Changing Epidemiology and Outcomes of Hemolytic Uremic Syndrome in Children: A Prospective National Cohort Study from the Polish Pediatric HUS Registry and the Polish Registry of Renal Replacement Therapy in Children
Abstract
1. Introduction
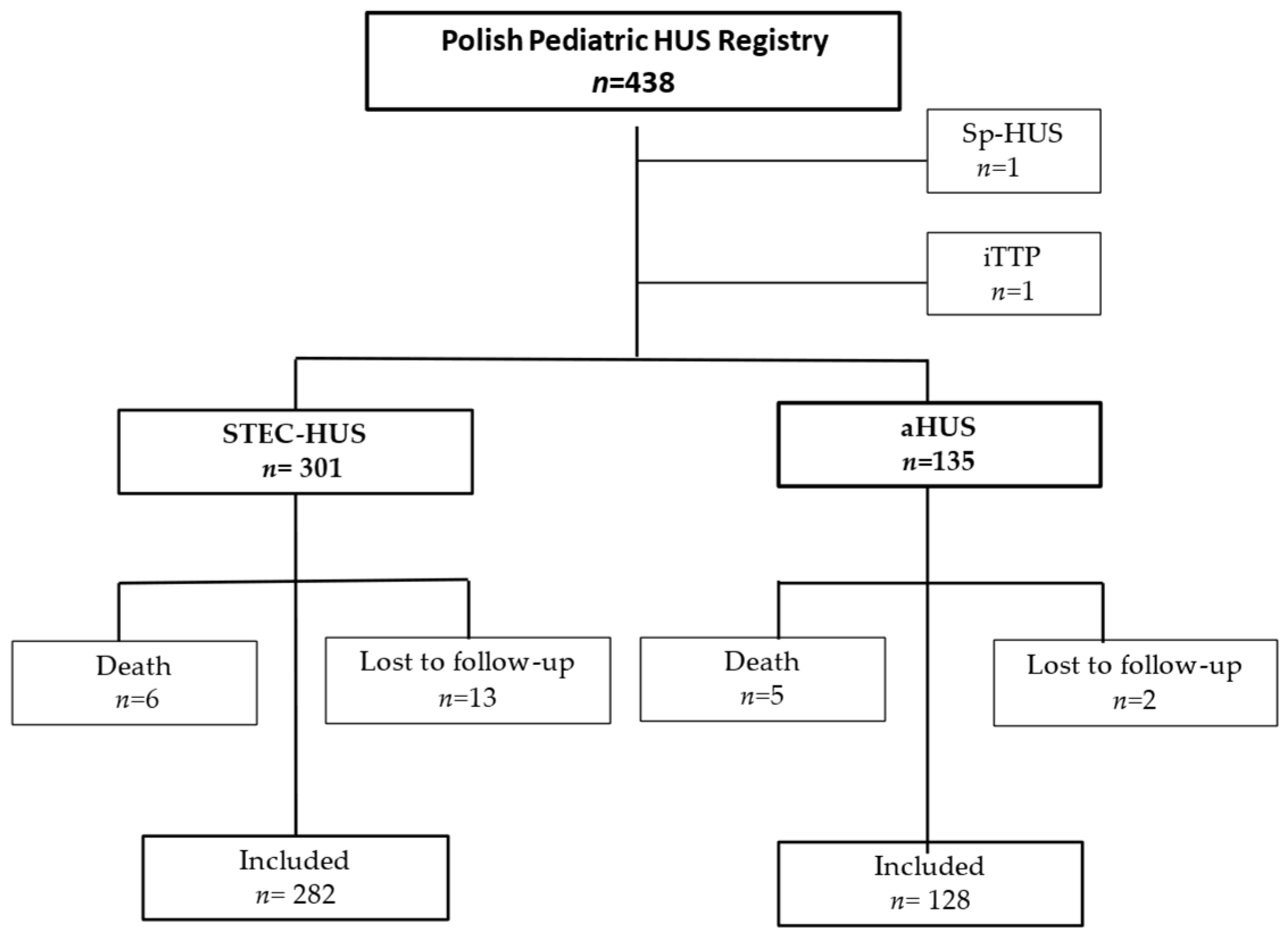
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
2.3. Outcome Analysis
3. Results
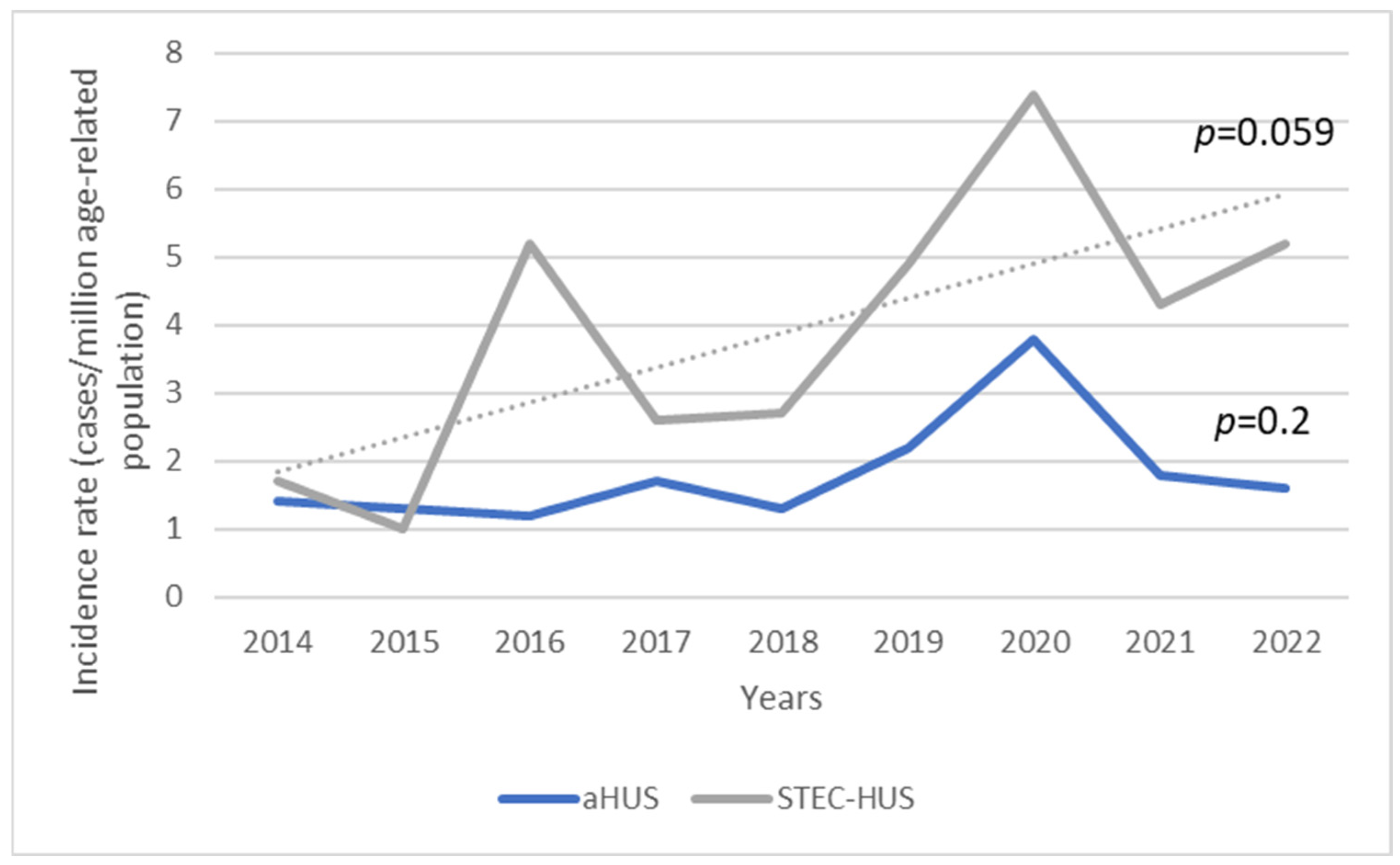
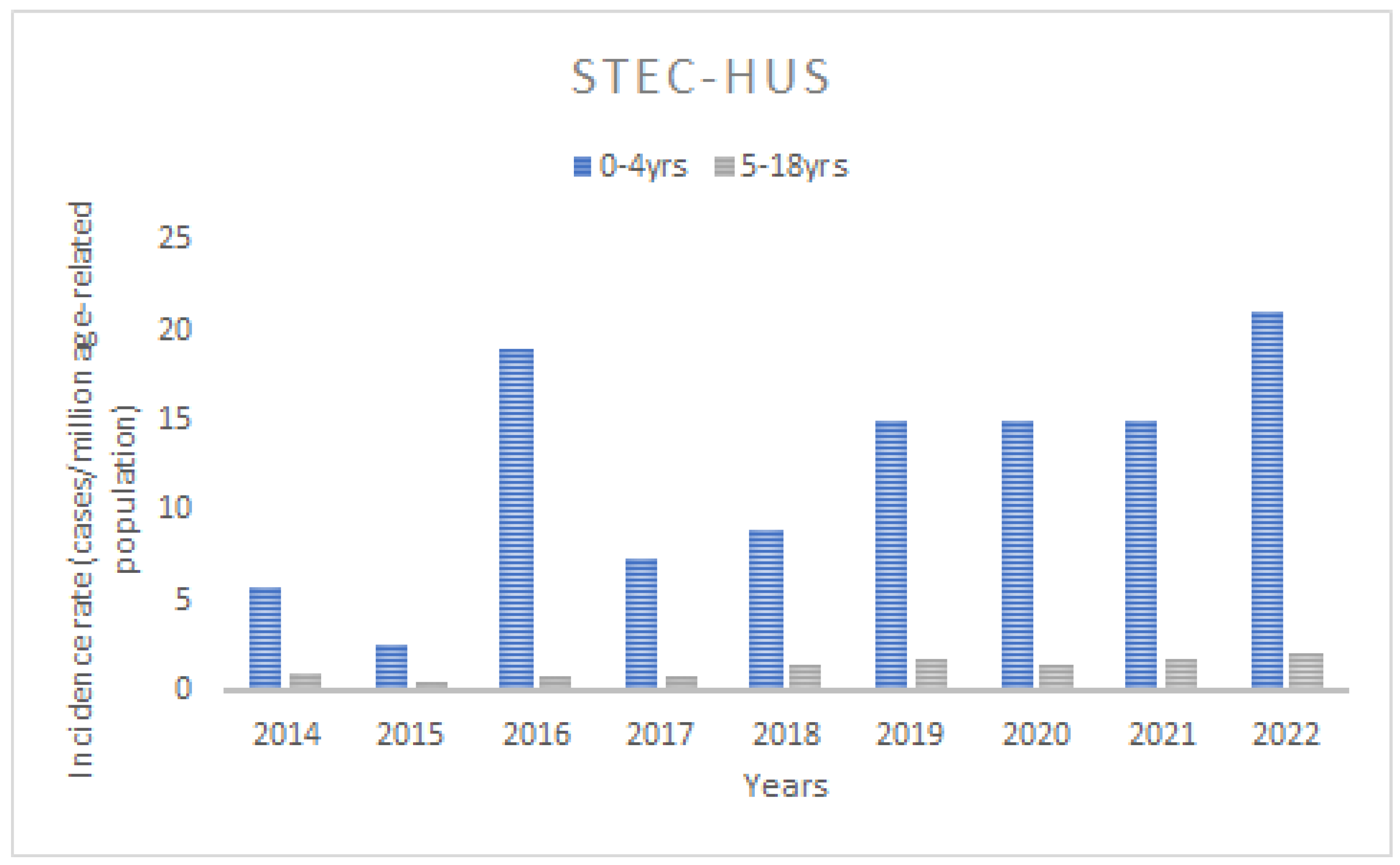
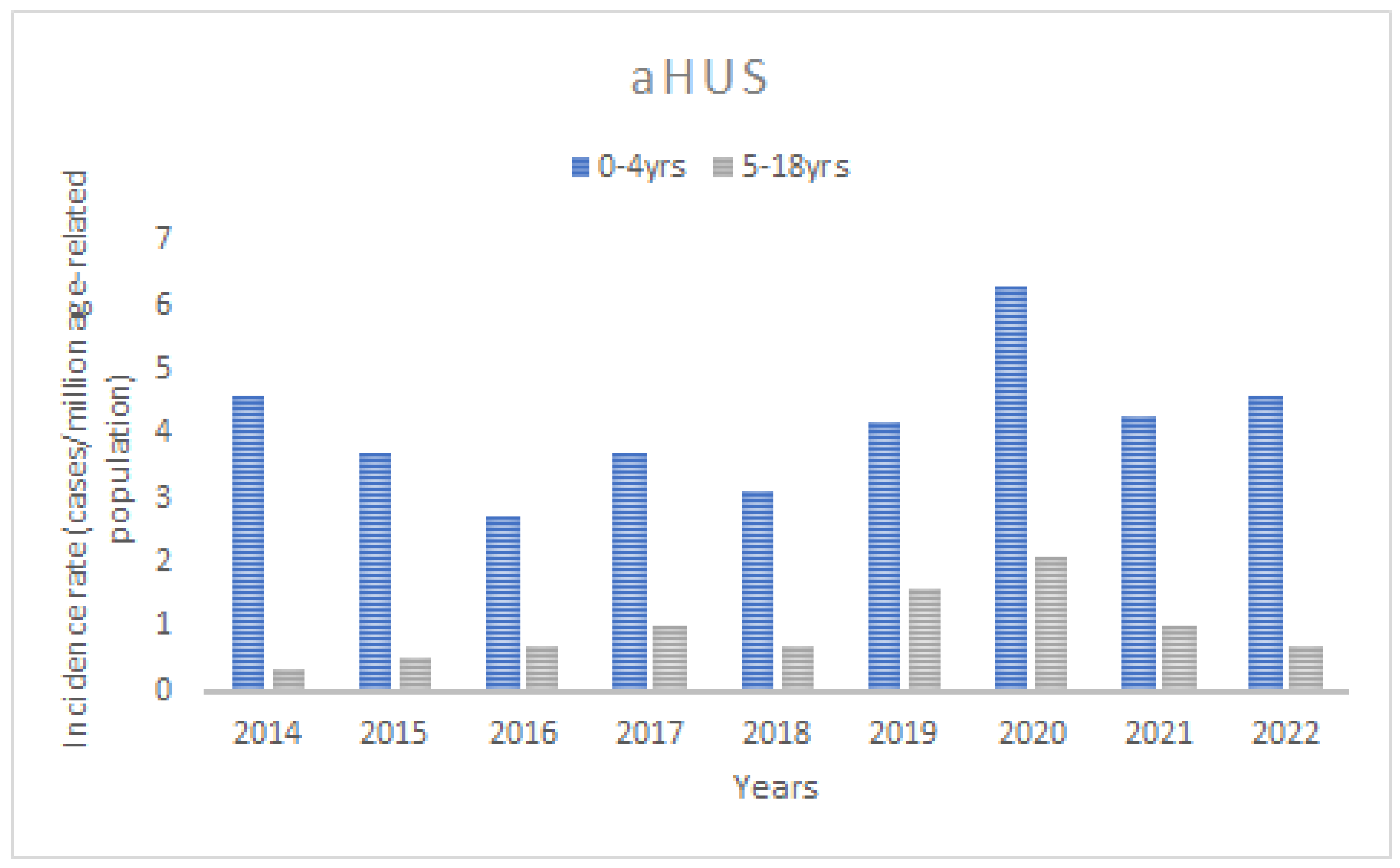
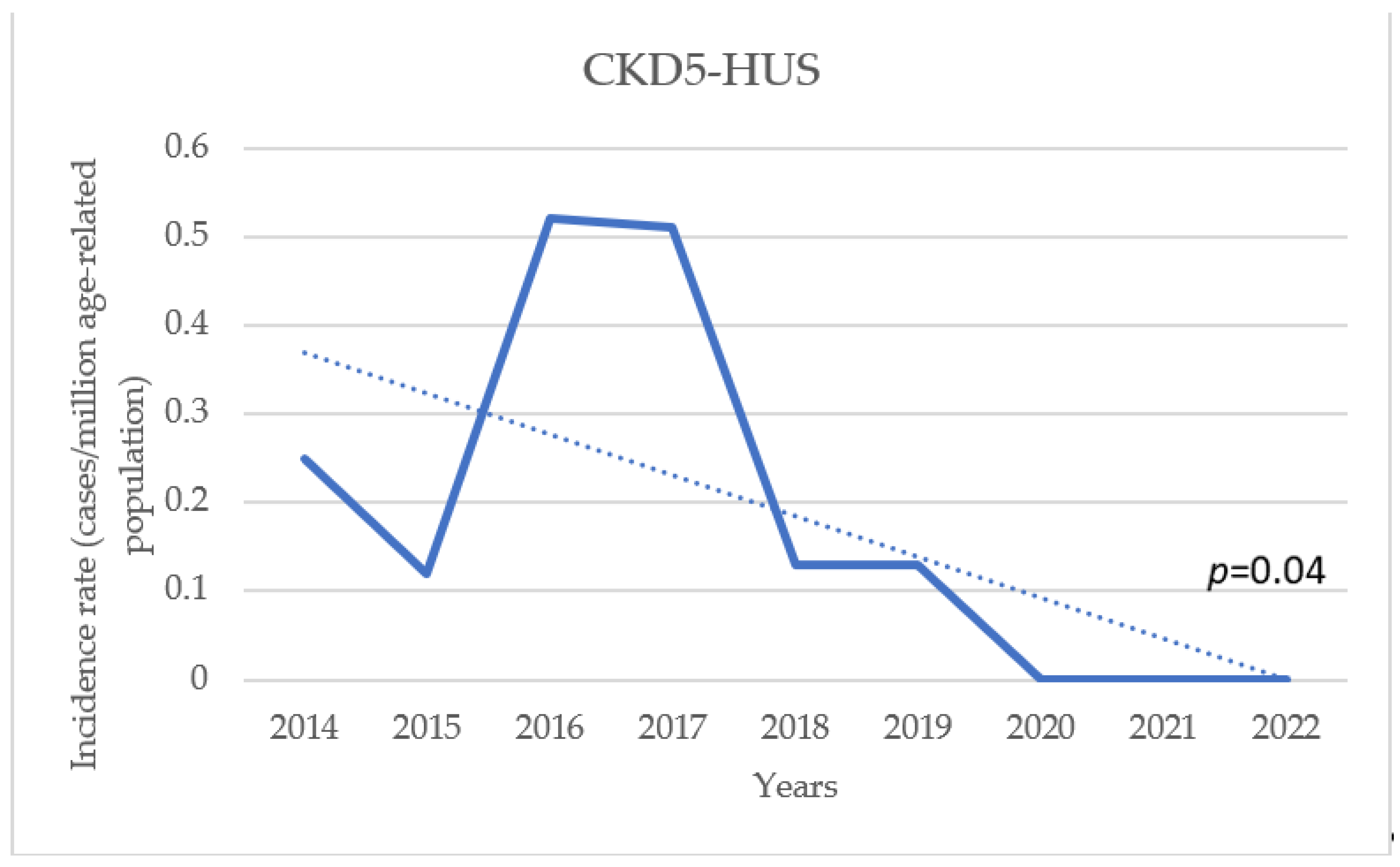
3.1. Incidence
3.2. Initial Manifestation
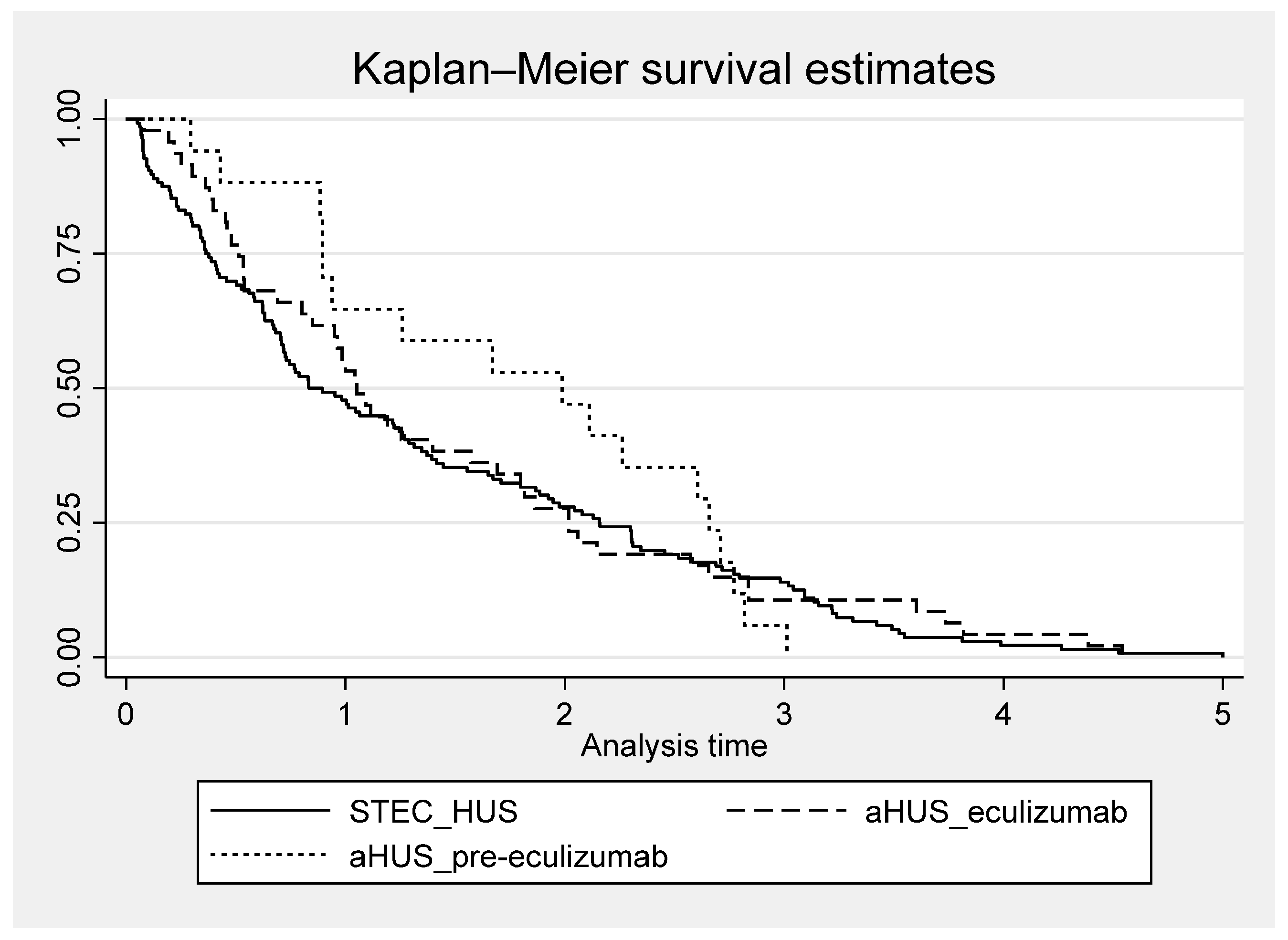
3.3. Outcome
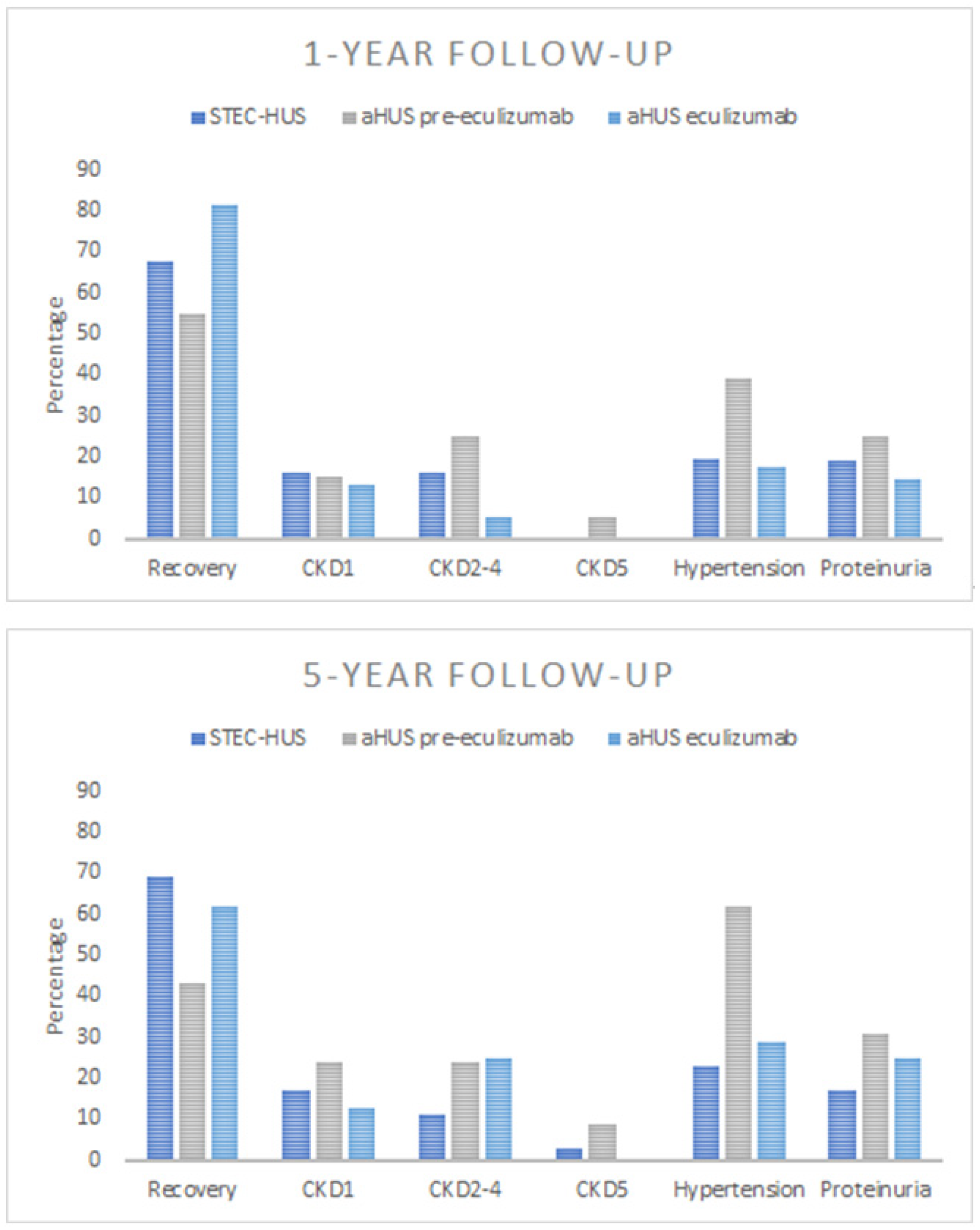
3.3.1. Recovery
3.3.2. Kidney Sequelae
3.3.3. Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palma, L.M.P.; Vaisbich-Guimarães, M.H.; Sridharan, M.; Tran, C.L.; Sethi, S. Thrombotic microangiopathy in children. Pediatr. Nephrol. 2022, 37, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Remuzzi, G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009, 361, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Caprioli, J.; Bresin, E.; Mossali, C.; Pianetti, G.; Gamba, S.; Daina, E.; Fenili, C.; Castelletti, F.; Sorosina, A.; et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin. J. Am. Soc. Nephrol. 2010, 5, 1844–1859. [Google Scholar] [CrossRef] [PubMed]
- Wijnsma, K.L.; Duineveld, C.; Wetzels, J.F.M.; van de Kar, N. Eculizumab in atypical hemolytic uremic syndrome: Strategies toward restrictive use. Pediatr. Nephrol. 2019, 34, 2261–2277. [Google Scholar] [CrossRef]
- Fremeaux-Bacchi, V.; Fakhouri, F.; Garnier, A.; Bienaimé, F.; Dragon-Durey, M.A.; Ngo, S.; Moulin, B.; Servais, A.; Provot, F.; Rostaing, L.; et al. Genetics and outcome of atypical hemolytic uremic syndrome: A nationwide French series comparing children and adults. Clin. J. Am. Soc. Nephrol. 2013, 8, 554–562. [Google Scholar] [CrossRef]
- Schaefer, F.; Ardissino, G.; Ariceta, G.; Fakhouri, F.; Scully, M.; Isbel, N.; Lommelé, Å.; Kupelian, V.; Gasteyger, C.; Greenbaum, L.A.; et al. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018, 94, 408–418. [Google Scholar] [CrossRef]
- Brocklebank, V.; Walsh, P.R.; Smith-Jackson, K.; Hallam, T.M.; Marchbank, K.J.; Wilson, V.; Bigirumurame, T.; Dutt, T.; Montgomery, E.K.; Malina, M.; et al. Atypical hemolytic uremic syndrome in the era of terminal complement inhibition: An observational cohort study. Blood 2023, 142, 1371–1386. [Google Scholar] [CrossRef]
- Goodship, T.H.; Cook, H.T.; Fakhouri, F.; Fervenza, F.C.; Frémeaux-Bacchi, V.; Kavanagh, D.; Nester, C.M.; Noris, M.; Pickering, M.C.; Rodríguez de Córdoba, S.; et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017, 91, 539–551. [Google Scholar] [CrossRef]
- Ashida, A.; Matsumura, H.; Shimono, A.; Fujii, Y.; Yamazaki, S. Comparison of outcomes after plasma therapy or eculizumab in pediatric patients with atypical hemolytic uremic syndrome. Clin. Exp. Nephrol. 2023, 27, 161–170. [Google Scholar] [CrossRef]
- Kato, H.; Nangaku, M.; Hataya, H.; Sawai, T.; Ashida, A.; Fujimaru, R.; Hidaka, Y.; Kaname, S.; Maruyama, S.; Yasuda, T.; et al. Clinical guides for atypical hemolytic uremic syndrome in Japan. Pediatr. Int. 2016, 58, 549–555. [Google Scholar] [CrossRef]
- Medina, P.J.; Sipols, J.M.; George, J.N. Drug-associated thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Curr. Opin. Hematol. 2001, 8, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ardissino, G.; Salardi, S.; Colombo, E.; Testa, S.; Borsa-Ghiringhelli, N.; Paglialonga, F.; Paracchini, V.; Tel, F.; Possenti, I.; Belingheri, M.; et al. Epidemiology of haemolytic uremic syndrome in children. Data from the North. Italian HUS network. Eur. J. Pediatr. 2016, 175, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Apostal, M.; Comstock, N.; Hurd, S.; Webb, T.H.; Mickelson, S.; Scheftel, J.; Smith, G.; Shiferaw, B.; Boothe, E.; et al. Strategies for surveillance of pediatric hemolytic uremic syndrome: Foodborne Diseases Active Surveillance Network (FoodNet), 2000–2007. Clin. Infect. Dis. 2012, 54 (Suppl. S5), S424–S431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Byrne, L.; Douglas, A.; Launders, N.; Godbole, G.; Lynn, R.; Inward, C.; Jenkins, C. Haemolytic uraemic syndrome in children England, Wales, Northern Ireland, and Ireland: A prospective cohort study. Epidemiol. Infect. 2023, 151, e160. [Google Scholar] [CrossRef]
- Monteverde, M.L.; Panero, N.; Chaparro, A.B.; Locane, F.; Sarkis, C.; Mattio, S.A.; Ibañez, J.P. A decrease in the incidence of Shiga toxin-related hemolytic uremic syndrome as a cause of kidney transplantation at an argentine referral center. Pediatr. Transplant. 2023, 27, e14489. [Google Scholar] [CrossRef]
- Alfandary, H.; Rinat, C.; Gurevich, E.; Eisenstein, I.; Goldberg, O.; Kropach, N.; Landau, D. Hemolytic Uremic Syndrome: A Contemporary Pediatric Experience. Nephron 2020, 144, 109–117. [Google Scholar] [CrossRef]
- Caprioli, J.; Noris, M.; Brioschi, S.; Pianetti, G.; Castelletti, F.; Bettinaglio, P.; Mele, C.; Bresin, E.; Cassis, L.; Gamba, S.; et al. Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 2006, 108, 1267–1279. [Google Scholar] [CrossRef]
- Loirat, C.; Frémeaux-Bacchi, V. Atypical hemolytic uremic syndrome. Orphanet. J. Rare Dis. 2011, 6, 60. [Google Scholar] [CrossRef]
- Walle, J.V.; Delmas, Y.; Ardissino, G.; Wang, J.; Kincaid, J.F.; Haller, H. Improved renal recovery in patients with atypical hemolytic uremic syndrome following rapid initiation of eculizumab treatment. J. Nephrol. 2017, 30, 127–134. [Google Scholar] [CrossRef]
- Glover, E.K.; Smith-Jackson, K.; Brocklebank, V.; Wilson, V.; Walsh, P.R.; Montgomery, E.K.; Wong, E.K.S.; Johnson, S.; Malina, M.; Kavanagh, D.; et al. Assessing the Impact of Prophylactic Eculizumab on Renal Graft Survival in Atypical Hemolytic Uremic Syndrome. Transplantation 2023, 107, 994–1003. [Google Scholar] [CrossRef]
- Mody, R.K.; Gu, W.; Griffin, P.M.; Jones, T.F.; Rounds, J.; Shiferaw, B.; Tobin-D’Angelo, M.; Smith, G.; Spina, N.; Hurd, S.; et al. Postdiarrheal hemolytic uremic syndrome in United States children: Clinical spectrum and predictors of in-hospital death. J. Pediatr. 2015, 166, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Alconcher, L.F.; Lucarelli, L.I.; Bronfen, S. Long-term kidney outcomes in non-dialyzed children with Shiga-toxin Escherichia coli associated hemolytic uremic syndrome. Pediatr. Nephrol. 2023, 38, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Ake, J.A.; Jelacic, S.; Ciol, M.A.; Watkins, S.L.; Murray, K.F.; Christie, D.L.; Klein, E.J.; Tarr, P.I. Relative nephroprotection during Escherichia coli O157, H7 infections: Association with intravenous volume expansion. Pediatrics 2005, 115, e673–e680. [Google Scholar] [CrossRef] [PubMed]
- Ardissino, G.; Daccò, V.; Testa, S.; Civitillo, C.F.; Tel, F.; Possenti, I.; Belingheri, M.; Castorina, P.; Bolsa-Ghiringhelli, N.; Tedeschi, S.; et al. Hemoconcentration: A major risk factor for neurological involvement in hemolytic uremic syndrome. Pediatr. Nephrol. 2015, 30, 345–352. [Google Scholar] [CrossRef]
- Rosales, A.; Hofer, J.; Zimmerhackl, L.B.; Jungraithmayr, T.C.; Riedl, M.; Giner, T.; Strasak, A.; Orth-Höller, D.; Würzner, R.; Karch, H. Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin. Infect. Dis. 2012, 54, 1413–1421. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef]
- McKee, R.S.; Schnadower, D.; Tarr, P.I.; Xie, J.; Finkelstein, Y.; Desai, N.; Lane, R.D.; Bergmann, K.R.; Kaplan, R.L.; Hariharan, S.; et al. Predicting Hemolytic Uremic Syndrome and Renal Replacement Therapy in Shiga Toxin-producing Escherichia coli-infected Children. Clin. Infect. Dis. 2020, 70, 1643–1651. [Google Scholar] [CrossRef]
- Keir, L.S. Shiga toxin associated hemolytic uremic syndrome. Hematol. Oncol. Clin. N. Am. 2015, 29, 525–539. [Google Scholar] [CrossRef]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Sawyer, C.; Vishram, B.; Jenkins, C.; Jorgensen, F.; Byrne, L.; Mikhail, A.F.W.; Dallman, T.J.; Carroll, K.; Ahyow, L.; Vahora, Q.; et al. Epidemiological investigation of recurrent outbreaks of haemolytic uraemic syndrome caused by Shiga toxin-producing Escherichia coli serotype O55, H7 in England, 2014–2018. Epidemiol. Infect. 2021, 149, e108. [Google Scholar] [CrossRef]
- Krogvold, L.; Henrichsen, T.; Bjerre, A.; Brackman, D.; Dollner, H.; Gudmundsdottir, H.; Syversen, G.; Næss, P.A.; Bangstad, H.J. Clinical aspects of a nationwide epidemic of severe haemolytic uremic syndrome (HUS) in children. Scand. J. Trauma. Resusc. Emerg. Med. 2011, 19, 44. [Google Scholar] [CrossRef]
- Bruyand, M.; Mariani-Kurkdjian, P.; Gouali, M.; de Valk, H.; King, L.A.; Le Hello, S.; Bonacorsi, S.; Loirat, C. Hemolytic uremic syndrome due to Shiga toxin-producing Escherichia coli infection. Med. Mal. Infect. 2018, 48, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Ardissino, G.; Tel, F.; Testa, S.; Paglialonga, F.; Longhi, S.; Martelli, L.; Consolo, S.; Picicco, D.; Dodaro, A.; Daprai, L.; et al. A simple prognostic index for Shigatoxin-related hemolytic uremic syndrome at onset: Data from the ItalKid-HUS network. Eur. J. Pediatr. 2018, 177, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Balestracci, A.; Martin, S.M.; Toledo, I.; Alvarado, C.; Wainsztein, R.E. Dehydration at admission increased the need for dialysis in hemolytic uremic syndrome children. Pediatr. Nephrol. 2012, 27, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Scallan, E.; Jones-Bitton, A.; Sargeant, J.M.; Stapleton, J.; Angulo, F.J.; Yeung, D.H.; Kirk, M.D. Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: A systematic review and knowledge synthesis. Foodborne Pathog. Dis. 2014, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Mohandas, J.; McDonald, S.P.; Hawley, C.M.; Badve, S.V.; Boudville, N.; Brown, F.G.; Clayton, P.A.; Wiggins, K.J.; Bannister, K.M.; et al. End-stage kidney disease due to haemolytic uraemic syndrome—Outcomes in 241 consecutive ANZDATA registry cases. BMC Nephrol. 2012, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Bonthuis, M.; Harambat, J.; Bérard, E.; Cransberg, K.; Duzova, A.; Garneata, L.; Herthelius, M.; Lungu, A.C.; Jahnukainen, T.; Kaltenegger, L.; et al. Recovery of Kidney Function in Children Treated with Maintenance Dialysis. Clin. J. Am. Soc. Nephrol. 2018, 13, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Alconcher, L.F.; Lucarelli, L.I.; Bronfen, S.; Meni Battaglia, L.; Balestracci, A. Dynamic evolution of kidney function in patients with STEC-hemolytic uremic syndrome followed for more than 15 years: Unexpected changes. Pediatr. Nephrol. 2024. [Google Scholar] [CrossRef]
- Pundzienė, B.; Dobilienė, D.; Čerkauskienė, R.; Mitkienė, R.; Medzevičienė, A.; Darškuvienė, E.; Jankauskienė, A. Long-term follow-up of children with typical hemolytic uremic syndrome. Medicina 2015, 51, 146–151. [Google Scholar] [CrossRef]
- Caletti, M.G.; Missoni, M.; Vezzani, C.; Grignoli, M.; Piantanida, J.J.; Repetto, H.A.; Exeni, R.; Rasse, S.M. Effect of diet, enalapril, or losartan in post-diarrheal hemolytic uremic syndrome nephropathy. Pediatr. Nephrol. 2011, 26, 1247–1254. [Google Scholar] [CrossRef]
- Caletti, M.G.; Balestracci, A.; Missoni, M.; Vezzani, C. Additive antiproteinuric effect of enalapril and losartan in children with hemolytic uremic syndrome. Pediatr. Nephrol. 2013, 28, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Ardissino, G.; Tel, F.; Possenti, I.; Testa, S.; Consonni, D.; Paglialonga, F.; Salardi, S.; Borsa-Ghiringhelli, N.; Salice, P.; Tedeschi, S.; et al. Early Volume Expansion and Outcomes of Hemolytic Uremic Syndrome. Pediatrics 2016, 137, e2015215. [Google Scholar] [CrossRef] [PubMed]
- Oakes, R.S.; Siegler, R.L.; McReynolds, M.A.; Pysher, T.; Pavia, A.T. Predictors of fatality in postdiarrheal hemolytic uremic syndrome. Pediatrics 2006, 117, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Loos, S.; Oh, J.; van de Loo, L.; Kemper, M.J.; Blohm, M.; Schild, R. Hemoconcentration and predictors in Shiga toxin-producing E. coli-hemolytic uremic syndrome (STEC-HUS). Pediatr. Nephrol. 2021, 36, 3777–3783. [Google Scholar] [CrossRef]
- Fakhouri, F.; Zuber, J.; Frémeaux-Bacchi, V.; Loirat, C. Haemolytic uraemic syndrome. Lancet 2017, 390, 681–696. [Google Scholar] [CrossRef]
| aHUS (n = 135) | STEC-HUS (n = 301) | p-Value | |
|---|---|---|---|
| Gender Female Male | 67 (49.6%) 68 (50.4%) | 164 (54.5%) 137 (45.5%) | 0.401 |
| Age at Onset, Years (IQR) | 3.8 (1.7 -6.7) | 2.2 (1.3-4.7) | 0.002 |
| Initial Symptoms Diarrhoea Blood in stool Respiratory tract infection Fever | 70 (51.8%) 25 (18.5%) 46 (34.1%) 61 (45.2%) | 286 (95%) 148 (49.2%) 32 (10.6%) 121 (40.2%) | <0.001 |
| Hypertension at Onset | 81 (60%) | 137 (45.8%) | 0.011 |
| KRT at Onset | 82 (60.7%) | 166 (55.2%) | 0.414 |
| KRT Modality at Onset | <0.001 | ||
| Peritoneal dialysis | 24 (17.8%) | 102 (33.9%) | |
| Hemodialysis/hemodiafiltration | 49 (36.3%) | 59 (19.6%) | |
| PD and HD/HDF | 8 (5.9%) | 6 (2%) | |
| Extrarenal Manifestation Neurological Pancreatitis Cardiac involvement | 49 (36.2%) 35 (25.9%) 25 (18.5%) 11 (8.1%) | 60 (19.9%) 42(13.9%) 25 (8.3%) 6 (2%) | 0.004 |
| Eculizumab Treatment at Onset | 73 (54%) * | 2 (0.7%) | 0.001 |
| aHUS | STEC-HUS | p-Value | |
|---|---|---|---|
| Mortality Rate | 5/135 (3.7%) | 6/301 (2%) | 0.29 |
| Cause of Death | 0.13 | ||
| Cardiac arrest/sudden death | 2 (40%) | 0 | |
| Heart failure | 1 (20%) | 0 | |
| Respiratory failure | 1 (20%) | 1 (16.6%) | |
| Neurological complications | 1 (20%) | 5 (83.3%) | |
| Death during Acute Episode of HUS | 2 (40%) | 6 (100%) | 0.06 |
| Death According to KRT Modality PD CVVHDF No KRT | 1 (20%) 0 4 (80%) | 2 (33.3%) 3 (50%) 1 (16.6%) | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagożdżon, I.; Szczepańska, M.; Leszczyńska, B.; Jarmużek, W.; Miklaszewska, M.; Tkaczyk, M.; Medyńska, A.; Wieczorkiewicz-Płaza, A.; Zachwieja, J.; Protas, P.; et al. Changing Epidemiology and Outcomes of Hemolytic Uremic Syndrome in Children: A Prospective National Cohort Study from the Polish Pediatric HUS Registry and the Polish Registry of Renal Replacement Therapy in Children. J. Clin. Med. 2024, 13, 6499. https://doi.org/10.3390/jcm13216499
Zagożdżon I, Szczepańska M, Leszczyńska B, Jarmużek W, Miklaszewska M, Tkaczyk M, Medyńska A, Wieczorkiewicz-Płaza A, Zachwieja J, Protas P, et al. Changing Epidemiology and Outcomes of Hemolytic Uremic Syndrome in Children: A Prospective National Cohort Study from the Polish Pediatric HUS Registry and the Polish Registry of Renal Replacement Therapy in Children. Journal of Clinical Medicine. 2024; 13(21):6499. https://doi.org/10.3390/jcm13216499
Chicago/Turabian StyleZagożdżon, Ilona, Maria Szczepańska, Beata Leszczyńska, Wioleta Jarmużek, Monika Miklaszewska, Marcin Tkaczyk, Anna Medyńska, Anna Wieczorkiewicz-Płaza, Jacek Zachwieja, Piotr Protas, and et al. 2024. "Changing Epidemiology and Outcomes of Hemolytic Uremic Syndrome in Children: A Prospective National Cohort Study from the Polish Pediatric HUS Registry and the Polish Registry of Renal Replacement Therapy in Children" Journal of Clinical Medicine 13, no. 21: 6499. https://doi.org/10.3390/jcm13216499
APA StyleZagożdżon, I., Szczepańska, M., Leszczyńska, B., Jarmużek, W., Miklaszewska, M., Tkaczyk, M., Medyńska, A., Wieczorkiewicz-Płaza, A., Zachwieja, J., Protas, P., Rosińska, P., Jacher, U., Trembecka-Dubel, E., Zwolińska, D., & Żurowska, A. (2024). Changing Epidemiology and Outcomes of Hemolytic Uremic Syndrome in Children: A Prospective National Cohort Study from the Polish Pediatric HUS Registry and the Polish Registry of Renal Replacement Therapy in Children. Journal of Clinical Medicine, 13(21), 6499. https://doi.org/10.3390/jcm13216499







