Real-World Data on Effectiveness and Safety of First-Line Use of Caplacizumab in Italian Centers for the Treatment of Thrombotic Thrombocytopenic Purpura: The Roscapli Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
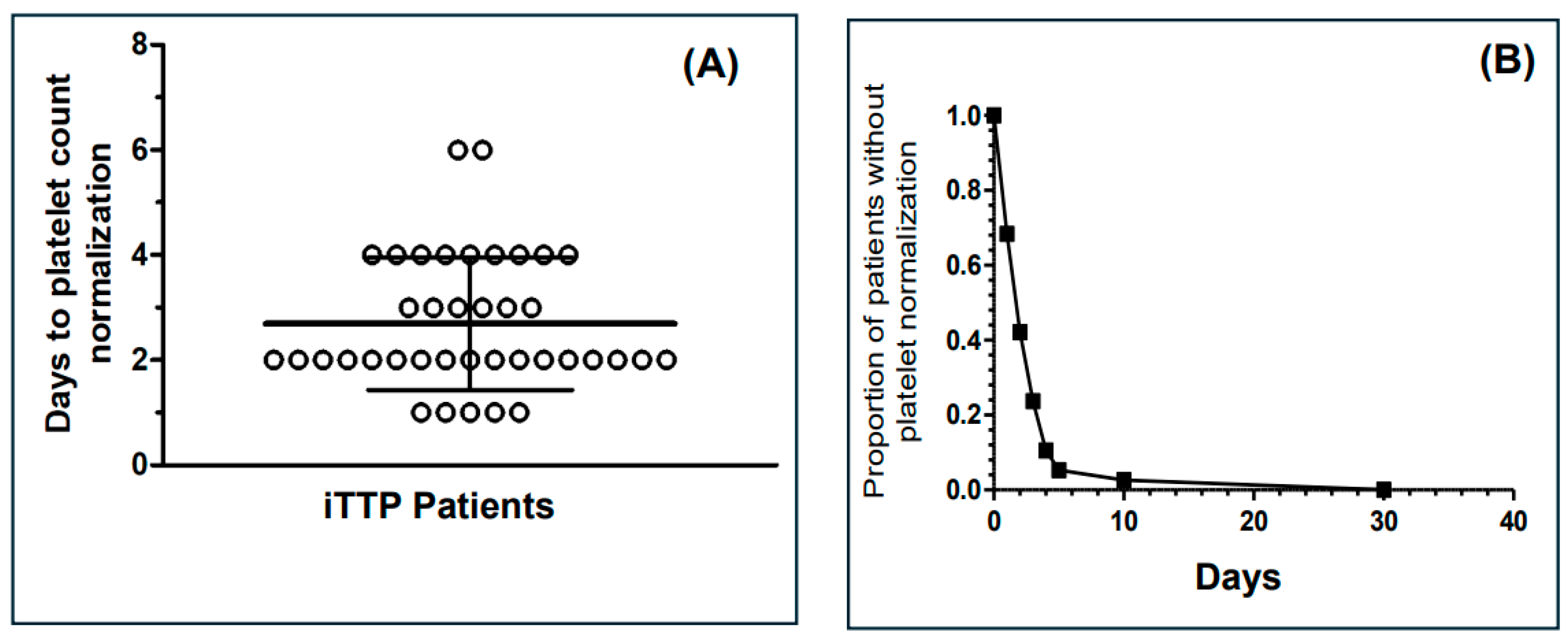
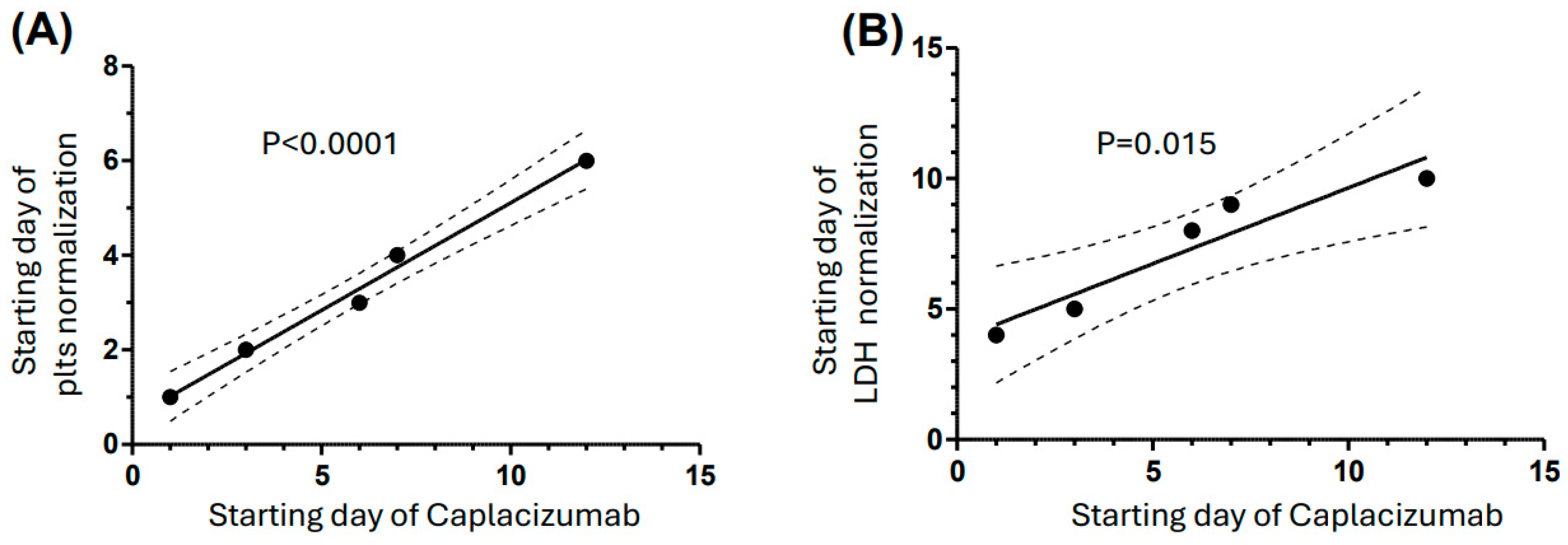
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- George, J.N. Clinical practice. Thrombotic thrombocytopenic purpura. N. Engl. J. Med. 2006, 354, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Rock, G.A.; Shumak, K.H.; Buskard, N.A.; Blanchette, V.S.; Kelton, J.G.; Nair, R.C.; Spasoff, R.A. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N. Engl. J. Med. 1991, 325, 393–397. [Google Scholar] [CrossRef]
- Peyvandi, F.; Scully, M.; Kremer Hovinga, J.A.; Cataland, S.; Knobl, P.; Wu, H.; Artoni, A.; Westwood, J.P.; Mansouri Taleghani, M.; Jilma, B.; et al. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2016, 374, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knobl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Coppo, P.; Bubenheim, M.; Azoulay, E.; Galicier, L.; Malot, S.; Bige, N.; Poullin, P.; Provot, F.; Martis, N.; Presne, C.; et al. A regimen with caplacizumab, immunosuppression, and plasma exchange prevents unfavorable outcomes in immune-mediated TTP. Blood 2021, 137, 733–742. [Google Scholar] [CrossRef]
- Dutt, T.; Shaw, R.J.; Stubbs, M.; Yong, J.; Bailiff, B.; Cranfield, T.; Crowley, M.P.; Desborough, M.; Eyre, T.A.; Gooding, R.; et al. Real-world experience with caplacizumab in the management of acute TTP. Blood 2021, 137, 1731–1740. [Google Scholar] [CrossRef]
- Volker, L.A.; Kaufeld, J.; Miesbach, W.; Brahler, S.; Reinhardt, M.; Kuhne, L.; Muhlfeld, A.; Schreiber, A.; Gaedeke, J.; Tolle, M.; et al. Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura. Blood Adv. 2020, 4, 3085–3092. [Google Scholar] [CrossRef]
- Kuhne, L.; Kaufeld, J.; Volker, L.A.; Wendt, R.; Schonermarck, U.; Hagele, H.; Osterholt, T.; Eichenauer, D.A.; Bieringer, M.; von Bergwelt-Baildon, A.; et al. Alternate-day dosing of caplacizumab for immune-mediated thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2022, 20, 951–960. [Google Scholar] [CrossRef]
- Knoebl, P.; Cataland, S.; Peyvandi, F.; Coppo, P.; Scully, M.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Minkue Mi Edou, J.; et al. Efficacy and safety of open-label caplacizumab in patients with exacerbations of acquired thrombotic thrombocytopenic purpura in the HERCULES study. J. Thromb. Haemost. 2020, 18, 479–484. [Google Scholar] [CrossRef]
- Agosti, P.; De Leo, P.; Capecchi, M.; Ferrari, B.; Mancini, I.; Gattillo, S.; Trisolini, S.M.; Rinaldi, E.; Podda, G.M.; Prezioso, L.; et al. Caplacizumab use for immune thrombotic thrombocytopenic purpura: The Milan thrombotic thrombocytopenic purpura registry. Res. Pract. Thromb. Haemost. 2023, 7, 102185. [Google Scholar] [CrossRef]
- Angelucci, E.; Artoni, A.; Fianchi, L.; Dovizio, M.; Iacolare, B.; Saragoni, S.; Esposti, L.D. Real-World Data Analysis of Patients Affected by Immune-Mediated Thrombotic Thrombocytopenic Purpura in Italy. J. Clin. Med. 2024, 13, 1342. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Keogh, L.; Dickens, E.L.; Dutt, T.; Grainger, J.; Gregory, R.; Mapplebeck, C.; Richards, M.; Stokley, S.; Salta, S.; et al. Caplacizumab in paediatric immune thrombotic thrombocytopenic purpura (iTTP): The UK TTP Registry experience. Blood Adv. 2024, 8, 4563–4567. [Google Scholar] [CrossRef]
- Volker, L.A.; Brinkkoetter, P.T.; Knobl, P.N.; Krstic, M.; Kaufeld, J.; Menne, J.; Buxhofer-Ausch, V.; Miesbach, W. Treatment of acquired thrombotic thrombocytopenic purpura without plasma exchange in selected patients under caplacizumab. J. Thromb. Haemost. 2020, 18, 3061–3066. [Google Scholar] [CrossRef] [PubMed]
- Chander, D.P.; Loch, M.M.; Cataland, S.R.; George, J.N. Caplacizumab Therapy without Plasma Exchange for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 381, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, L.; Knoebl, P.; Eller, K.; Thaler, J.; Sperr, W.R.; Gleixner, K.V.; Osterholt, T.; Kaufeld, J.K.; Menne, J.; Buxhofer-Ausch, V.; et al. Management of Immune Thrombotic Thrombocytopenic Purpura without Therapeutic Plasma Exchange. Blood 2024, 144, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Cataland, S.R.; Coppo, P.; de la Rubia, J.; Friedman, K.D.; George, J.N.; Knoebl, P.N.; Kremer Hovinga, J.A.; Lammle, B.; Matsumoto, M.; et al. Redefining outcomes in immune TTP: An international working group consensus report. Blood 2021, 137, 1855–1861. [Google Scholar] [CrossRef]
- Scully, M.; Cataland, S.; Coppo, P.; de la Rubia, J.; Friedman, K.D.; Kremer Hovinga, J.; Lammle, B.; Matsumoto, M.; Pavenski, K.; Sadler, E.; et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J. Thromb. Haemost. 2017, 15, 312–322. [Google Scholar] [CrossRef]
- Izquierdo, C.P.; Mingot-Castellano, M.E.; Fuentes, A.E.K.; Garcia-Arroba Peinado, J.; Cid, J.; Jimenez, M.M.; Valcarcel, D.; Gomez-Segui, I.; de la Rubia, J.; Martin, P.; et al. Real-world effectiveness of caplacizumab vs the standard of care in immune thrombotic thrombocytopenic purpura. Blood Adv. 2022, 6, 6219–6227. [Google Scholar] [CrossRef]
- Van de Louw, A.; Mariotte, E.; Darmon, M.; Cohrs, A.; Leslie, D.; Azoulay, E. Outcomes in 1096 patients with severe thrombotic thrombocytopenic purpura before the Caplacizumab era. PLoS ONE 2021, 16, e0256024. [Google Scholar] [CrossRef]
- Peng, J.Y.; Wang, S.Y.; Chen, M.Q.; Liu, C.X.; Zhao, Y.T.; Xu, T.S.; Wu, Q.L. Efficacy and relative safety of caplacizumab in immune-mediated thrombotic thrombocytopenic purpura: A systematic review and meta-analysis. Blood Coagul. Fibrinolysis 2024, 35, 271–278. [Google Scholar] [CrossRef]
- McCarthy, L.J.; Danielson, C.F.; Skipworth, E.M.; Peters, S.L.; Miraglia, C.C.; Antony, A.C. Myocardial infarction/injury is relatively common at presentation of acute thrombotic thrombocytopenic purpura: The Indiana University experience. Ther. Apher. 2002, 6, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Dutt, T. Mind and matter: The neurological complications of thrombotic thrombocytopenic purpura. Br. J. Haematol. 2022, 197, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Yarranton, H.; Liesner, R.; Cavenagh, J.; Hunt, B.; Benjamin, S.; Bevan, D.; Mackie, I.; Machin, S. Regional UK TTP registry: Correlation with laboratory ADAMTS 13 analysis and clinical features. Br. J. Haematol. 2008, 142, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Yu, J.; Brown, J.; Wei, A.; Selvakumar, S.; Gerber, G.F.; Moliterno, A.R.; Streiff, M.B.; Kraus, P.; Logue, C.M.; et al. Silent cerebral infarction during immune TTP remission: Prevalence, predictors, and impact on cognition. Blood 2023, 142, 325–335. [Google Scholar] [CrossRef]
- Gandhi, K.; Aronow, W.S.; Desai, H.; Amin, H.; Sharma, M.; Lai, H.M.; Singh, P. Cardiovascular manifestations in patients with thrombotic thrombocytopenic purpura: A single-center experience. Clin. Cardiol. 2010, 33, 213–216. [Google Scholar] [CrossRef]
- Di Minno, G.; Ravasio, R. Cost-effectiveness analysis of caplacizumab in the new standard of care for immune Thrombotic Thrombocytopenic Purpura in Italy. Glob. Reg. Health Technol. Assess. 2021, 8, 43–52. [Google Scholar] [CrossRef]
- Lim, W.; Vesely, S.K.; George, J.N. The role of rituximab in the management of patients with acquired thrombotic thrombocytopenic purpura. Blood 2015, 125, 1526–1531. [Google Scholar] [CrossRef]
- Zheng, X.L.; Vesely, S.K.; Cataland, S.R.; Coppo, P.; Geldziler, B.; Iorio, A.; Matsumoto, M.; Mustafa, R.A.; Pai, M.; Rock, G.; et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2020, 18, 2496–2502. [Google Scholar] [CrossRef]
- Zheng, X.L.; Vesely, S.K.; Cataland, S.R.; Coppo, P.; Geldziler, B.; Iorio, A.; Matsumoto, M.; Mustafa, R.A.; Pai, M.; Rock, G.; et al. Good practice statements (GPS) for the clinical care of patients with thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2020, 18, 2503–2512. [Google Scholar] [CrossRef]
- Abou-Ismail, M.Y.; Zhang, C.; Presson, A.P.; Chaturvedi, S.; Antun, A.G.; Farland, A.M.; Woods, R.; Metjian, A.; Park, Y.A.; de Ridder, G.; et al. A descriptive analysis of fatal outcomes in immune thrombotic thrombocytopenic purpura in the USTMA TTP Registry. Blood Adv. 2024, 8, 620–623. [Google Scholar] [CrossRef]
- Sawler, D.; Parker, A.; Britto, J.; Goodyear, M.D.; Sun, H.L. Time from suspected thrombotic thrombocytopenic purpura to initiation of plasma exchange and impact on survival: A 10-year provincial retrospective cohort study. Thromb. Res. 2020, 193, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Deford, C.C.; Reese, J.A.; Schwartz, L.H.; Perdue, J.J.; Kremer Hovinga, J.A.; Lammle, B.; Terrell, D.R.; Vesely, S.K.; George, J.N. Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood 2013, 122, 2023–2029; quiz 2142. [Google Scholar] [CrossRef] [PubMed]
- Volker, L.A.; Kaufeld, J.; Balduin, G.; Merkel, L.; Kuhne, L.; Eichenauer, D.A.; Osterholt, T.; Hagele, H.; Kann, M.; Grundmann, F.; et al. Impact of first-line use of caplacizumab on treatment outcomes in immune thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2023, 21, 559–572. [Google Scholar] [CrossRef] [PubMed]
| Gender (F:M, %) | Age (yr) | BMI | Hb (gr/dL) | Plt (×109/L) | WBC (×109/L) | LDH (U/L) | Total Bilirubin (mg/dL) | hsTnI (pg/mL) | AD13 § (U/dL) | AD13 ° Inhibitor titer (U/dL) | ALT (U/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (78.9:21.1) | 43 (39–56) | 25.4 (20.7–29.9) | 8.75 (7.1–16.20) | 17 (9–28) | 8.89 (5.96–10.64) | 634 (320–1064) | 1.9 (1.4–3.15) | 224 (103–312) | 0.005 (0–0.4) | 24.5 (14–67.8) | 20 (12.5–27) |
| Parameter | Result (Number of Cases or Days, and % or IQR) |
|---|---|
| Death | 0 (0%) |
| TTP relapse | 2 (5.3%) |
| Serious Adverse Events (SAEs) | 0 (0%) |
| Exacerbations | 4 (10.5%) |
| Refractoriness | 0 (0%) |
| Number of PEX days (median, IQR) | 9 (8–10) |
| Days of stay in ICU | 2 days for 1 patient |
| Days of hospitalization (median, IQR) | 12 (9–18) |
| Safety assessments, as reported descriptively by the clinicians at each center | Nothing to report |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fianchi, L.; Bonanni, M.; Borchiellini, A.; Valeri, F.; Giuffrida, G.; Grasso, S.; Fozza, C.; Ponta, M.; Tiscia, G.L.; Grandone, E.; et al. Real-World Data on Effectiveness and Safety of First-Line Use of Caplacizumab in Italian Centers for the Treatment of Thrombotic Thrombocytopenic Purpura: The Roscapli Study. J. Clin. Med. 2024, 13, 6561. https://doi.org/10.3390/jcm13216561
Fianchi L, Bonanni M, Borchiellini A, Valeri F, Giuffrida G, Grasso S, Fozza C, Ponta M, Tiscia GL, Grandone E, et al. Real-World Data on Effectiveness and Safety of First-Line Use of Caplacizumab in Italian Centers for the Treatment of Thrombotic Thrombocytopenic Purpura: The Roscapli Study. Journal of Clinical Medicine. 2024; 13(21):6561. https://doi.org/10.3390/jcm13216561
Chicago/Turabian StyleFianchi, Luana, Matteo Bonanni, Alessandra Borchiellini, Federica Valeri, Gaetano Giuffrida, Stephanie Grasso, Claudio Fozza, Michele Ponta, Giovanni L. Tiscia, Elvira Grandone, and et al. 2024. "Real-World Data on Effectiveness and Safety of First-Line Use of Caplacizumab in Italian Centers for the Treatment of Thrombotic Thrombocytopenic Purpura: The Roscapli Study" Journal of Clinical Medicine 13, no. 21: 6561. https://doi.org/10.3390/jcm13216561
APA StyleFianchi, L., Bonanni, M., Borchiellini, A., Valeri, F., Giuffrida, G., Grasso, S., Fozza, C., Ponta, M., Tiscia, G. L., Grandone, E., Vianelli, N., Dedola, A., Pirozzi, T., Sacco, M., Lancellotti, S., & De Cristofaro, R. (2024). Real-World Data on Effectiveness and Safety of First-Line Use of Caplacizumab in Italian Centers for the Treatment of Thrombotic Thrombocytopenic Purpura: The Roscapli Study. Journal of Clinical Medicine, 13(21), 6561. https://doi.org/10.3390/jcm13216561









