Diagnostic Accuracy Performance of Fluorescence In Situ Hybridization (FISH) for Biliary Strictures: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. FISH Assay
2.3. Study Outcomes
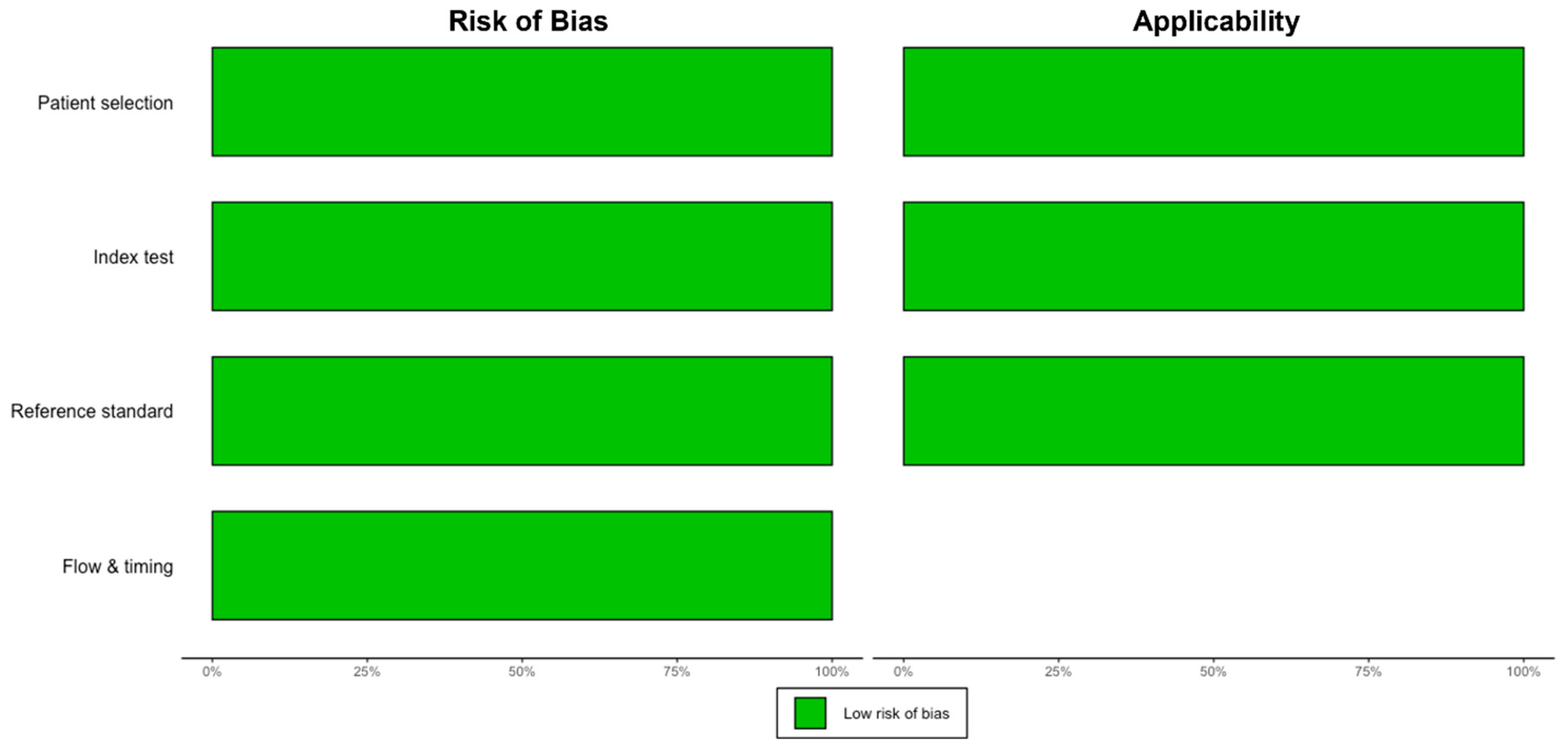
2.4. Study Selection, Data Extraction, and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Diagnostic Accuracy of FISH in Biliary Stricture
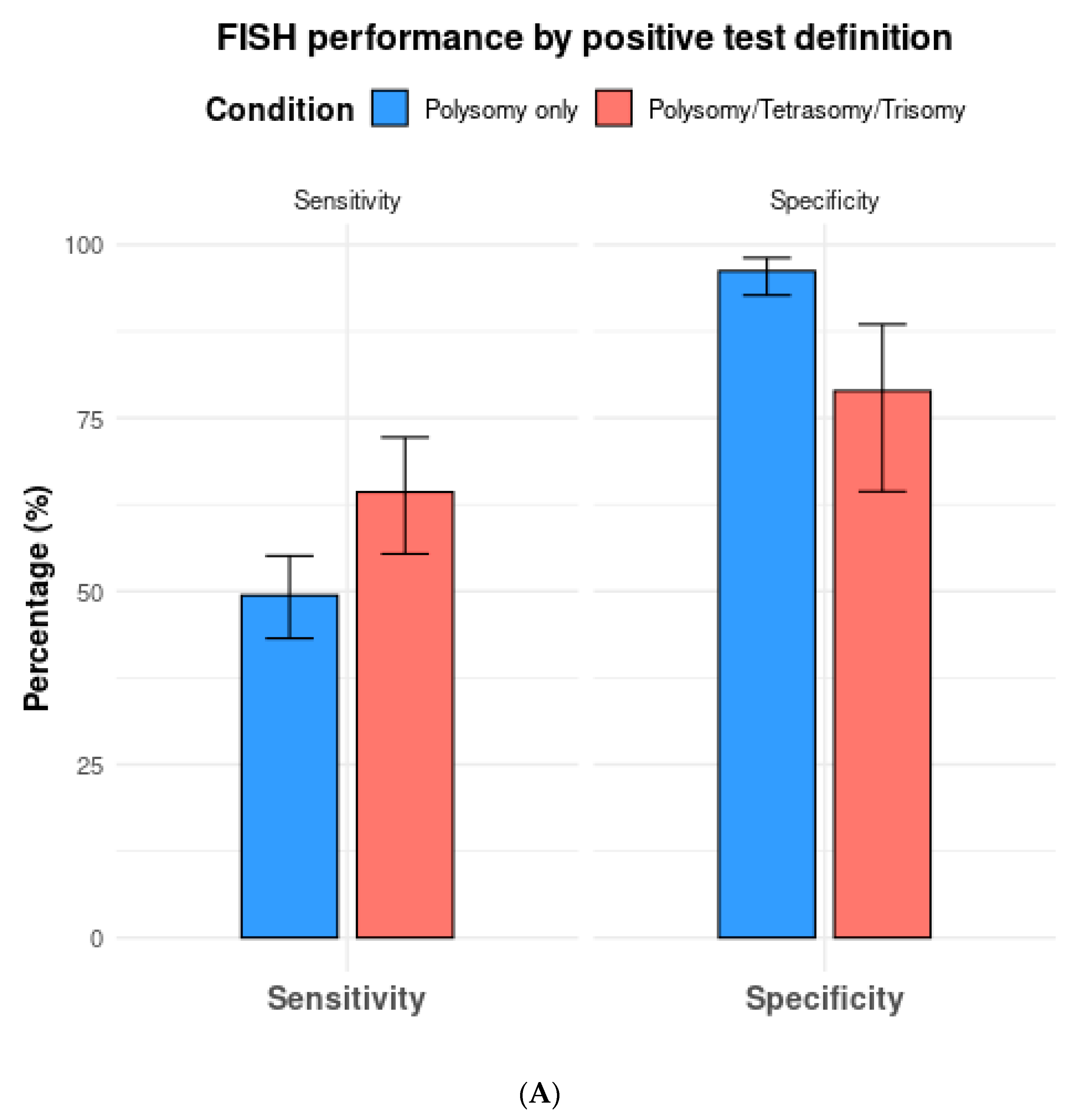
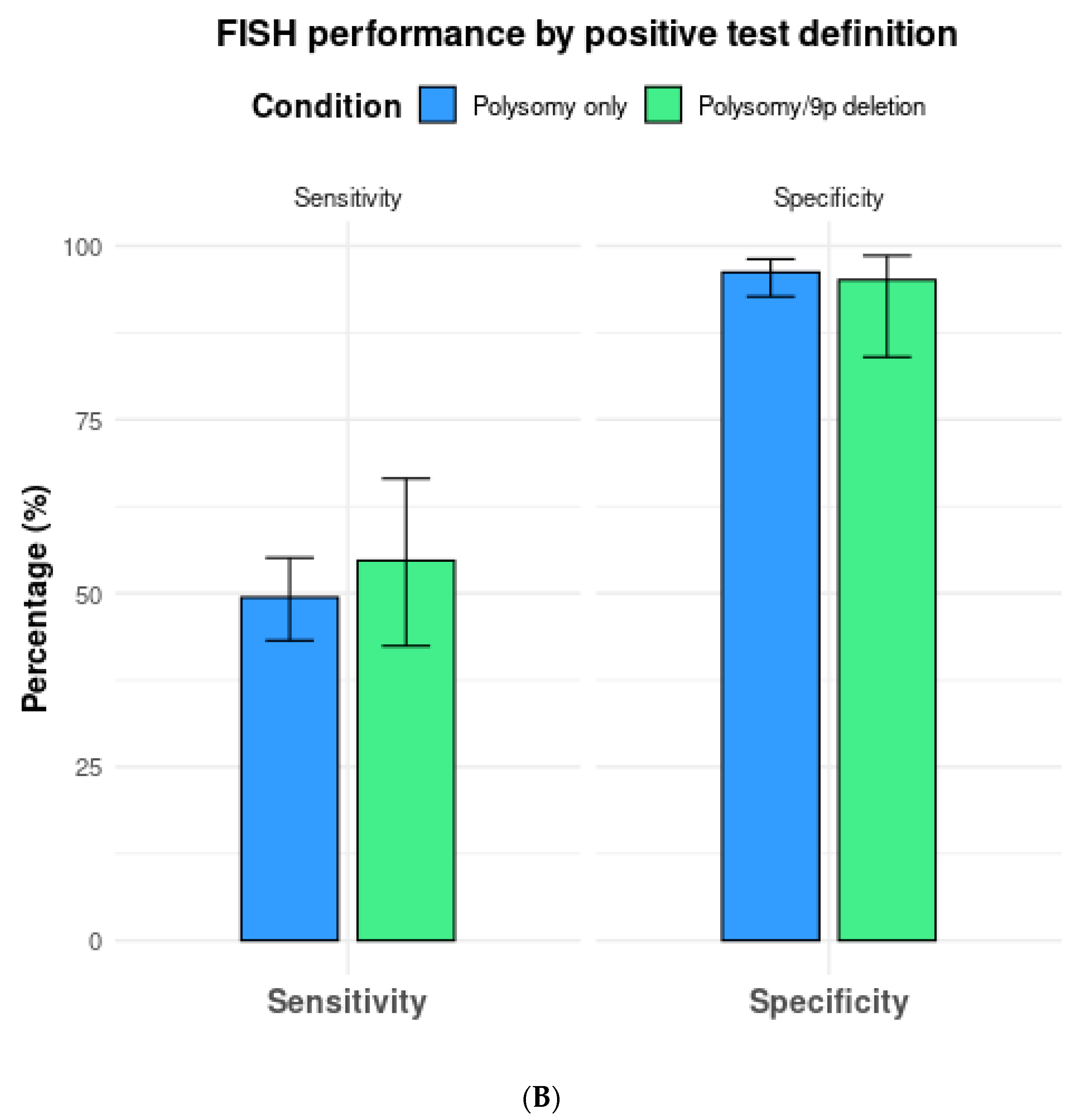
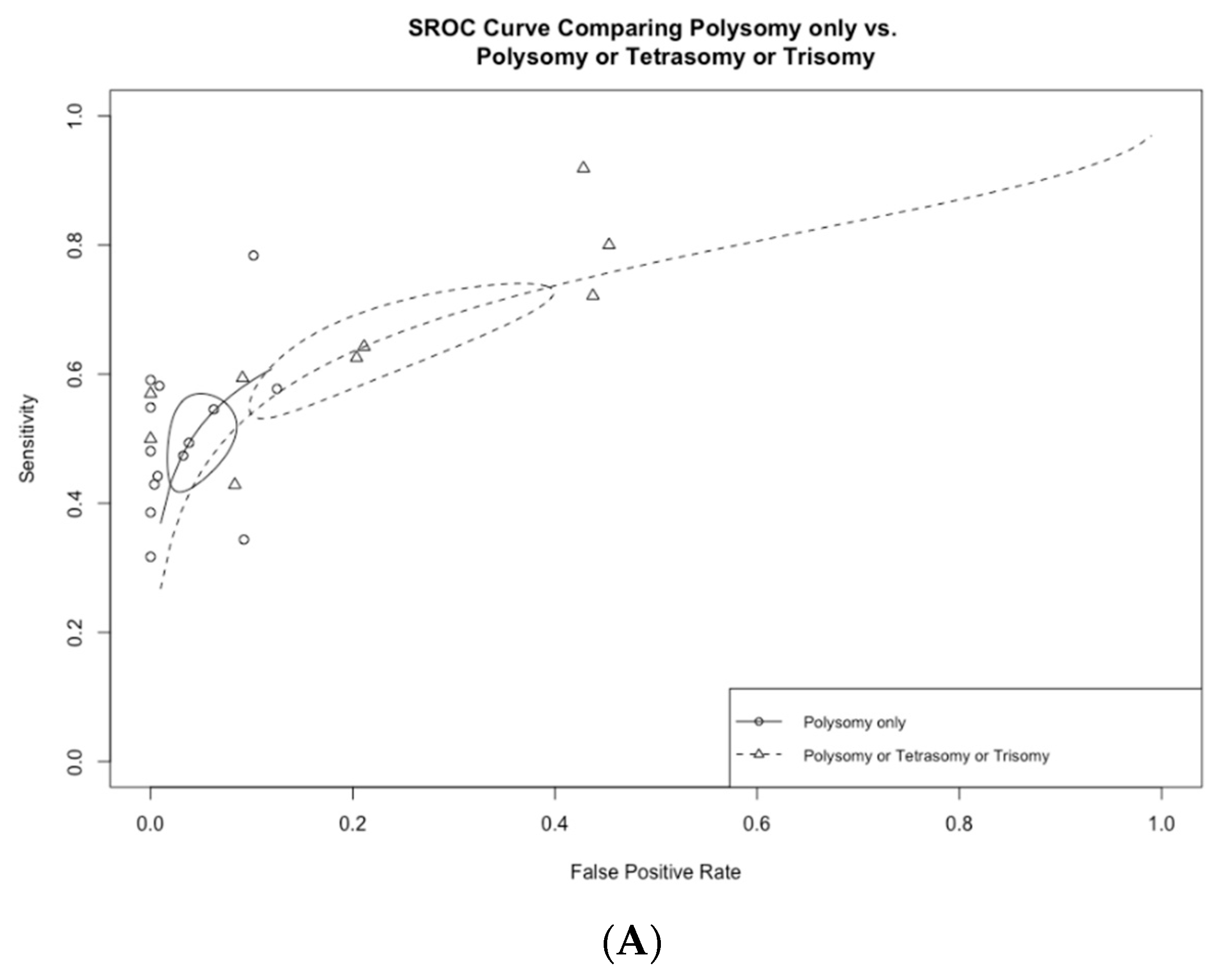
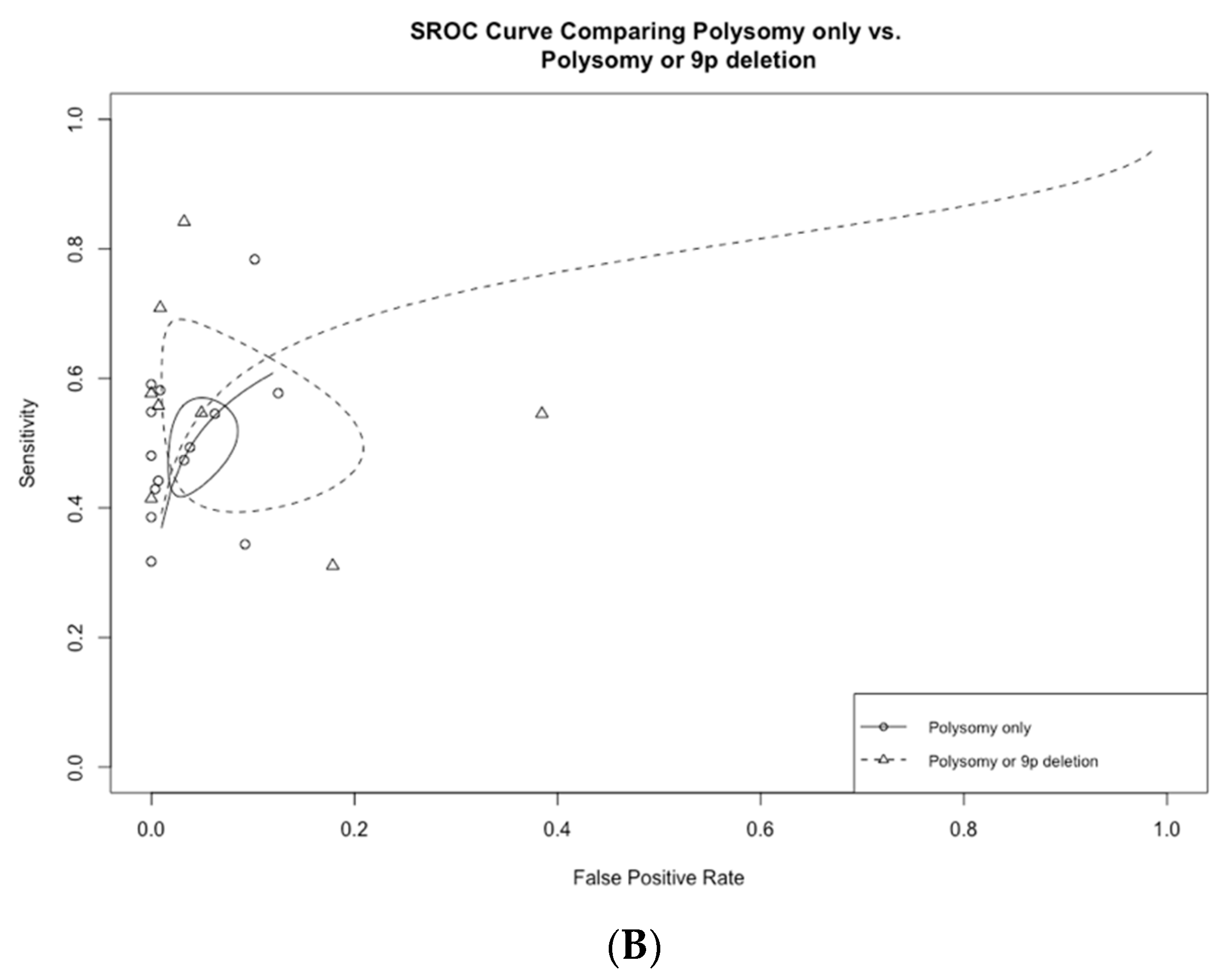
3.3. Subgroup Analysis Based on FISH Definition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| DOR | Diagnostic Odds Ratio |
| DTA | Diagnostic Test Accuracy |
| FISH | Fluorescence In Situ Hybridization |
| LR | Likelihood Ratio |
| PB | Pancreaticobiliary |
| PSC | Primary Sclerosing Cholangitis |
| ROC | Receiver Operating Characteristic Curve |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Studies-2 |
| SROC | Summary Receiver Operating Characteristic Curve |
References
- Dorrell, R.; Pawa, S.; Zhou, Y.; Lalwani, N.; Pawa, R. The Diagnostic Dilemma of Malignant Biliary Strictures. Diagnostics 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Tummala, P.; Munigala, S.; Eloubeidi, M.A.; Agarwal, B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: Prevalence of malignancy and potential role of EUS-FNA. J. Clin. Gastroenterol. 2013, 47, 532–537. [Google Scholar] [CrossRef]
- Burnett, A.S.; Calvert, T.J.; Chokshi, R.J. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J. Surg. Res. 2013, 184, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Elmunzer, B.J.; Maranki, J.L.; Gómez, V.; Tavakkoli, A.; Sauer, B.G.; Limketkai, B.N.; Brennan, E.A.; Attridge, E.M.; Brigham, T.J.; Wang, A.Y. ACG Clinical Guideline: Diagnosis and Management of Biliary Strictures. Off. J. Am. Coll. Gastroenterol.|ACG 2023, 118, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Kipp, B.R.; Stadheim, L.M.; Halling, S.A.; Pochron, N.L.; Harmsen, S.; Nagorney, D.M.; Sebo, T.J.; Therneau, T.M.; Gores, G.J.; de Groen, P.C.; et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am. J. Gastroenterol. 2004, 99, 1675–1681. [Google Scholar] [CrossRef]
- Han, S.; Tatman, P.; Mehrotra, S.; Wani, S.; Attwell, A.R.; Edmundowicz, S.A.; Brauer, B.C.; Wagh, M.S.; Hammad, H.T.; Shah, R.J. Combination of ERCP-Based Modalities Increases Diagnostic Yield for Biliary Strictures. Dig. Dis. Sci. 2021, 66, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.G.; Witt, B. Cytologic Diagnosis of Biliary Strictures: FISH or Cut the Sensitivity Rate? Dig. Dis. Sci. 2018, 63, 549–550. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; the PRISMA-DTA Group. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Deeks, J.J.; Bossuyt, P.M.; Leeflang, M.M.; Takwoingi, Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Cooley, M.A.; Schneider, A.R.; Barr Fritcher, E.G.; Milosevic, D.; Levy, M.J.; Bridgeman, A.R.; Martin, J.A.; Petersen, B.T.; Abu Dayyeh, B.K.; Storm, A.C.; et al. Utility of methylated DNA markers for the diagnosis of malignant biliary strictures. Hepatology 2024, 21, 21. [Google Scholar] [CrossRef]
- Barr Fritcher, E.G.; Voss, J.S.; Jenkins, S.M.; Lingineni, R.K.; Clayton, A.C.; Roberts, L.R.; Halling, K.C.; Talwalkar, J.A.; Gores, G.J.; Kipp, B.R. Primary sclerosing cholangitis with equivocal cytology: Fluorescence in situ hybridization and serum CA 19-9 predict risk of malignancy. Cancer Cytopathol. 2013, 121, 708–717. [Google Scholar] [CrossRef]
- Moreno Luna, L.E.; Kipp, B.; Halling, K.C.; Sebo, T.J.; Kremers, W.K.; Roberts, L.R.; Barr Fritcher, E.G.; Levy, M.J.; Gores, G.J. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 2006, 131, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Holmes, I.M.; Chen-Yost, H.I.; Smola, B.; Lew, M.; Betz, B.L.; Brown, N.A.; Pang, J. Performance of fluorescence in situ hybridization in biliary brushings with equivocal cytology: An institutional experience. J. Am. Soc. Cytopathol. 2024, 13, 285–290. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.W.; Choi, S.H.; Huh, J.; Park, S.H. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis. Korean J. Radiol. 2015, 16, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Barr Fritcher, E.G.; Kipp, B.R.; Halling, K.C.; Oberg, T.N.; Bryant, S.C.; Tarrell, R.F.; Gores, G.J.; Levy, M.J.; Clayton, A.C.; Sebo, T.J.; et al. A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures. Gastroenterology 2009, 136, 2180–2186. [Google Scholar] [CrossRef]
- Eaton, J.E.; Barr Fritcher, E.G.; Gores, G.J.; Atkinson, E.J.; Tabibian, J.H.; Topazian, M.D.; Gossard, A.A.; Halling, K.C.; Kipp, B.R.; Lazaridis, K.N. Biliary multifocal chromosomal polysomy and cholangiocarcinoma in primary sclerosing cholangitis. Am. J. Gastroenterol. 2015, 110, 299–309. [Google Scholar] [CrossRef]
- Nanda, A.; Brown, J.M.; Berger, S.H.; Lewis, M.M.; Barr Fritcher, E.G.; Gores, G.J.; Keilin, S.A.; Woods, K.E.; Cai, Q.; Willingham, F.F. Triple modality testing by endoscopic retrograde cholangiopancreatography for the diagnosis of cholangiocarcinoma. Ther. Adv. Gastroenterol. 2015, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.C.; Zheng, Z.; McDonald, T.; Le, L.J.P.; Dias-Santagata, D.; Borger, D.; Batten, J.; Vernovsky, K.; Sweeney, B.; Arpin, R.N.; et al. Next-Generation Sequencing and Fluorescence in Situ Hybridization Have Comparable Performance Characteristics in the Analysis of Pancreaticobiliary Brushings for Malignancy. J. Mol. Diagn. 2016, 18, 124–130. [Google Scholar] [CrossRef]
- Gonda, T.A.; Viterbo, D.; Gausman, V.; Kipp, C.; Sethi, A.; Poneros, J.M.; Gress, F.; Park, T.; Khan, A.; Jackson, S.A.; et al. Mutation Profile and Fluorescence In Situ Hybridization Analyses Increase Detection of Malignancies in Biliary Strictures. Clin. Gastroenterol. Hepatol. 2017, 15, 913–919.e911. [Google Scholar] [CrossRef]
- Brooks, C.; Gausman, V.; Kokoy-Mondragon, C.; Munot, K.; Amin, S.P.; Desai, A.; Kipp, C.; Poneros, J.; Sethi, A.; Gress, F.G.; et al. Role of Fluorescent In Situ Hybridization, Cholangioscopic Biopsies, and EUS-FNA in the Evaluation of Biliary Strictures. Dig. Dis. Sci. 2018, 63, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J. UroVysion Multi-Target Fluorescence in situ Hybridization Assay for the Detection of Malignant Bile Duct Brushing Specimens: A Comparison with Routine Cytology. Acta Cytol. 2018, 62, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, V.M.; Mullady, D.K.; Das, K.; Lang, G.; Hollander, T.G.; Murad, F.M.; Jackson, S.A.; Toney, N.A.; Finkelstein, S.D.; Edmundowicz, S.A. The Diagnostic Yield of Malignancy Comparing Cytology, FISH, and Molecular Analysis of Cell Free Cytology Brush Supernatant in Patients With Biliary Strictures Undergoing Endoscopic Retrograde Cholangiography (ERC): A Prospective Study. J. Clin. Gastroenterol. 2019, 53, 686–692. [Google Scholar] [CrossRef]
- Mettman, D.; Saeed, A.; Shold, J.; Laury, R.; Ly, A.; Khan, I.; Golem, S.; Olyaee, M.; O’Neil, M. Refined pancreatobiliary UroVysion criteria and an approach for further optimization. Cancer Med. 2021, 10, 5725–5738. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; la Sancha, C.; Saad, M.; Alkashash, A.; Ullah, A.; Alruwaii, F.; Velasquez Zarate, L.; Cramer, H.M.; Wu, H.H. The Role of Fluorescence In Situ Hybridization in Pancreatobiliary Brushing Cytology: A Large Retrospective Review with Histologic Correlation. Diagnostics 2022, 12, 2486. [Google Scholar] [CrossRef]
- Gonda, T.A.; Glick, M.P.; Sethi, A.; Poneros, J.M.; Palmas, W.; Iqbal, S.; Gonzalez, S.; Nandula, S.V.; Emond, J.C.; Brown, R.S.; et al. Polysomy and p16 deletion by fluorescence in situ hybridization in the diagnosis of indeterminate biliary strictures. Gastrointest. Endosc. 2012, 75, 74–79. [Google Scholar] [CrossRef]
- Smoczynski, M.; Jablonska, A.; Matyskiel, A.; Lakomy, J.; Dubowik, M.; Marek, I.; Biernat, W.; Limon, J. Routine brush cytology and fluorescence in situ hybridization for assessment of pancreatobiliary strictures. Gastrointest. Endosc. 2012, 75, 65–73. [Google Scholar] [CrossRef]
- Boldorini, R.; Paganotti, A.; Andorno, S.; Orlando, S.; Mercalli, F.; Orsello, M.; Ballarè, M.; Magnani, C.; Sartori, M. A multistep cytological approach for patients with jaundice and biliary strictures of indeterminate origin. J. Clin. Pathol. 2015, 68, 283–287. [Google Scholar] [CrossRef]
- Chaiteerakij, R.; Barr Fritcher, E.G.; Angsuwatcharakon, P.; Ridtitid, W.; Chaithongrat, S.; Leerapun, A.; Baron, T.H.; Kipp, B.R.; Henry, M.R.; Halling, K.C.; et al. Fluorescence in situ hybridization compared with conventional cytology for the diagnosis of malignant biliary tract strictures in Asian patients. Gastrointest. Endosc. 2016, 83, 1228–1235. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, X.; Yang, A.; Yao, F.; Guo, T.; Wu, D.; Wu, S.; Qiu, H.; Zhou, W.; Huo, Z.; et al. Fluorescence In Situ Hybridization with the UroVysion Kit for the Detection of Biliary Cancer in Chinese Patients. Clin. Lab. 2017, 63, 407–413. [Google Scholar] [CrossRef]
- Zoundjiekpon, V.D.; Falt, P.; Zapletalova, J.; Vanek, P.; Kurfurstova, D.; Slobodova, Z.; Skanderova, D.; Korinkova, G.; Skalicky, P.; Lovecek, M.; et al. Fluorescence In Situ Hybridization in Primary Diagnosis of Biliary Strictures: A Single-Center Prospective Interventional Study. Biomedicines 2023, 11, 755. [Google Scholar] [CrossRef]
- Baroud, S.; Sahakian, A.J.; Sawas, T.; Storm, A.C.; Martin, J.A.; Abu Dayyeh, B.K.; Topazian, M.D.; Levy, M.J.; Roberts, L.R.; Gores, G.J.; et al. Impact of trimodality sampling on detection of malignant biliary strictures compared with patients with primary sclerosing cholangitis. Gastrointest. Endosc. 2022, 95, 884–892. [Google Scholar] [CrossRef] [PubMed]
- DeHaan, R.D.; Kipp, B.R.; Smyrk, T.C.; Abraham, S.C.; Roberts, L.R.; Halling, K.C. An assessment of chromosomal alterations detected by fluorescence in situ hybridization and p16 expression in sporadic and primary sclerosing cholangitis-associated cholangiocarcinomas. Hum. Pathol. 2007, 38, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A. Cellular and molecular biology of biliary tract cancers. Surg. Oncol. Clin. N. Am. 2002, 11, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Njei, B.; Venkatesh, P.G.K.; Vargo, J.J.; Parsi, M.A. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: A systematic review and meta-analysis. Gastrointest. Endosc. 2014, 79, 943–950.e3. [Google Scholar] [CrossRef] [PubMed]
- Harbhajanka, A.; Michael, C.W.; Janaki, N.; Gokozan, H.N.; Wasman, J.; Bomeisl, P.; Yoest, J.; Sadri, N. Tiny but mighty: Use of next generation sequencing on discarded cytocentrifuged bile duct brushing specimens to increase sensitivity of cytological diagnosis. Mod. Pathol. 2020, 33, 2019–2025. [Google Scholar] [CrossRef]
- Singhi, A.D.; Nikiforova, M.N.; Chennat, J.; Papachristou, G.I.; Khalid, A.; Rabinovitz, M.; Das, R.; Sarkaria, S.; Ayasso, M.S.; Wald, A.I.; et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut 2020, 69, 52–61. [Google Scholar] [CrossRef]
- Arechederra, M.; Rullan, M.; Amat, I.; Oyon, D.; Zabalza, L.; Elizalde, M.; Latasa, M.U.; Mercado, M.R.; Ruiz-Clavijo, D.; Saldana, C.; et al. Next-generation sequencing of bile cell-free DNA for the early detection of patients with malignant biliary strictures. Gut 2022, 71, 1141–1151. [Google Scholar] [CrossRef]
- Nakaoka, T.; Saito, Y.; Saito, H. Aberrant DNA Methylation as a Biomarker and a Therapeutic Target of Cholangiocarcinoma. Int. J. Mol. Sci. 2017, 18, 1111. [Google Scholar] [CrossRef]
- Vedeld, H.M.; Grimsrud, M.M.; Andresen, K.; Pharo, H.D.; von Seth, E.; Karlsen, T.H.; Honne, H.; Paulsen, V.; Farkkila, M.A.; Bergquist, A.; et al. Early and accurate detection of cholangiocarcinoma in patients with primary sclerosing cholangitis by methylation markers in bile. Hepatology 2022, 75, 59–73. [Google Scholar] [CrossRef]
- Andresen, K.; Boberg, K.M.; Vedeld, H.M.; Honne, H.; Jebsen, P.; Hektoen, M.; Wadsworth, C.A.; Clausen, O.P.; Lundin, K.E.; Paulsen, V.; et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology 2015, 61, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Lapitz, A.; Fabris, L. Comparative performance of methylation DNA markers, brushing cytology, and FISH in diagnosing malignant biliary strictures. Hepatology 2024. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Tyberg, A.; Slivka, A.; Adler, D.G.; Desai, A.P.; Sejpal, D.V.; Pleskow, D.K.; Bertani, H.; Gan, S.I.; Shah, R.; et al. Digital Single-operator Cholangioscopy (DSOC) Improves Interobserver Agreement (IOA) and Accuracy for Evaluation of Indeterminate Biliary Strictures: The Monaco Classification. J. Clin. Gastroenterol. 2022, 56, e94–e97. [Google Scholar] [CrossRef]
- Sethi, A.; Chen, Y.K.; Austin, G.L.; Brown, W.R.; Brauer, B.C.; Fukami, N.N.; Khan, A.H.; Shah, R.J. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: A single-center experience. Gastrointest. Endosc. 2011, 73, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch VJ, H.W. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar]
- Ji, H.; Barr Fritcher, E.G.; Yin, J.; Bainter, T.M.; Zemla, T.J.; Gores, G.J.; Halling, K.C.; Kipp, B.R.; Roberts, L.R. Evaluating the Significance of Pancreatobiliary Fluorescence In Situ Hybridization Polysomy on Prognosis in De Novo Cholangiocarcinoma. Clin. Transl. Gastroenterol. 2022, 13, e00523. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. MetaArXiv 2020. [Google Scholar] [CrossRef]

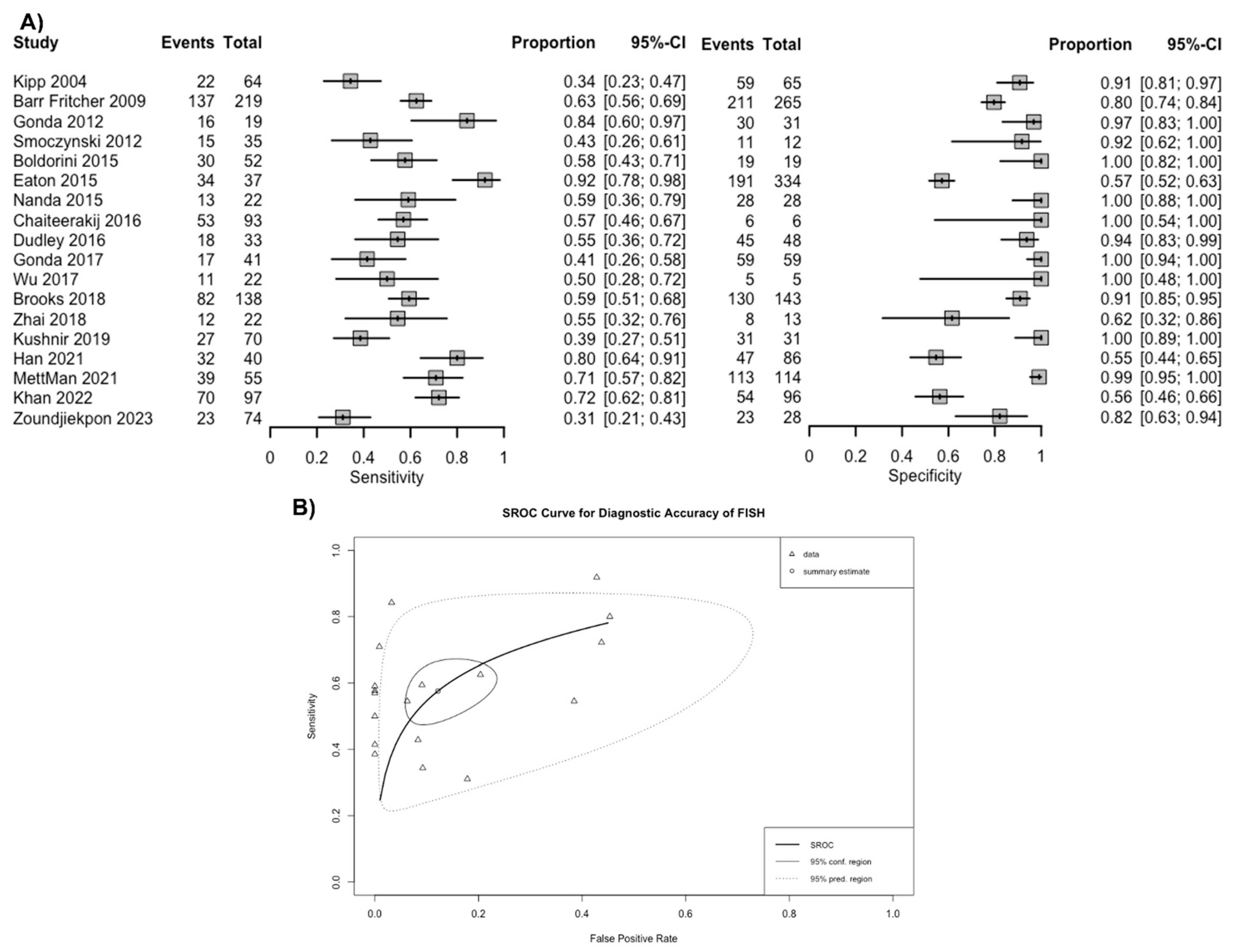
| Study | Location | Study Period | Study Threshold for Diagnostic FISH | FISH Samples (n) | Malignancy (n) | Cholangiocarcinoma (n) |
|---|---|---|---|---|---|---|
| Kipp 2004 [5] | USA | June 2000–June 2002 | Polysomy only | 129 | 64 | 39 |
| Barr Fritcher 2009 [17] | USA | October 2003–March 2006 | Polysomy, Tetrasomy, or Trisomy Polysomy only | 484 | 219 | 152 |
| Gonda 2012 [27] | USA | February 2008–February 2010 | Polysomy or 9p deletion Polysomy only | 50 | 19 | 16 |
| Smoczynski 2012 [28] | Poland | September 2008–August 2010 | Polysomy or Trisomy | 47 | 35 | 11 |
| Boldorini 2015 [29] | Italy | June 2007–September 2009 | Polysomy or 9p deletion Polysomy only | 71 | 52 | NA |
| Eaton 2015 [18] | USA | January 2005–July 2013 | Polysomy, Tetrasomy, or Trisomy Polysomy only | 371 | 37 | 37 |
| Nanda 2015 [19] | USA | December 2008–November 2012 | Polysomy only | 50 | 22 | 22 |
| Chaiteerakij 2016 [30] | Thailand | March 2010–December 2013 | Polysomy or Trisomy with 9p deletion Polysomy only | 99 | 93 | 58 |
| Dudley 2016 [20] | USA | April 2014–January 2015 | Polysomy only | 81 | 33 | 10 |
| Gonda 2017 [21] | USA | June 2012–June 2014 | Polysomy or 9p deletion Polysomy only | 100 | 41 | 13 |
| Wu 2017 [31] | China | October 2008–June 2009 | Polysomy or Trisomy | 27 | 22 | 17 |
| Brooks 2018 [22] | USA | 2006–2016 | Polysomy or Trisomy or 9p deletion Polysomy or 9p deletion Polysomy only | 281 | 138 | 55 |
| Zhai 2018 [23] | USA | Not Reported | Polysomy or 9p deletion | 35 | 22 | 3 |
| Kushnir 2019 [24] | USA | November 2013–February 2016 | Polysomy only | 101 | 70 | 33 |
| Han 2021 [6] | USA | January 2008–July 2015 | Polysomy, Tetrasomy, or 9p deletion | 126 | 40 | NA |
| MettMan 2021 [25] | USA | October 2014–November 2019 | Polysomy or 9p deletion Polysomy only | 169 | 55 | NA |
| Khan 2022 [26] | USA | January 2001–September 2019 | Polysomy or Trisomy Polysomy only | 193 | 97 | NA |
| Zoundjiekpon 2023 [32] | Czech Republic | April 2019–January 2021 | Polysomy or 9p deletion | 102 | 74 | 26 |
| Sensitivity | Specificity | Positive LR | Negative LR | DOR | |
|---|---|---|---|---|---|
| All included studies | |||||
| All thresholds (n = 18) | 57.6 (49.4–65.4) | 87.8 (79.2–93.2) | 4.9 (2.9–8.1) | 0.49 (0.40–0.57) | 10.3 (5.4–17.9) |
| Polysomy only (n = 13) | 49.4 (43.2–55.1) | 96.2 (92.7–98.1) | 13.7 (7.0–24.5) | 0.53 (0.47–0.59) | 26.1 (12.9–47.2) |
| Polysomy, tetrasomy, or trisomy (n = 8) | 64.3 (55.4–72.2) | 78.9 (64.4–88.5) | 2.8 (1.8–4.4) | 0.47 (0.41–0.53) | 6.0 (3.5–9.7) |
| Polysomy or 9p deletion (n = 7) | 54.7 (42.4–66.5) | 95.1 (84.0–98.6) | 14.0 (3.1–42.6) | 0.48 (0.35–0.65) | 31.7 (5.0–109.0) |
| Studies with head-to-head comparison of different thresholds | |||||
| Polysomy only (n = 5) | 52.9 (44.2–61.4) | 96.3 (87.6–99.0) | 17.1 (4.8–45.7) | 0.49 (0.42–0.57) | 34.2 (10.1–86.1) |
| Polysomy, tetrasomy, or trisomy (n = 5) | 65.7 (57.3–73.1) | 77.1 (59.0–88.8) | 3.0 (1.8–5.3) | 0.45 (0.39–0.52) | 6.8 (3.6–11.7) |
| Polysomy only (n = 5) | 46.3 (38.6–54.2) | 98.3 (96.0–99.3) | 29.2 (11.6–61.8) | 0.55 (0.47–0.63) | 54.1 (20.1–118.0) |
| Polysomy or 9p deletion (n = 5) | 61.0 (47.7–72.9) | 98.4 (96.0–99.4) | 43.0 (16.0–94.8) | 0.40 (0.28–0.53) | 110.0 (40.2–242.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggarwal, M.; Simadibrata, D.M.; Kipp, B.R.; Prokop, L.J.; Barr Fritcher, E.G.; Schneider, A.; Cooley, M.A.; Gores, G.J.; Eaton, J.; Roberts, L.R.; et al. Diagnostic Accuracy Performance of Fluorescence In Situ Hybridization (FISH) for Biliary Strictures: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 6457. https://doi.org/10.3390/jcm13216457
Aggarwal M, Simadibrata DM, Kipp BR, Prokop LJ, Barr Fritcher EG, Schneider A, Cooley MA, Gores GJ, Eaton J, Roberts LR, et al. Diagnostic Accuracy Performance of Fluorescence In Situ Hybridization (FISH) for Biliary Strictures: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(21):6457. https://doi.org/10.3390/jcm13216457
Chicago/Turabian StyleAggarwal, Manik, Daniel M. Simadibrata, Benjamin R. Kipp, Larry J. Prokop, Emily G. Barr Fritcher, Amber Schneider, Matthew A. Cooley, Gregory J. Gores, John Eaton, Lewis R. Roberts, and et al. 2024. "Diagnostic Accuracy Performance of Fluorescence In Situ Hybridization (FISH) for Biliary Strictures: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 21: 6457. https://doi.org/10.3390/jcm13216457
APA StyleAggarwal, M., Simadibrata, D. M., Kipp, B. R., Prokop, L. J., Barr Fritcher, E. G., Schneider, A., Cooley, M. A., Gores, G. J., Eaton, J., Roberts, L. R., & Chandrasekhara, V. (2024). Diagnostic Accuracy Performance of Fluorescence In Situ Hybridization (FISH) for Biliary Strictures: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(21), 6457. https://doi.org/10.3390/jcm13216457






