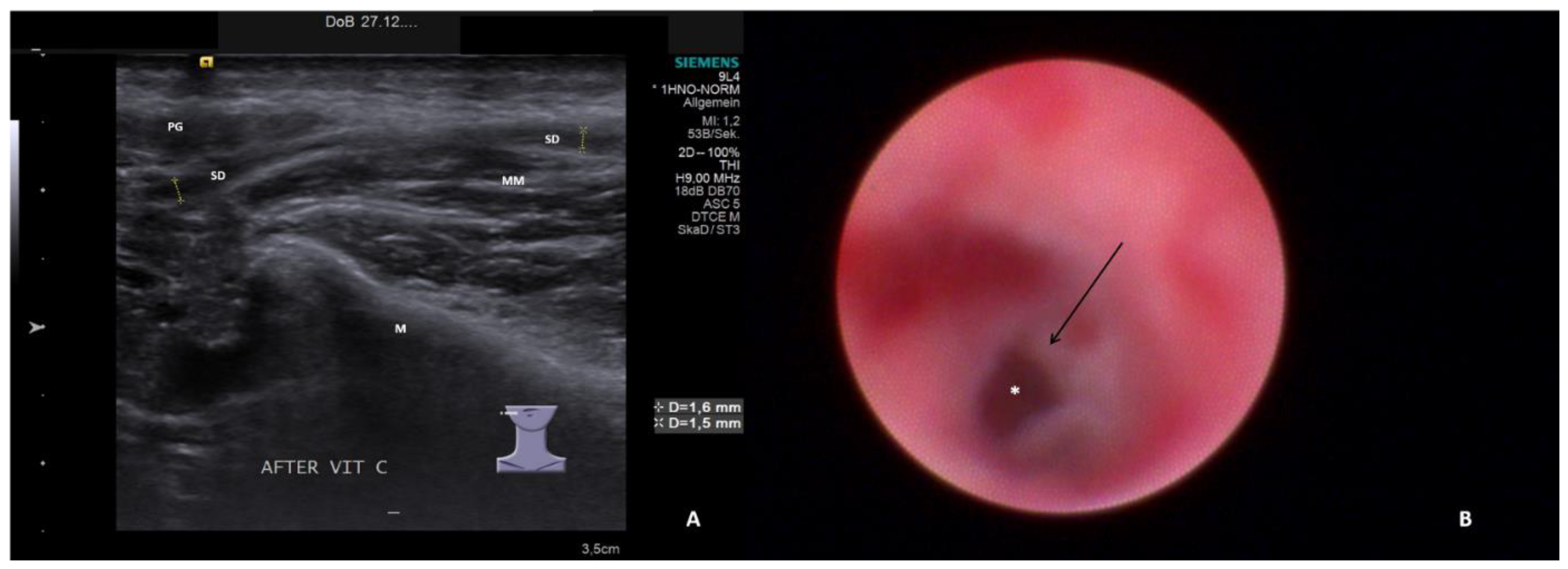
- Chronic sialodochitis (SD: inflammation, paleness of the duct wall, plaques, discharge; Figure 1B; Supplementary Video S1)
- Sialodochitis with a tendency toward duct narrowing (SD + Sten: SD and thickness of the duct wall with a tendency toward luminal narrowing; Figure 2B; Supplementary Video S2)
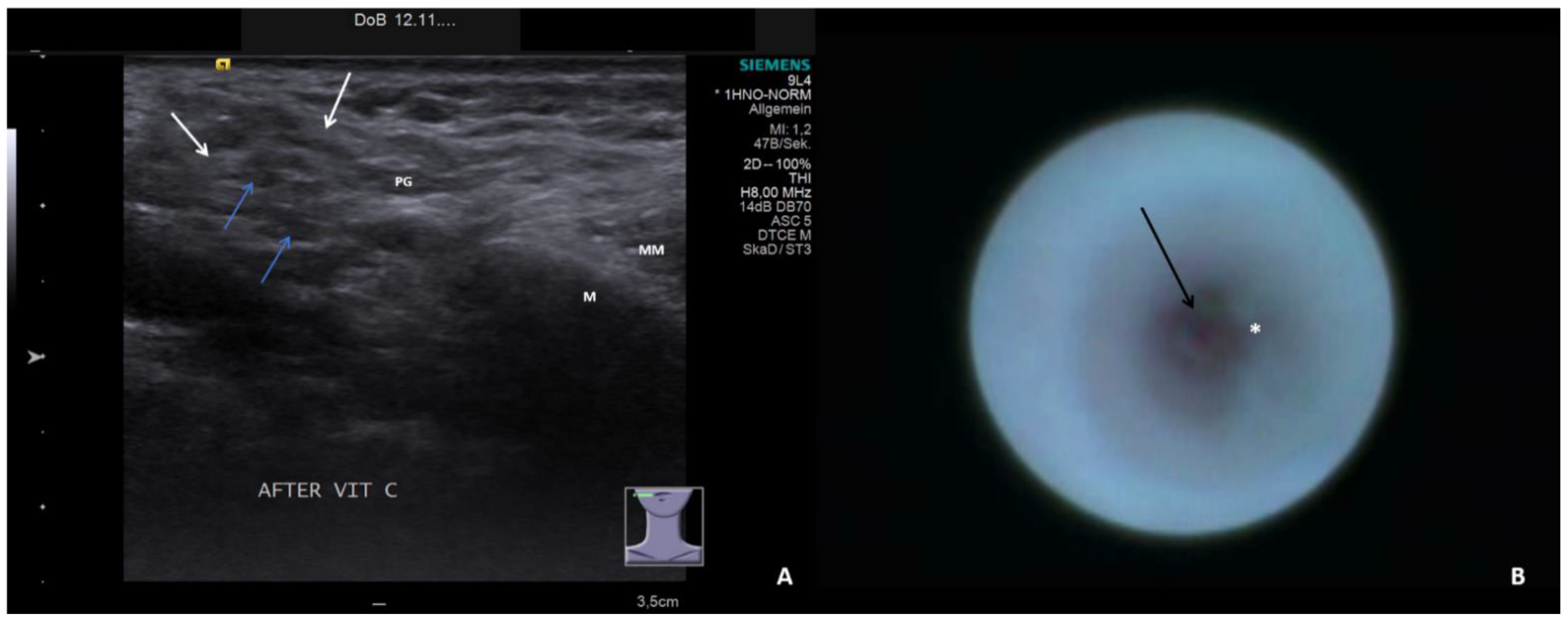
- Short duct stenosis (≤1 cm; Figure 3B; Supplementary Video S3)
- Segmental duct stenosis (≥1 cm)
- Long duct stenosis (≥3 cm; Figure 4B; Supplementary Video S4)
- Complete duct stenosis (no lumen visible, but visible lumen proximal to the stenosis)
- Duct obliteration (lumen obstructed by fibrotic, cobweb-like material, no epithelialized lumen visible; Figure 5B; Supplementary Video S5) as a sign of gland atrophy (diffuse long stenosis and/or complete duct stenosis with proximal duct obliteration)
Statistical Analysis
3. Results
3.1. Ultrasound and Sialendoscopy Findings and Correlations/Associations
3.2. Duct Dilation and Maximum Ultrasound Findings
3.3. Duct Dilation and Maximum Sialendoscopy Findings
3.4. Changes between First and Last Ultrasound and Sialendoscopy
4. Discussion
- Normal parenchyma with unremarkable findings, SD, SD + Sten on sialendoscopy: the prognosis tends to be good and treatment measures are indicated. Treatment consists of conservative measures (gland massage with sialogouges) and (sialendoscopic-controlled) irrigation of the duct system with cortisone.
- Hypoechoic glands and SD, SD + Sten, or any kind of stenosis: a relatively good prognosis can be expected. Treatment measures are worthwhile. In addition to the afore-mentioned measures, interventional sialendoscopy with the opening and dilation of a stenosis with or without stent implantation can be indicated.
- Heterogeneous parenchyma and presence of SD + Sten or any kind of stenosis: the prognosis tends to be uncertain. Treatment may be tried, particularly if duct dilation is present, but RAIS may impair the subsequent course. Conservative measures, irrigation of the duct system, and interventional sialendoscopy may belong to the therapeutic measures.
- Heterogeneous parenchyma and complete or long stenosis, or incipient signs of duct obliteration: progression is more likely. The success of any therapy may not be promising (at least in the long term), particularly if no duct dilation is visible. While conservative measures like irrigation of the duct system may be performed, the benefit of interventional sialendoscopy is questionable.
- Atrophic parenchyma and duct stenosis, or duct obliteration with or without duct dilation: no further therapeutic measures (except conservative) are indicated.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adramerinas, M.; Andreadis, D.; Vahtsevanos, K.; Poulopoulos, A.; Pazaitou-Panayiotou, K. Sialadenitis as a complication of radioiodine therapy in patients with thyroid cancer: Where do we stand? Hormones 2021, 20, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Riachy, R.; Ghazal, N.; Haidar, M.B.; Elamine, A.; Nasrallah, M.P. Early Sialadenitis After Radioactive Iodine Therapy for Differentiated Thyroid Cancer: Prevalence and Predictors. Int. J. Endocrinol. 2020, 2020, 8649794. [Google Scholar] [CrossRef] [PubMed]
- An, Y.-S.; Yoon, J.-K.; Lee, S.J.; Song, H.-S.; Yoon, S.-H.; Jo, K.-S. Symptomatic late-onset sialadenitis after radioiodine therapy in thyroid cancer. Ann. Nucl. Med. 2013, 27, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.S.; An, Y.-S.; Lee, S.J.; Soh, E.-Y.; Lee, J.; Chung, Y.-S.; Kim, D.J.; Yoon, S.-H.; Lee, D.H.; Yoon, J.-K. Significance of Salivary Gland Radioiodine Retention on Post-ablation 131I Scintigraphy as a Predictor of Salivary Gland Dysfunction in Patients with Differentiated Thyroid Carcinoma. Nucl. Med. Mol. Imaging 2014, 48, 203–211. [Google Scholar] [CrossRef][Green Version]
- Lee, H.N.; An, J.Y.; Lee, K.M.; Kim, E.J.; Choi, W.S.; Kim, D.Y. Salivary gland dysfunction after radioactive iodine (I-131) therapy in patients following total thyroidectomy: Emphasis on radioactive iodine therapy dose. Clin. Imaging 2015, 39, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lee, J.W.; Kim, S.Y.; Han, S.W.; Bae, W.K. Prediction of risk for symptomatic sialadenitis by post-therapeutic dual 131I scintigraphy in patients with differentiated thyroid cancer. Ann. Nucl. Med. 2013, 27, 700–709. [Google Scholar] [CrossRef]
- Van Nostrand, D. Sialoadenitis secondary to 131I therapy for well-differentiated thyroid cancer. Oral Dis. 2011, 17, 154–161. [Google Scholar] [CrossRef]
- Hyer, S.; Kong, A.; Pratt, B.; Harmer, C. Salivary gland toxicity after radioiodine therapy for thyroid cancer. Clin. Oncol. 2007, 19, 83–86. [Google Scholar] [CrossRef]
- Nakada, K.; Ishibashi, T.; Takei, T.; Hirata, K.; Shinohara, K.; Katoh, S.; Zhao, S.; Tamaki, N.; Noguchi, Y.; Noguchi, S. Does lemon candy decrease salivary gland damage after radioiodine therapy for thyroid cancer? J. Nucl. Med. 2005, 46, 261–266. [Google Scholar]
- Mandel, S.J.; Mandel, L.; Mok, Y.; Pang, Y.H.; Teh, M.; Petersson, F.; Choi, J.-S.; An, H.-Y.; Park, I.S.; Kim, Y.-M.; et al. Radioactive Iodine and the Salivary Glands. Thyroid 2003, 13, 265–271. [Google Scholar] [CrossRef]
- Caglar, M.; Tuncel, M.; Alpar, R. Scintigraphic Evaluation of Salivary Gland Dysfunction in Patients with Thyroid Cancer After Radioiodine Treatment. Clin. Nucl. Med. 2002, 27, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Solans, R.; Bosch, J.A.; Galofré, P.; Porta, F.; Roselló, J.; Selva-O’Callagan, A.; Vilardell, M. Salivary and lacrimal gland dysfunction (sicca syndrome) after radioiodine therapy. J. Nucl. Med. 2001, 42, 738–743. [Google Scholar] [PubMed]
- Newkirk, K.A.; Ringel, M.D.; Wartofsky, L.; Burman, K.D. The Role of Radioactive Iodine in Salivary Gland Dysfunction. Ear Nose Throat J. 2000, 79, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Bader, J.B.; Schaefer, A.; Finke, C.; Kirsch, C.M. Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J. Nucl. Med. 1998, 39, 1551–1554. [Google Scholar] [PubMed]
- Stephens, L.C.; Schultheiss, T.E.; Price, R.E.; Ang, K.K.; Peters, L.J. Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer 1991, 67, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Allweiss, P.; Braunstein, G.D.; Katz, A.; Waxman, A. Sialadenitis following I-131 therapy for thyroid carcinoma: Concise communication. J. Nucl. Med. 1984, 25, 755–758. [Google Scholar] [PubMed]
- Helman, J.; Turner, R.J.; Fox, P.C.; Baum, B.J. 99mTc-pertechnetate uptake in parotid acinar cells by the Na+/K+/Cl- co-transport system. J. Clin. Investig. 1987, 79, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, R.R.; Agopiantz, M.; Elhanbali, O.; Demore, B.; Cuny, T.; Demarquet, L.; Ndiaye, C.; Barbe, F.; Brunaud, L.; Weryha, G.; et al. Iodine Metabolism and Thyroid Physiology: Current Concepts. Thyroid 1997, 7, 177–181. [Google Scholar] [CrossRef]
- Jhiang, S.M.; Cho, J.Y.; Ryu, K.Y.; DeYoung, B.R.; Smanik, P.A.; McGaughy, V.R.; Fischer, A.H.; Mazzaferri, E.L. An immunohistochemical study of Na+/I- symporter in human thyroid tissues and salivary gland tissues. Endocrinology 1998, 139, 4416–4419. [Google Scholar] [CrossRef]
- De la Vieja, A.; Dohan, O.; Levy, O.; Carrasco, N.; Andrade, B.M.; Araujo, R.L.; Perry, R.L.S.; Souza, E.C.L.; Cazarin, J.M.; Carvalho, D.P.; et al. Molecular Analysis of the Sodium/Iodide Symporter: Impact on Thyroid and Extrathyroid Pathophysiology. Physiol. Rev. 2000, 80, 1083–1105. [Google Scholar] [CrossRef]
- Nahlieli, O.; Nazarian, Y. Sialadenitis following radioiodine therapy—A new diagnostic and treatment modality. Oral Dis. 2006, 12, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Han, G.S.; Lee, S.H.; Lee, D.Y.; Kim, Y. Sialoendoscopic Treatment for Radioiodine Induced Sialadenitis. Laryngoscope 2007, 117, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Brozzi, F.; Rago, T.; Bencivelli, W.; Bianchi, F.; Santini, P.; Vitti, P.; Pinchera, A.; Ceccarelli, C. Salivary glands ultrasound examination after radioiodine-131 treatment for differentiated thyroid cancer. J. Endocrinol. Investig. 2013, 36, 153–156. [Google Scholar] [CrossRef]
- Kim, D.W. Ultrasonographic Features of the Major Salivary Glands after Radioactive Iodine Ablation in Patients with Papillary Thyroid Carcinoma. Ultrasound Med. Biol. 2015, 41, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.S.; Kim, D.W.; Baek, H.J. Association of Xerostomia and Ultrasonographic Features of the Major Salivary Glands After Radioactive Iodine Ablation for Papillary Thyroid Carcinoma. Am. J. Roentgenol. 2016, 207, 1077–1081. [Google Scholar] [CrossRef]
- Horvath, E.; Skoknic, V.; Majlis, S.; Tala, H.; Silva, C.; Castillo, E.; Whittle, C.; Niedmann, J.P.; González, P. Radioiodine-Induced Salivary Gland Damage Detected by Ultrasonography in Patients Treated for Papillary Thyroid Cancer: Radioactive Iodine Activity and Risk. Thyroid 2020, 30, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.A.S.; López, R.V.M.; Ozório, G.A.; de Freitas, R.M.C.; Willegaignon, J.; Sapienza, M.T.; Chammas, M.C.; Coura-Filho, G.B. Ultrasonography Echotexture as a surrogate for Sialadenitis secondary to 131I Radioiodine Therapy for differentiated Thyroid Cancer: A review and metaanalysis. Clinics 2020, 75, e1843. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.A.S.; López, R.V.M.; de Freitas, R.M.C.; Willegaignon, J.; Sapienza, M.T.; Chammas, M.C.; Coura-Filho, G.B. Evaluation of Parotid Salivary Gland Echo Texture by Ultrasound Examinations and Correlation with Whole-Body Scintigraphy After Radioiodine Therapy in Patients with Differentiated Thyroid Carcinoma. J. Ultrasound Med. 2020, 39, 1811–1818. [Google Scholar] [CrossRef]
- Koch, M.; Fauck, V.; Sievert, M.; Mantsopoulos, K.; Iro, H.; Mueller, S. Ultrasound Changes in Salivary Glands after Radioactive Iodine Treatmrent in Benign Diseases and Differentiated Cancer of Thyroid Glands in Consideration of Dose and Time Dependency. Eur. J. Ultrasound, 2023; accepted for publication. [Google Scholar]
- Bomeli, S.R.; Schaitkin, B.; Carrau, R.L.; Walvekar, R.R. Interventional sialendoscopy for treatment of radioiodine-induced sialadenitis. Laryngoscope 2009, 119, 864–867. [Google Scholar] [CrossRef]
- Prendes, B.L.; Orloff, L.A.; Eisele, D.W. Therapeutic sialendoscopy for the management of radioiodine sialadenitis. Arch. Otolaryngol.–Head Neck Surg. 2012, 138, 15–19. [Google Scholar] [CrossRef]
- De Luca, R.; Vicidomini, A.; Trodella, M.; Tartaro, G.; Colella, G. Sialoendoscopy: A viable treatment for I131 induced sialoadenitis. Br. J. Oral Maxillofac. Surg. 2014, 52, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Bhayani, M.K.; Acharya, V.; Kongkiatkamon, S.; Farah, S.; Roberts, D.B.; Sterba, J.; Chambers, M.S.; Lai, S.Y.; Kim, J.W.; Kim, J.M.; et al. Sialendoscopy for Patients with Radioiodine-Induced Sialadenitis and Xerostomia. Thyroid 2015, 25, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-B.; Xi, H.; Zhou, Q.; Zhang, L.-M. Sialendoscopy-Assisted Treatment for Radioiodine-Induced Sialadenitis. J. Oral Maxillofac. Surg. 2015, 73, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, J.; Bin Hong, S.; Hyun, I.Y.; Lim, J. Salivary gland function after sialendoscopy for treatment of chronic radioiodine-induced sialadenitis. Head Neck 2016, 38, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cung, T.-D.; Lai, W.; Svider, P.F.; Hanba, C.; Samantray, J.; Folbe, A.J.; Shkoukani, M.; Raza, S.N. Sialendoscopy in the Management of Radioiodine Induced Sialadenitis: A Systematic Review. Ann. Otol. Rhinol. Laryngol. 2017, 126, 768–773. [Google Scholar] [CrossRef]
- Canzi, P.; Cacciola, S.; Capaccio, P.; Pagella, F.; Occhini, A.; Pignataro, L.; Benazzo, M. Interventional sialendoscopy for radioiodine-induced sialadenitis: Quo vadis? Acta Otorhinolaryngol. Ital. 2017, 37, 155–159. [Google Scholar] [CrossRef]
- Li, X.; Su, J.Z.; Zhang, Y.Y.; Zhang, L.Q.; Zhang, Y.Q.; Liu, D.G.; Yu, G.Y. Inflammation grading and sialoendoscopic treatment of (131)I radioiodine-induced sialadenitis. Beijing Da Xue Xue Bao Yi Xue Ban 2020, 52, 586–590. [Google Scholar]
- Koch, M.; Zenk, J.; Iro, H. Algorithms for Treatment of Salivary Gland Obstructions. Otolaryngol. Clin. N. Am. 2009, 42, 1173–1192. [Google Scholar] [CrossRef]
- Koch, M.; Iro, H. Extended and treatment-oriented classification of parotid duct stenosis. Laryngoscope 2017, 127, 366–371. [Google Scholar] [CrossRef]
- Koch, M.; Zenk, J.; Iro, H. Stenosis and stenosis-like lesions in the submandibular duct: Detailed clinical and sialendoscopy-based analysis and proposal for a classification. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 486–495. [Google Scholar] [CrossRef]
- Kowalczyk, D.M.; Jordan, J.R.; Stringer, S.P. Cost-effectiveness of sialendoscopy versus medical management for radioiodine-induced sialadenitis. Laryngoscope 2018, 128, 1822–1828. [Google Scholar] [CrossRef] [PubMed]


| Findings | All (n = 107) | PGs (n = 95) | SMGs (n = 12) |
|---|---|---|---|
| Maximum US findings at first examination | |||
| Normal parenchyma | 10 (9.3%) | 10 (10.5%) | 0 |
| Hypoechoic parenchyma | 45 (42.1%) | 38 (40.0%) | 7 (58.3%) |
| Heterogeneous parenchyma | 36 (33.6%) | 31 (32.6%) | 5 (41.7%) |
| Atrophic parenchyma | 16 (15.0%) | 16 (16.8%) | 0 |
| Duct dilation | 66 (61.7%) | 61 (64.2%) | 5 (41.7%) |
| Maximum SE findings at first examination | |||
| Normal | 2 (1.9%) | 1 (1.1%) | 1 (8.3%) |
| Sialodochitis | 18 (16.8%) | 16 (16.8%) | 2 (16.7%) |
| Sialodochitis with tendency to stenosis | 18 (16.8%) | 15 (15.8%) | 3 (25.0%) |
| Stenosis (short) | 26 (24.3%) | 20 (21.1%) | 6 (50.0%) |
| Stenosis (segmental) | 2 (1.9%) | 2 (2.1%) | 0 |
| Stenosis (long) | 15 (14.0%) | 15 (15.8%) | 0 |
| Stenosis (complete, without obliteration) | 2 (1.9%) | 2 (2.1%) | 0 |
| Duct obliteration | 24 (1.9%) | 24 (25.3%) | 0 |
| Maximum US findings at last examination | All (n = 64) | PGs (n = 58) | SMGs (n = 6) |
| Normal parenchyma | 8 (12.5%) | 8 (13.8%) | 0 |
| Hypoechoic parenchyma | 11 (17.2%) | 9 (15.5%) | 2 (33.3%) |
| Heterogeneous parenchyma | 19 (29.7%) | 16 (27.57%) | 2 (33.3%) |
| Atrophic parenchyma | 26 (40.6%) | 25 (43.10%) | 2 (33.3%) |
| Duct dilation | 21 (32.8%) | 21 (36.2%) | 0 |
| Maximum SE findings at last examination | |||
| Normal | 0 | 0 | 0 |
| Sialodochitis | 8 (13.8%) | 8 (13.8%) | 0 |
| Sialodochitis with tendency to stenosis | 8 (13.8%) | 6 (10.3%) | 2 (33.3%) |
| Stenosis (short) | 8 (13.8%) | 6 (10.3%) | 2 (33.3%) |
| Stenosis (segmental) | 0 | 0 | 0 |
| Stenosis (long) | 7 (10.9%) | 7 (12.1%) | 0 |
| Stenosis (complete, without obliteration) | 1 (1.6%) | 0 | 1 (16.7%) |
| Duct obliteration | 32 (50.0%) | 31 (53.4%) | 1 (16.7%) |
| SE Findings | Normal | Sialodochitis | Sialodochitis with Tendency to Stenosis | Stenosis (Short) | Stenosis (Segmental) | Stenosis (Long) | Stenosis (Complete, Without Obliteration) | Duct Obliteration | Total |
|---|---|---|---|---|---|---|---|---|---|
| First US examination: maximum US findings | |||||||||
| All glands | |||||||||
| Normal parenchyma | 1 | 9 | – | – | – | – | – | – | 10 (9.3%) |
| Hypoechoic parenchyma | 1 | 8 | 14 | 14 | 2 | 5 | 1 | – | 45 (42.1%) |
| Heterogeneous parenchyma | – | 1 | 4 | 11 | – | 8 | 1 | 11 | 36 (33.6%) |
| Atrophic parenchyma | – | – | – | 1 | – | 2 | – | 13 | 16 (14.95%) |
| Total | 2 (1.9%) | 18 (16.8%) | 18 (16.8%) | 26 (24.3%) | 2 (1.9%) | 15 (14.0%) | 2 (1.9%) | 24 (22.4%) | 107 (100%) |
| All PGs | |||||||||
| Normal parenchyma | 1 | 9 | – | – | – | – | – | – | 10 (10.5%) |
| Hypoechoic parenchyma | – | 7 | 12 | 11 | 2 | 5 | 1 | – | 38 (40.0%) |
| Heterogeneous parenchyma | – | – | 3 | 8 | – | 8 | 1 | 11 | 31 (32.6%) |
| Atrophic parenchyma | – | – | – | 1 | – | 2 | – | 13 | 16 (16.8%) |
| Total | 1 (1.1%) | 16 (16.8%) | 15 (15.8%) | 20 (21.1%) | 2 (2.1%) | 15 (15.8%) | 2 (2.1%) | 24 (25.3%) | 95 (100%) |
| All SMGs | |||||||||
| Normal parenchyma | – | – | – | – | – | – | – | – | – |
| Hypoechoic parenchyma | 1 | 1 | 2 | 3 | – | – | – | – | 7 (58.3%) |
| Heterogeneous parenchyma | – | 1 | 1 | 3 | – | – | – | – | 5 (41.7%) |
| Atrophic parenchyma | – | – | – | – | – | – | – | – | – |
| Total | 1 | 2 | 3 | 6 | – | – | – | – | 12 (100%) |
| Last US examination: maximum US findings | |||||||||
| All glands | |||||||||
| Normal parenchyma | – | 8 | – | – | – | – | – | – | 8 (12.5%) |
| Hypoechoic parenchyma | – | – | 6 | 4 | – | 1 | – | – | 11 (17.2%) |
| Heterogeneous parenchyma | – | – | 2 | 4 | – | 6 | – | 6 | 18 (28.1%) |
| Atrophic parenchyma | – | – | – | – | – | – | 1 | 26 | 27 (42.2%) |
| Total | – | 8 (12.5%) | 8 (12.5%) | 8 (12.5%) | – | 7 (10.9%) | 1 (1.6%) | 32 (50.0%) | 64 (100%) |
| All PGs | |||||||||
| Normal parenchyma | – | 8 | – | – | – | – | – | – | 8 (13.8%) |
| Hypoechoic parenchyma | – | – | 5 | 3 | – | 1 | – | – | 9 (15.5%) |
| Heterogeneous parenchyma | – | – | 1 | 3 | – | 6 | – | 6 | 16 (27.6%) |
| Atrophic parenchyma | – | – | – | – | – | 25 | 25 (43.1%) | ||
| Total | – | 8 (13.8%) | 6 (10.3%) | 6 (10.3%) | – | 7 (12.1%) | – | 31 (53.4%) | 58 (100%) |
| All SMGs | |||||||||
| Normal parenchyma | – | – | – | – | – | – | – | – | – |
| Hypoechoic parenchyma | – | – | 1 | 1 | – | – | – | – | 2 (33.3%) |
| Heterogeneous parenchyma | – | – | 1 | 1 | – | – | – | – | 2 (33.3%) |
| Atrophic parenchyma | – | – | – | – | – | – | 1 | 1 | 2 (33.3%) |
| Total | – | – | 2 (33.3%) | 2 (33.3%) | – | – | 1 (16.7%) | 1 (16.7%) | 6 (100%) |
| Maximum US Findings | Normal Parenchyma | Hypoechoic Parenchyma | Heterogeneous Parenchyma | Atrophic Parenchyma | Total |
|---|---|---|---|---|---|
| First examination, duct dilation | |||||
| All glands | 4/10 (40%) | 37/45 (82.2%) | 24/26 (66.7%) | 1/16 (6.3%) | 66/107 (61.7%) |
| PGs | 4/10 (40%) | 34/38 (89.5%) | 22/31 (70.97%) | 1/16 (6.3%) | 61/95 (64.2%) |
| SMGs | – | 3/7 (42.9%) | 2/5 (40.0%) | – | 5/12 (41.7%) |
| Last examination, duct dilation | |||||
| All glands | 1/8 (12.5%) | 6/11 (54.5%) | 10/18 (55.5%) | 4/27 (14.8%) | 21/64 (32.8%) |
| PGs | 1/8 (12.5%) | 6/9 (66.7%) | 10/16 (62.5%) | 4/25 (16.0%) | 21/58 (36.2%) |
| SMGs | – | 0/2 | 0/2 | 0/2 | 0/6 |
| SE Finding Ductdilation/ SE Finding Gland (n; %) | Normal | Sialodochitis | Sialodochitis with Tendency to Stenosis | Stenosis (Short) | Stenosis (Segmental) | Stenosis (Long) | Stenosis (Complete, without Obliteration) | Duct Obliteration | Duct Dilation/Finding Total |
|---|---|---|---|---|---|---|---|---|---|
| First US examination | |||||||||
| PGs (n; %) | 0/1 | 9/16 (56.3%) | 12/15 (80.%) | 19/20 (95%) | 2/2 | 12/15 (80%) | 2/2 | 5/24 (20.8%) | 61/95 (64.2%) |
| SMGs (n; %) | 1/1 | 1/2 | 1/3 (33.3%) | 2/6 (33.3%) | – | – | – | – | 5/12 (41.7%) |
| all glands (n; %) | 1/2 | 10/18 (55.6%) | 13/18 (72.2%) | 21/26 (80.8%) | 2/2 | 12/15 (80%) | 2/2 | 5/24 (20.8%) | 66/107 (61.7%) |
| Last US examination | |||||||||
| PGs (n; %) | – | 1/8 (12.5%) | 5/6 (83.3%) | 5/6 (83.3%) | – | 4/7 (57.1%) | – | 6/31 (19.4%) | 21/58 (36.2%) |
| SMGs (n; %) | – | – | 0/2 | 0/2 | – | 0/1 | 0/1 | 0/6 | |
| all glands (n; %) | – | 1/8 (12.5%) | 5/8 (62.5%) | 5/8 (62.5%) | – | 4/7 (57.1%) | 0/1 | 6/32 (18.8%) | 21/64 (32.8%) |
| US Findings | PGs (n, %) | SMGs (n, %) | SE Findings | PGs (n, %) | SMGs (n, %) |
|---|---|---|---|---|---|
| Deterioration: change from → to | Deterioration: change from → to | ||||
| Hypoechoic → heterogeneous | 9 | 1 | SD → SD + tendency to stenosis | 3 | – |
| Hypoechoic → atrophic | 8 | 1 | SD → short stenosis | 1 | – |
| Heterogenous → atrophic | 11 | 1 | SD + tendency to stenosis → short stenosis | 1 | – |
| SD + tendency to stenosis → long stenosis | 2 | – | |||
| SD + tendency to stenosis → complete stenosis | – | 1 | |||
| Short stenosis → long stenosis | 2 | – | |||
| SD → duct obliteration | 1 | – | |||
| SD + tendency to stenosis → duct obliteration | 4 | – | |||
| Short stenosis → duct obliteration | 5 | 1 | |||
| Segmental stenosis → duct obliteration | 1 | – | |||
| Long stenosis → duct obliteration | 8 | – | |||
| Complete stenosis → duct obliteration | 1 | – | |||
| 28 (48.3%) | 3 (50%) | 29 (50%) | 2 (33.3%) | ||
| Unchanged | Unchanged | ||||
| Normal | 4 | – | SD | 5 | – |
| Hypoechoic | 8 | 1 | SD + tendency to stenosis | – | 1 |
| Heterogeneous | 7 | 1 | Short stenosis | 4 | 3 |
| Atrophic | 6 | – | Long stenosis | 2 | – |
| Duct obliteration | 11 | – | |||
| 25 (43.1%) | 2 (33.3%) | 22 (37.9%) | 4 (66.7%) | ||
| Improved: change from → to | Improved: change from → to | ||||
| Hypoechoic → normal | 4 | – | SD + tendency to stenosis → SD | 3 | – |
| Heterogeneous → hypoechoic | 1 | 1 | Short stenosis → SD + tendency to stenosis | 2 | – |
| Segmental stenosis → SD + tendency to stenosis | 1 | – | |||
| Complete stenosis → long stenosis | 1 | – | |||
| 5 (8.6%) | 1 (16.7%) | 7 (12.1%) | – | ||
| Total | 58 (100%) | 6 (100%) | 58 (100%) | 6 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, M.; Müller, S.; Mantsopoulos, K.; Iro, H.; Sievert, M. Ultrasound and Sialendoscopy Findings in Radioactive Iodine–Induced Sialadenitis: Comparative Analysis and Possible Impact on Management. J. Clin. Med. 2024, 13, 657. https://doi.org/10.3390/jcm13030657
Koch M, Müller S, Mantsopoulos K, Iro H, Sievert M. Ultrasound and Sialendoscopy Findings in Radioactive Iodine–Induced Sialadenitis: Comparative Analysis and Possible Impact on Management. Journal of Clinical Medicine. 2024; 13(3):657. https://doi.org/10.3390/jcm13030657
Chicago/Turabian StyleKoch, Michael, Sarina Müller, Konstantinos Mantsopoulos, Heinrich Iro, and Matti Sievert. 2024. "Ultrasound and Sialendoscopy Findings in Radioactive Iodine–Induced Sialadenitis: Comparative Analysis and Possible Impact on Management" Journal of Clinical Medicine 13, no. 3: 657. https://doi.org/10.3390/jcm13030657
APA StyleKoch, M., Müller, S., Mantsopoulos, K., Iro, H., & Sievert, M. (2024). Ultrasound and Sialendoscopy Findings in Radioactive Iodine–Induced Sialadenitis: Comparative Analysis and Possible Impact on Management. Journal of Clinical Medicine, 13(3), 657. https://doi.org/10.3390/jcm13030657







