Abstract
Background and Aims: This systematic review and meta-analysis aims to compare the performance of UroVysion® FISH based on the different definitions of a positive result used in published literature with the goal of determining the optimal FISH definition for detecting pancreaticobiliary malignancy. Methods: A systematic literature search identified studies from database inception to Sept 2024 that evaluated the diagnostic performance of FISH in determining malignancy among patients with biliary strictures. All thresholds for positive FISH, as defined by the individual study, were included in this review. Subgroup analysis was performed based on the definitions of positive FISH as follows: (1) polysomy only; (2) polysomy, tetrasomy, or trisomy; and (3) polysomy or 9p deletion. Results: Eighteen studies comprising 2516 FISH specimens were analyzed, including 1133 (45.0%) with malignancy. Using a threshold for positivity as defined in individual studies, the overall sensitivity of FISH was 57.6% (95% confidence interval [CI], 49.4–65.4%), and the overall specificity was 87.8% (95% CI, 79.2–93.2%). Subgroup analysis showed that polysomy as the threshold for positive FISH yielded a sensitivity of 49.4% (95% CI, 43.2–55.5%), with an increased specificity of 96.2% (95% CI, 92.7–98.1%), while polysomy + tetrasomy/trisomy as positive FISH resulted in an increased sensitivity of 64.3% (95% CI 55.4–72.2%) but a decreased specificity of 78.9% (95% CI 64.4–88.5%). The addition of 9p deletion to polysomy as the criteria for a positive test resulted in a non-significant increase in sensitivity (54.7% (95% CI 42.4–66.5%) while maintaining specificity (95.1% (95% CI 84.0–98.6%). Conclusions: Based on these findings, polysomy only or polysomy/9p deletion should be considered as the criterion for defining a positive FISH test to improve diagnostic sensitivity while maintaining high specificity.
1. Introduction
Biliary stricture can arise from both benign (such as primary sclerosing cholangitis [PSC], chronic pancreatitis, infection, autoimmune cholangiopathy, and bile duct injury) and malignant conditions (such as cholangiocarcinoma [CCA] or pancreatic ductal adenocarcinoma [PDAC]) [1]. Most biliary strictures are considered malignant [2]; however, providing a definitive diagnosis of malignancy remains a clinical challenge. Several cytological and molecular techniques have been developed to aid in the diagnosis of malignant biliary strictures. These include traditional brush cytology, fluorescence in situ hybridization (FISH), and novel molecular techniques, such as methylated DNA markers and next-generation sequencing (NGS). Brushings of the biliary tract for cytology, a commonly performed diagnostic modality, is known to have poor sensitivity for detecting malignancy in biliary strictures [3]. FISH is a molecular cytogenetic technique that can be used to assess aneuploidy and has become a reliable diagnostic marker for detecting malignancy in patients with biliary strictures, with improved sensitivity over cytology while maintaining specificity [4,5]. Therefore, due to the increased sensitivity, current guidelines have suggested using multimodality analysis or tissue analysis over brush cytology alone [4,6].
Various FISH abnormalities are found in patients with biliary strictures diagnosed with malignancy, such as the presence of polysomy, tetrasomy, trisomy, and 9p deletion, among others [7]. Currently, there is a lack of consensus regarding the threshold or definition of positive FISH. These thresholds significantly affect the overall performance of FISH in evaluating biliary strictures. Thus, the lack of consensus has resulted in varying sensitivities and specificities of FISH across studies, potentially impacting patient management. This study aims to summarize the diagnostic performance of FISH for detecting malignancy in patients with biliary strictures. Furthermore, we aim to compare the performance of FISH, based on the different definitions used in published literature, with the goal of determining the optimal FISH definition for detecting pancreaticobiliary malignancy in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP)-based tissue sampling.
2. Methods
This study was performed in accordance with the PRISMA guideline for diagnostic test accuracy (DTA) [8] and the Cochrane Handbook for Systematic Reviews of DTA [9]. The 27-item PRISMA diagnostic test accuracy checklist is provided in Supplementary Materials S1. Institutional review board approval was not needed for this study, due to the use of publicly available data.
2.1. Search Strategy and Selection Criteria
A comprehensive search of Ovid MEDLINE, Ovid Embase, Cochrane, and Scopus from database inception until September 2024 was performed by an expert librarian (JLP). (Search strategy is provided in Supplementary Materials S2) A priori inclusion criteria were full-text articles assessing the diagnostic accuracy of UroVysion® FISH in adult (≥18 years of age) patients with biliary strictures who underwent ERCP-based brushing and comparing the findings with a clinical or histopathological diagnosis as the gold standard. Studies utilizing pancreaticobiliary (PB) FISH probe sets were excluded, due to the inherent differences between the FISH tests [10]. When studies had overlapping data, the study with the most complete and greater number of cases was included to avoid the double counting of subjects. This was evident in a few publications reported by the same institution [11,12]. Two studies were excluded, as they only included patients with inconclusive brush cytology, thus introducing selection bias [11,13]. There were no restrictions to the language of the study.
2.2. FISH Assay
FISH in each study was performed using a commercial kit (UroVysion® probe set; Abbott Molecular Inc., Des Plaines, IL, USA) that contained labeled DNA probes in the pericentromeric regions of chromosomes 3, 7, and 17 and in the chromosomal band 9p21. Cells with a normal FISH pattern had 2 copies for each probe, which is consistent with a benign diagnosis. Three main abnormalities can be detected in cells using the FISH assay: (1) polysomy, 3 or more copies of 2 or more probes; (2) trisomy, 3 or more copies of 1 probe and 2 copies of the other 3 probes; and (3) tetrasomy, 4 copies of each probe. Additionally, the chromosomal band probe can show the loss of 9p21.
2.3. Study Outcomes
The outcome of interest was the diagnostic accuracy of FISH in evaluating biliary strictures, which included the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the curve (AUC). The definition of a positive FISH result was determined per study definition, such as but not limited to (1) polysomy only, (2) polysomy/tetrasomy/trisomy, or (3) polysomy/9p deletion.
2.4. Study Selection, Data Extraction, and Quality Assessment
Two reviewers (M.A. and D.M.S) independently performed study adjudication, assessing the title, abstract, and full-text articles based on the eligibility criteria. For the included studies, data extraction was performed using Microsoft Excel (Version 2408 Build 16.0.17928.20114) to obtain key information on the study design and characteristics, patient demographics, definition of a positive FISH, diagnostic outcomes, and methodological quality. The risk of bias in the included studies was assessed using the QUADAS-2 tool recommended by the Cochrane Collaboration [14]. This tool assessed four domains: (1) methods of patient selection, (2) index test, (3) reference standard, and (4) flow of timing. Any discrepancies in the study selection, data extraction, and quality assessment processes were resolved by consensus between the two reviewers.
2.5. Statistical Analysis
A 2 × 2 contingency table was constructed using the extracted data to assess diagnostic performance. A DTA meta-analysis was performed using the “mada” package of the R program (version 0.5.11) (Vienna, Austria). The pooled sensitivity, specificity, PLR, NLR, and DOR with the 95% confidence interval (95% CI) were obtained using a bivariate random-effects model, as heterogeneity between studies was expected in the DTA meta-analysis. Univariate models or separate meta-analyses of sensitivity and specificity estimates are known to underestimate the test accuracy, due to the failure to account for the trade-off between sensitivity and specificity [9]. As endorsed by the Cochrane Handbook of Systematic Reviews of DTA studies [9], statistical heterogeneity was not assessed using the I2 statistic. I2 is often not appropriate for use in systematic reviews of DTA studies (particularly when the bivariate model has been used) because it is a univariate measure that does not account for threshold effects or the expected inverse correlation between sensitivity and specificity [15]. Tests used to measure heterogeneity are known to have low statistical power in DTA reviews [16]. The use of prediction ellipses around SROC curves (the approximate bivariate equivalent of a prediction interval) is a suitable alternative for graphically depicting heterogeneity in DTA meta-analyses. The magnitude of observed heterogeneity is best depicted graphically, where such relationships can be observed by the scatter of points and from the prediction ellipse. Consequently, the hierarchical summary receiver operating characteristic curve (SROC) with prediction ellipses were constructed, and the AUC value was determined. Subgroup analysis was performed based on the study definitions of positive FISH: (1) polysomy only, (2) polysomy/tetrasomy/trisomy, and (3) polysomy/9p deletion. Furthermore, we also analyzed the findings in patients with a known PSC diagnosis. Publication bias was assessed visually using a funnel plot and quantitatively analyzed using Deek’s linear regression test.
3. Results
3.1. Study Characteristics
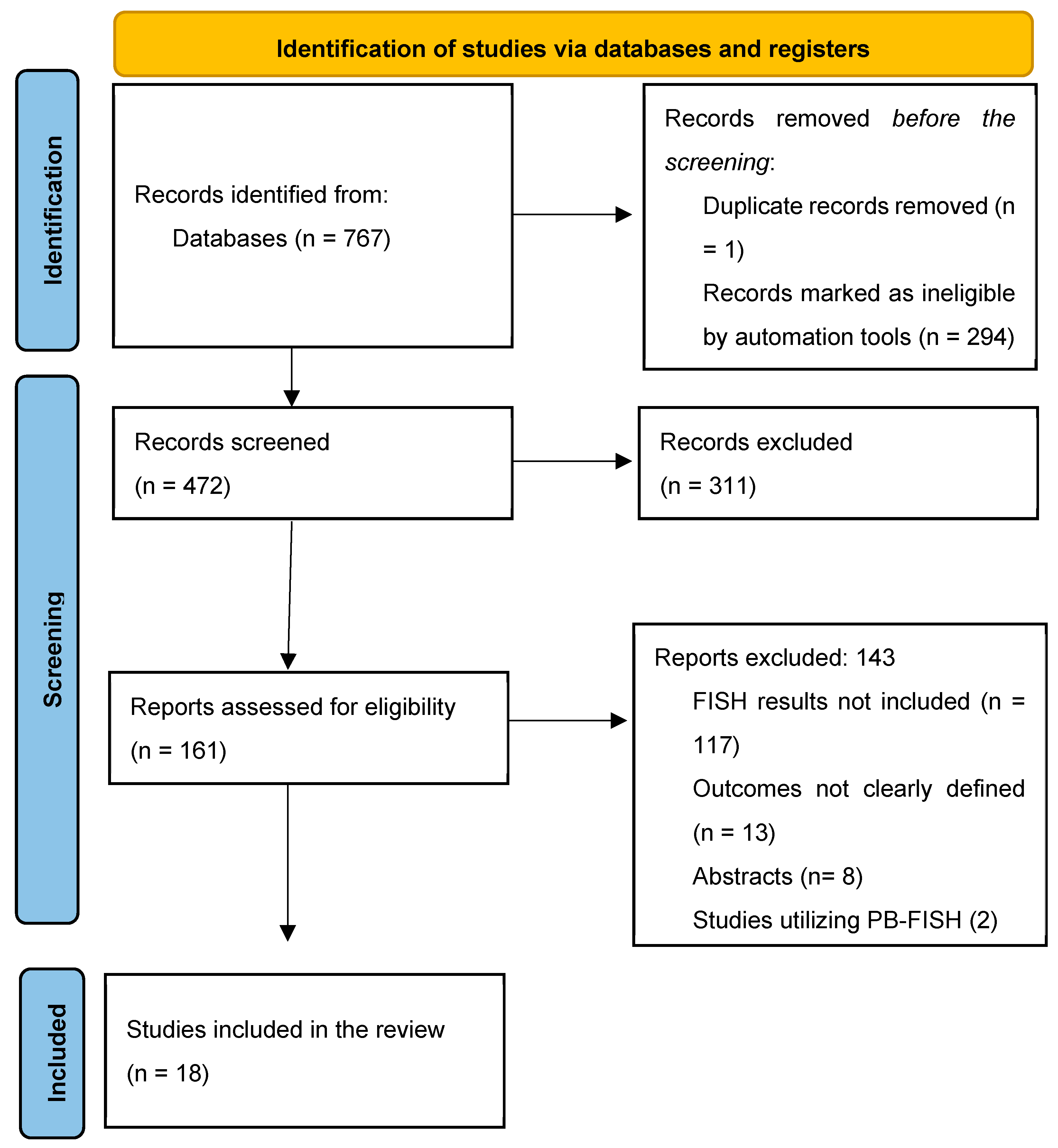
The initial search identified 472 titles, of which 157 were reviewed based on the full text for study eligibility (Figure 1). Overall, 18 studies (n = 2516 FISH specimens) were included, with malignancy identified in 1133 (45.0%) cases. All studies used the UroVysion® (Abbott Molecular) probe set. Most of the studies were from the United States of America (n = 13) [5,6,17,18,19,20,21,22,23,24,25,26,27]; the remaining were from Poland [28], Italy [29], Thailand [30], China [31], and the Czech Republic [32]. All but two [22,30] were single-center studies. Only three studies had isolated data for PSC patients [5,18,22]. Studies used various thresholds/definitions for positive FISH, including but not limited to polysomy only (n = 13), polysomy/tetrasomy/trisomy (n = 8), and polysomy/9p deletion (n = 7). The overall risk of bias in the studies included was low (Figure 2, Supplementary Materials S3). Other study details and definitions of positive FISH are outlined in Table 1, Supplementary Materials S4.
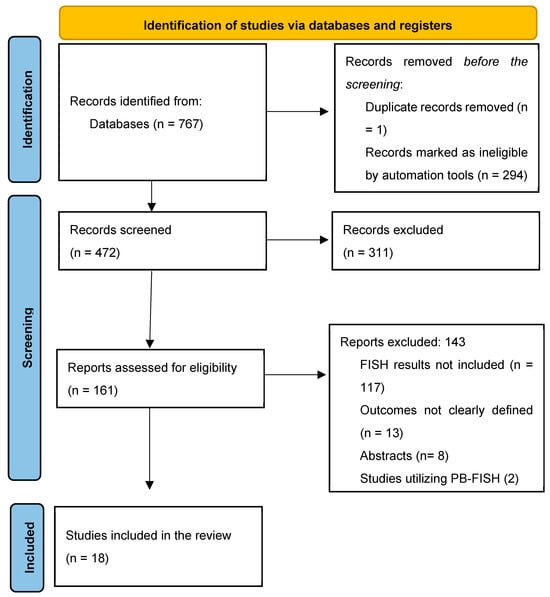
Figure 1.
PRISMA flow chart for study selection.
Figure 2.
Summary of risk of bias based on QUADAS-2.

Table 1.
Baseline characteristics of the included studies.
3.2. Diagnostic Accuracy of FISH in Biliary Stricture
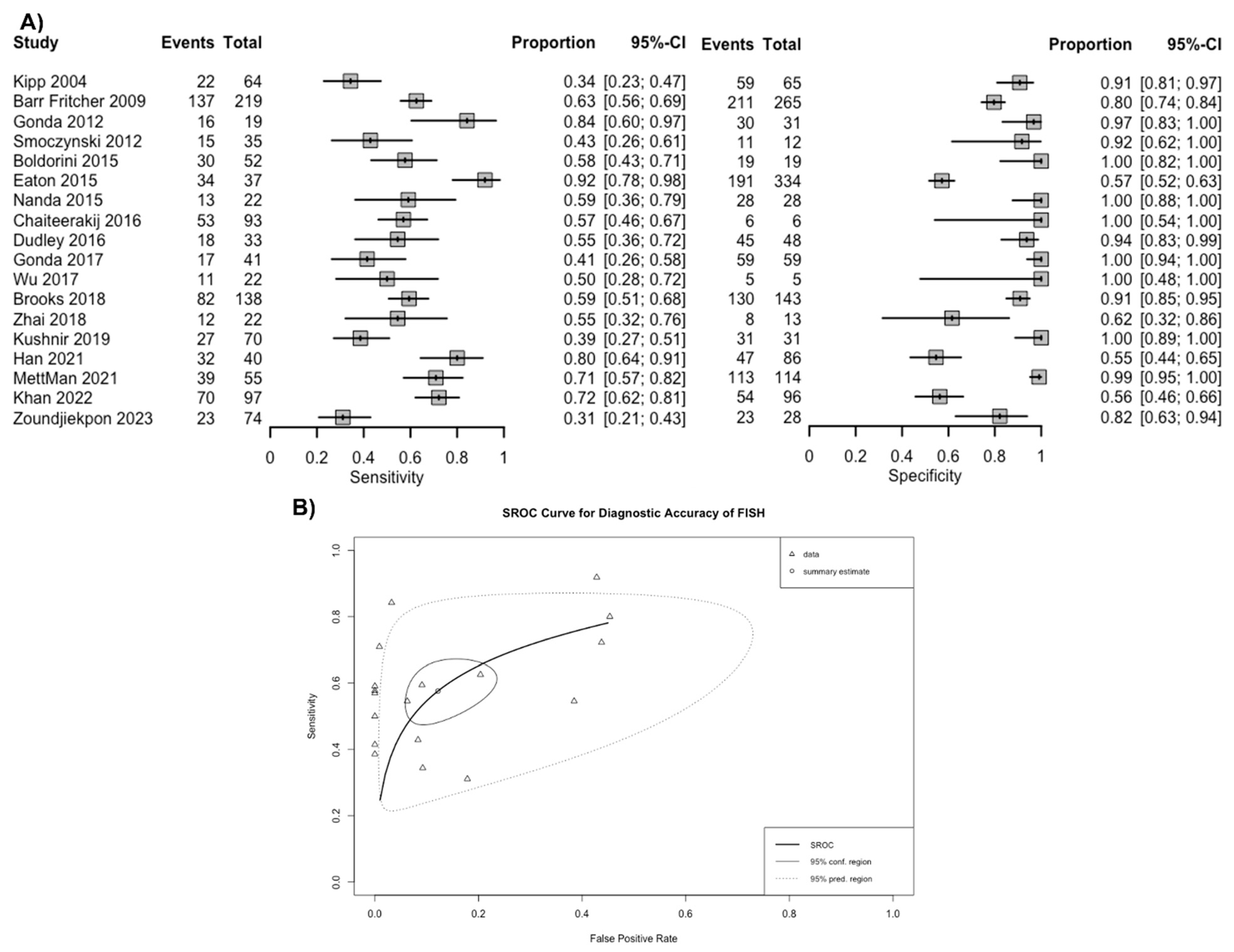
Overall, based on a positive threshold defined by individual studies, FISH had a sensitivity of 57.6% (95% CI 49.4–65.4%) and a specificity of 87.8% (95% CI 79.2–93.2%) for diagnosing malignancy in patients with biliary stricture (Figure 3A). The overall AUC was 0.76 (95% CI 0.74–0.78) (Figure 3B). The positive LR for FISH was 4.9 (95% CI 2.9–8.1), the negative LR was 0.49 (95% CI 0.40–0.57), and the DOR was 10.3 (95% CI 5.4–17.9). Based on the funnel plot and Deek’s linear regression test, publication bias was unlikely (p = 0.344) (Supplementary Materials S5).
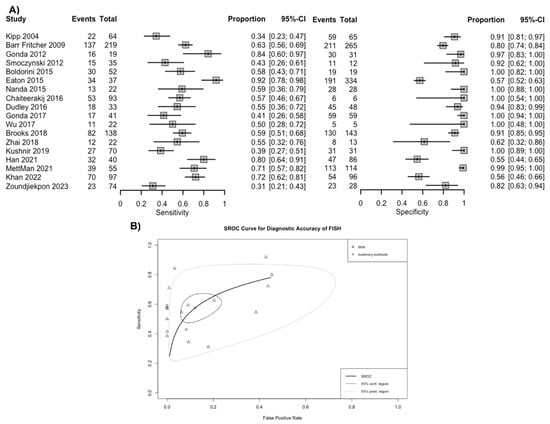
Figure 3.
(A) Sensitivity and specificity of fluorescence in situ hybridization (FISH) for the diagnosis of malignancy in biliary strictures and (B) summary receiver operating curves. Footnote: The spread of individual data points (triangles) and the size of the 95% prediction region compared to the confidence region both suggest there is notable heterogeneity [5,6,17,18,19,20,21,22,23,25,26,27,28,29,30,31,32].
3.3. Subgroup Analysis Based on FISH Definition
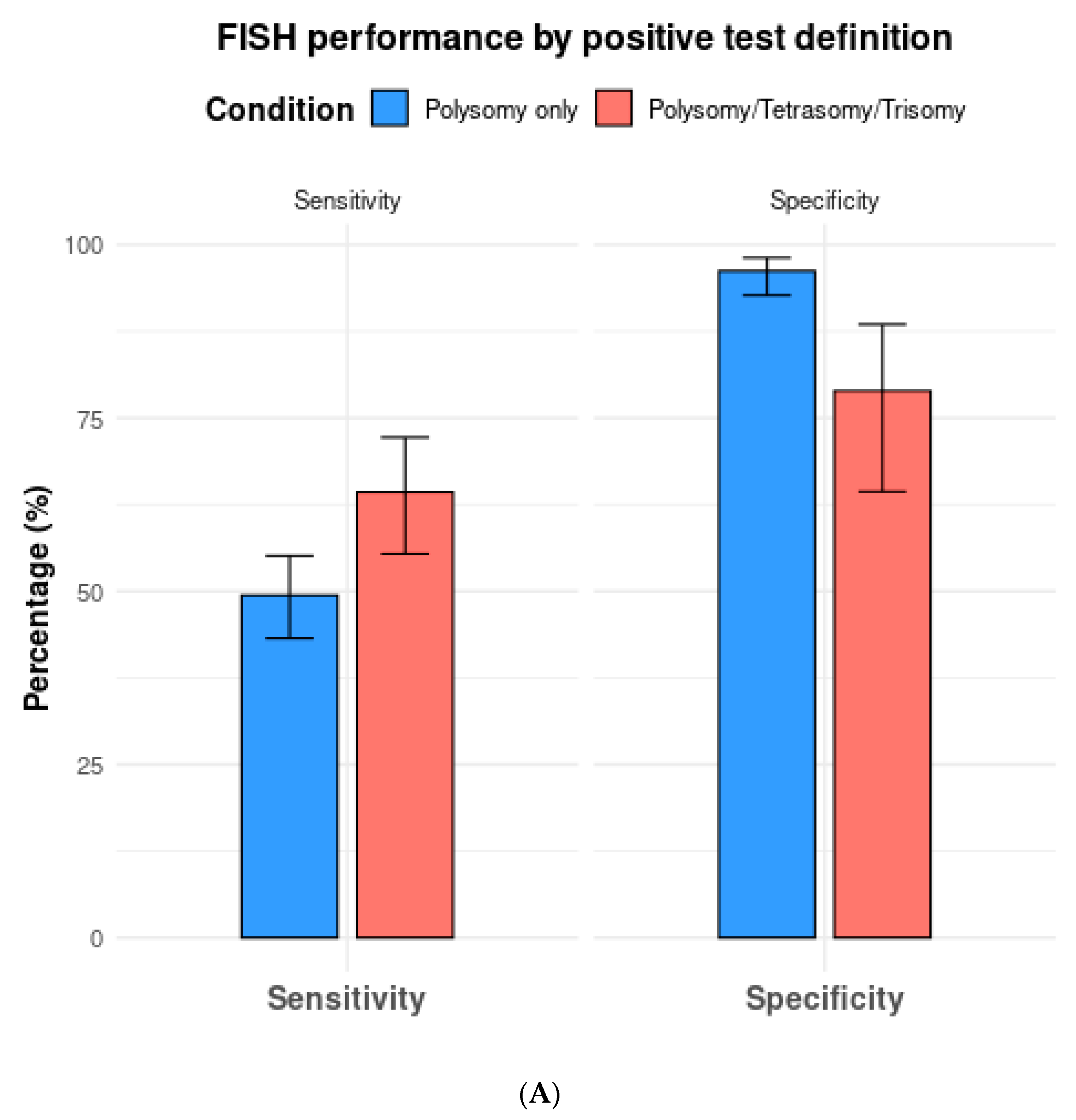
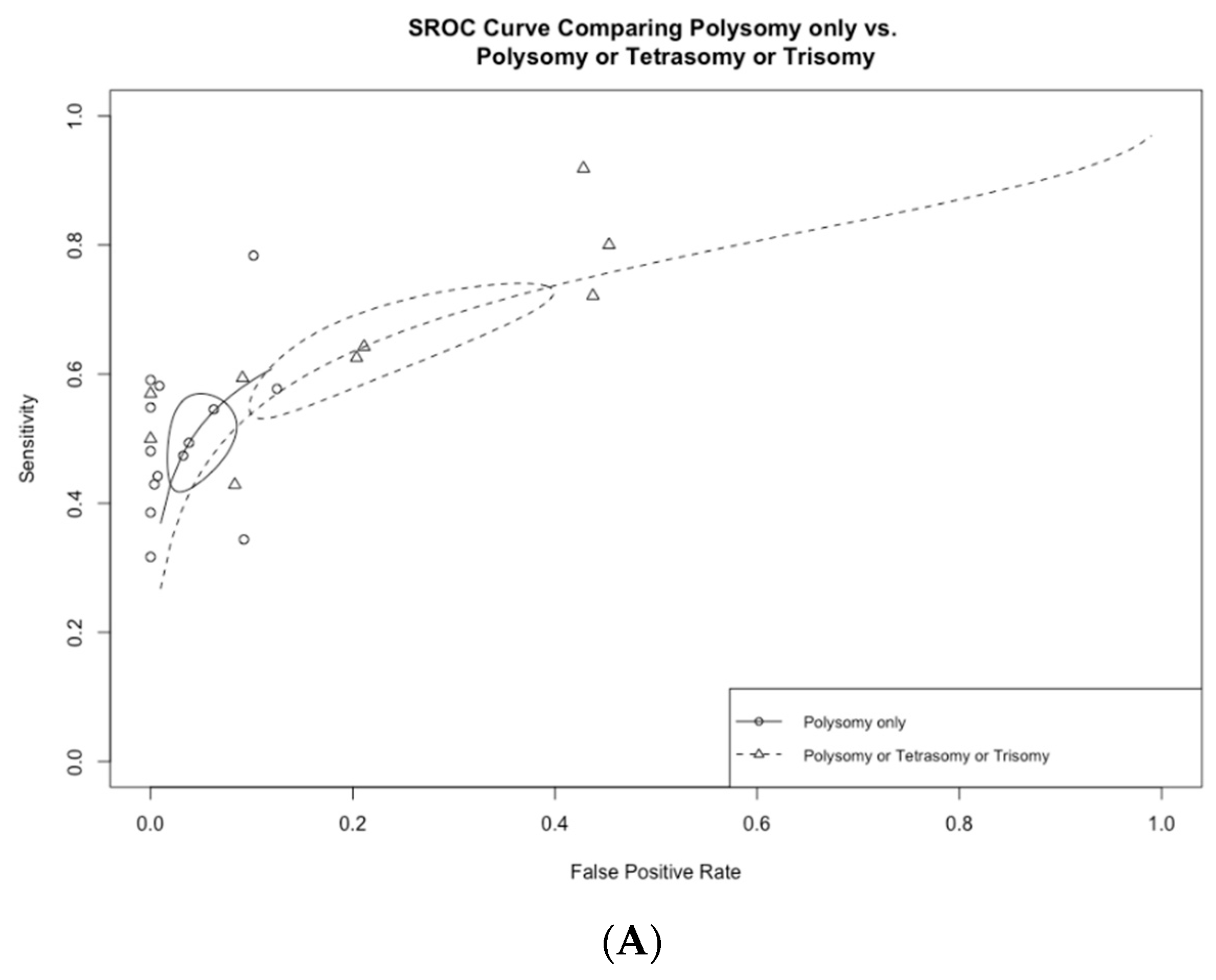
Among the 13 studies utilizing FISH polysomy as the threshold for diagnostic FISH, the overall test sensitivity was 49.4% (95% CI 43.2–55.5%), with a specificity of 96.2% (95% CI 92.7–98.1%) (Figure 4). The overall AUC was 0.74 (95% CI 0.72–0.76) (Figure 5A). On the other hand, in studies that considered polysomy, tetrasomy, or trisomy as positive FISH (n = 8), the overall sensitivity increased significantly (64.3%, 95% CI 55.4–72.2%) (Figure 4A). However, this increase in sensitivity was accompanied by a significant drop in specificity to 78.9% (95% CI 64.4–88.5%), with an overall AUC of 0.74 (95% CI 0.71–0.77).
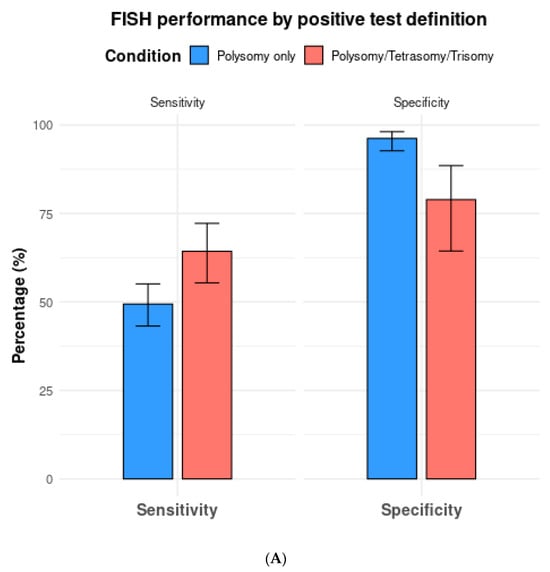
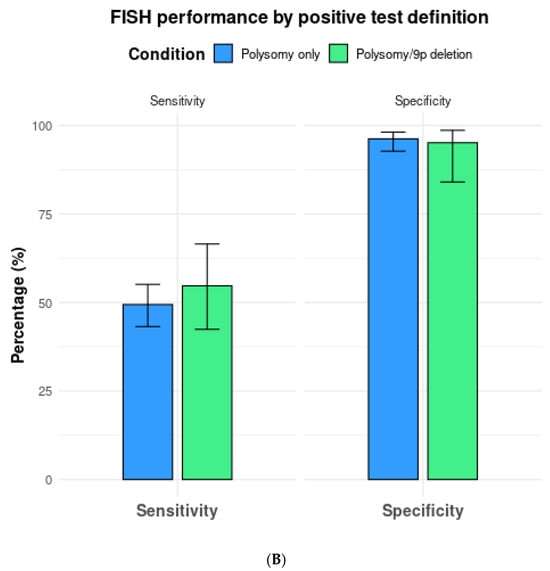
Figure 4.
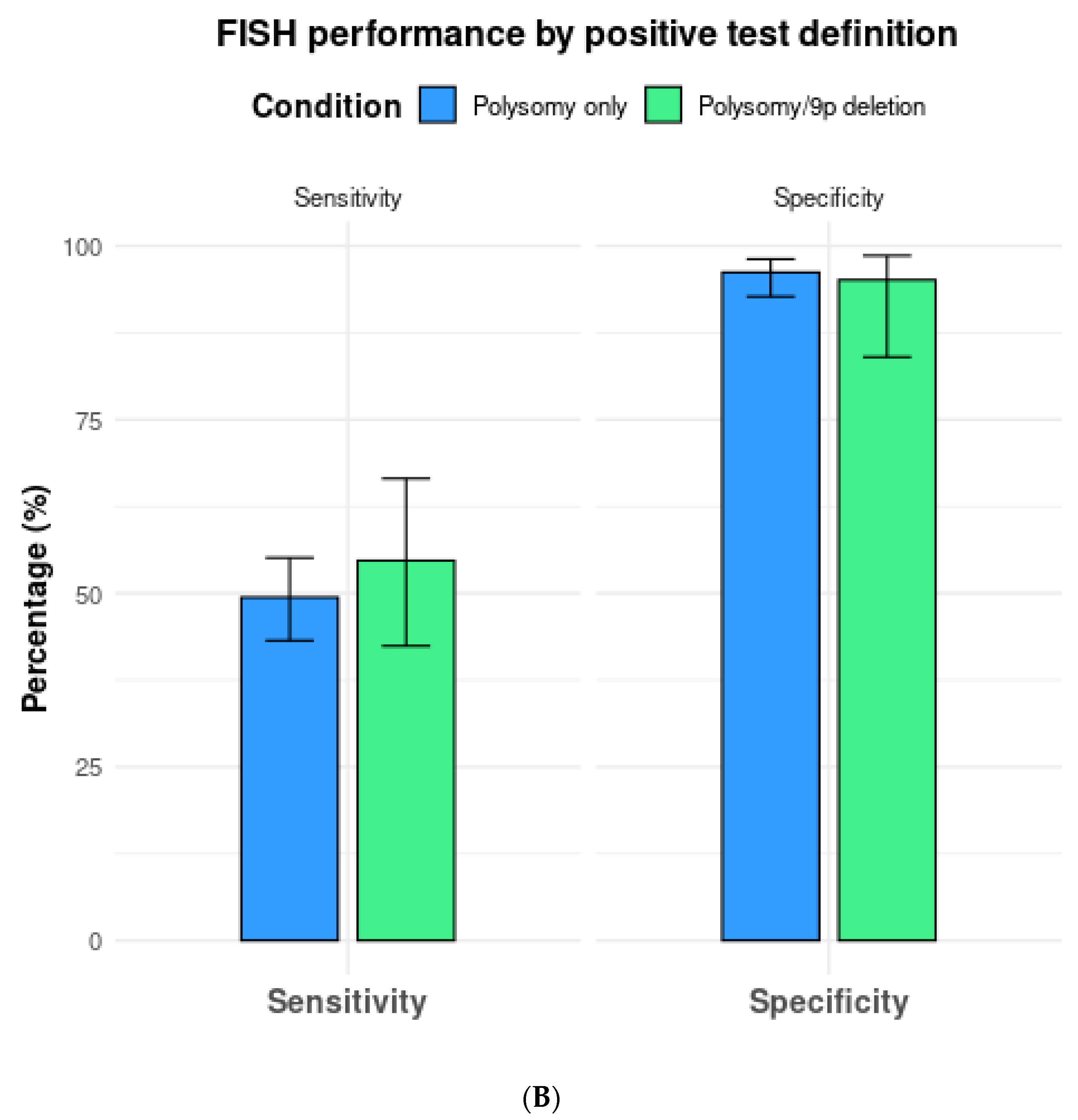
(A) Comparison of fluorescence in situ hybridization (FISH) performance based on positive test definitions of polysomy only vs. polysomy/tetrasomy/trisomy. (B) Comparison of fluorescence in situ hybridization (FISH) performance based on the positive test definitions of polysomy only vs. polysomy/9p deletion.
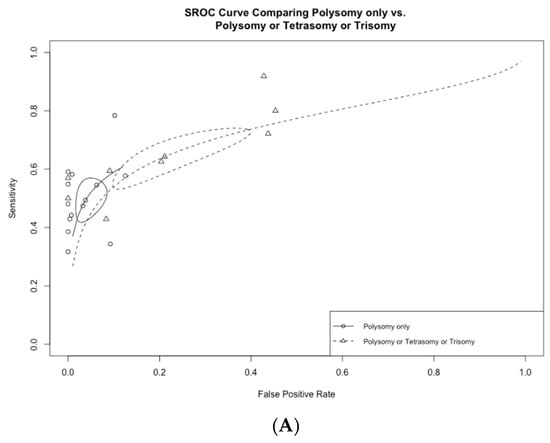
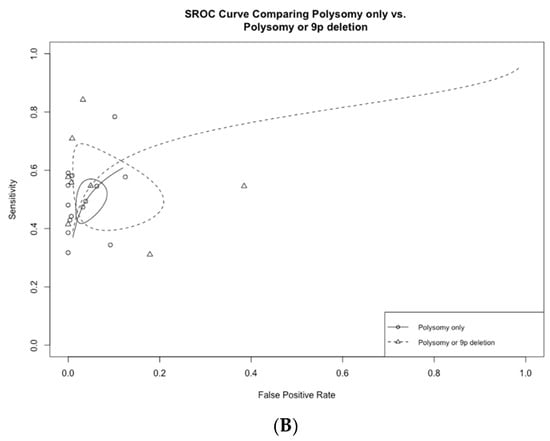
Figure 5.
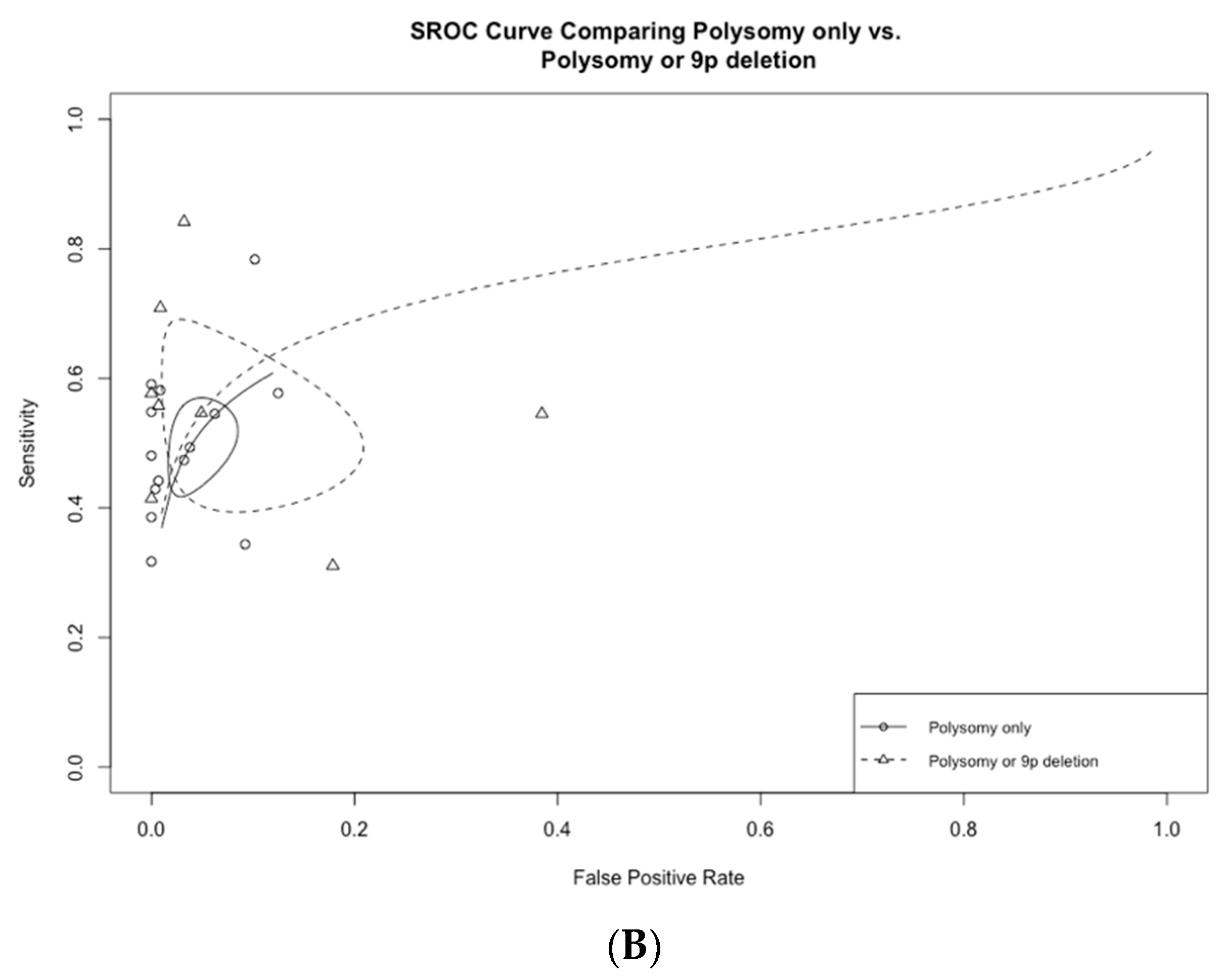
(A) Receiver operating curves comparing polysomy only vs. polysomy/tetrasomy/trisomy and (B) polysomy only vs. polysomy/9p deletion.
In a total of seven studies that included 9p deletions in addition to polysomy as the criteria for a positive test, overall sensitivity, specificity, and AUC were 54.7% (95% CI 42.4–66.5%), 95.1% (95% CI 84.0–98.6%), and 0.77 (0.73–0.81), respectively (Table 2, Figure 4B).

Table 2.
Diagnostic performance of fluorescence in situ hybridization (FISH) based on the definition of a positive test.
When only including studies that compared two or more cutoffs, the sensitivity and specificity of FISH with polysomy only as the diagnostic threshold (n = 5 studies) were 52.9% (95% CI 44.2–61.4%) and 96.3% (95% CI 87.6–99.0%), respectively. The respective sensitivity and specificity for polysomy, tetrasomy, or trisomy were 65.7% (95% CI 57.3–73.1%) and 77.1% (95% CI 59.0–88.8%) (Table 2).
Meanwhile, the sensitivity and specificity of FISH in polysomy only vs. polysomy/9p deletion (n = 5 studies) were 46.3% (95% CI 38.6–54.2%) and 98.3% (95% CI 96.0–99.3%) vs. 61.0% (95% CI 47.7–72.9%) and 98.4% (95% CI 96.0–99.4%).
For studies including only patients with PSC (n = 3) [5,18,22], the overall sensitivity, specificity, and AUC of a positive FISH in detecting malignancy for biliary stricture were 64.3% (95% CI 19.5–93.0%), 88.2% (95% CI 52.6–98.0%), and 0.85 (95% CI 0.77–0.93), respectively.
4. Discussion
Molecular testing by UroVysion® FISH has significantly improved the diagnosis of malignant biliary strictures and is currently recommended as part of multimodality sampling [4,33]. Although recommended, the definition of a diagnostic FISH test has not been standardized. Several abnormalities can be noted during the molecular FISH analysis (e.g., polysomy, tetrasomy, trisomy), thus creating a clinical dilemma that can dramatically affect a patient’s diagnosis and resulting management. In this systematic review and meta-analysis, the performance of FISH was found to vary significantly based on the definition of a positive test. A stricter threshold for diagnostic FISH tests, such as only considering polysomy, significantly increased specificity while maintaining sensitivity. Conversely, including tetrasomy or trisomy into the positive threshold significantly increased sensitivity but decreased specificity. Interestingly, adding 9p to the threshold improved sensitivity while maintaining specificity, compared to polysomy alone. Based on these findings, polysomy only or 9p deletion/polysomy should be considered as the criterion for defining a positive FISH test to improve diagnostic sensitivity while maintaining high specificity. It is important for molecular diagnostic testing for cancer to have a low rate of false-positive results (i.e., high specificity), which have significant clinical consequences. Thus, tetrasomy or trisomy should not be routinely regarded as a positive FISH test for malignancy based on the low specificity noted with this study.
The results of this meta-analysis reinforce the finding that FISH testing can increase the sensitivity of detecting malignant biliary strictures without reducing specificity [11]. Furthermore, FISH is known to be better at determining lesions deemed “suspicious for malignancy”. Despite the improved diagnostic performance, FISH testing should not be used as a standalone test; however, it is an excellent confirmatory test in addition to brush cytology for malignancy in patients with biliary stricture [24]. FISH results should be interpreted in the context of the individual patient and relevant clinical, laboratory (elevated CA19-9), and radiological features (e.g., dominant- or malignant-appearing stricture or an associated mass).
FISH polysomy and trisomy (most frequently CEP 7 probe) are the two most common FISH abnormalities within the biliary tract specimens. Cells demonstrating polysomy are chromosomally unstable by definition and hence are highly associated with malignancy. A polysomy FISH result is very specific for malignancy, with a PPV reaching 100% [12]. On the other hand, trisomy, particularly CEP 7, is observed in both neoplastic and non-neoplastic cells. The epidermal growth factor receptor gene is on chromosome 7 and is postulated to be involved in carcinogenesis. However, trisomy 7 has been observed in non-neoplastic inflammatory cells as well [34]. Thus, inclusion of trisomy into the definition of a positive test resulted in an increased sensitivity, with a significant drop in specificity. However, it must be noted that within the biliary tract, a subset, but not all, of patients with trisomy 7 will develop polysomy and, ultimately, CCA. Therefore, although not diagnostic, these patients should be closely monitored, and sampling should be repeated, as trisomy may reflect a pre-neoplastic state or dysplasia [34]. It must be noted that serial or multifocal polysomy is strongly associated with malignancy and should be factored into decision making in the context of the individual patient.
In this study, homozygous 9p deletion by FISH had a high specificity for malignancy, which was comparable to polysomy. In the FISH assay, the 9p21 probe spanned the P16 tumor suppressor gene, frequently inactivated in human cancers, including CCA [35]. Although rare (in one study, 4% of CCAs had homozygous 9p deletion), this finding should raise concern for malignancy, even in the absence of polysomy, as the homozygous loss of 9p21 would be expected to result in an absence of p16 expression.
Compared to a previous meta-analysis assessing the diagnostic accuracy of FISH that demonstrated a sensitivity of 68% and specificity of 70% for the diagnosis of cholangiocarcinoma in PSC patients [36], this study reported a lower sensitivity but higher specificity for the diagnosis of malignancy in PSC patients. Overall, the performance characteristics of FISH in PSC individuals was similar to the overall population. Of note, most of the studies included in the previous meta-analysis were reported from the same institution and had overlapping/similar time periods, thus presenting a serious risk of bias, such as double counting samples.
In addition to FISH, several molecular techniques have been developed over the last decade to personalize the diagnosis of malignancy in biliary strictures. NGS for the mutational analysis of both biliary brushings and bile has been demonstrated to be a sensitive diagnostic tool in both patients with and without PSC [37]. A 28-gene NGS panel demonstrated a diagnostic sensitivity of 75% for malignant strictures on both biliary brushings and biliary biopsies [38]. An additional advantage of NGS is the ability to identify actionable mutations that may guide therapy in these aggressive malignancies. Furthermore, the NGS of cell-free DNA in bile collected at the time of ERCP resulted in a sensitivity of 60% for the diagnosis of malignancy [39]. It must be noted that mutations may be seen in specimens from benign strictures, which creates a clinical dilemma regarding management, especially in the background of inflammation-induced cellular damage.
Aberrant DNA methylation, a key epigenetic event in carcinogenesis, has also been studied to aid in the diagnosis of malignant biliary strictures [40]. Digital droplet polymerase chain reaction (ddPCR)-based analysis of the methylation index of targeted genes has shown high sensitivity and specificity for malignancy in both brushings and bile specimens [41,42]. DNA methylation is purported to be less resource intensive and easier to perform than NGS and FISH. However, the clinical utility of these targeted assays can only be ascertained after external validations, which are ongoing [43]. Additionally, studies are needed to compare different molecular techniques and create appropriate decision trees for choosing the right test for the right clinical scenario.
Concurrently, advancements in per oral cholangioscopy for the direct visualization of the biliary tract has improved the classification of biliary strictures. Visual findings such as abnormal mucosal features, papillary projections, and abnormal vessels have been reported to have high sensitivity for the diagnosis of malignancy, with good interobserver agreement when using standard classification systems [44]. However per oral cholangioscopy has limited availability due to high cost and the technical expertise needed, with a trend towards higher adverse events in comparison with traditional ERCP-based sampling [45].
The strengths of this study include a rigorous selection of studies to avoid the risk of double counting. Furthermore, the study employed the most up-to-date DTA meta-analysis statistics endorsed by the Cochrane Collaboration [46]. However, there are several limitations to this study. All the FISH studies included in this meta-analysis used the UroVysion® probe set. A newer PB FISH test is purported to have an augmented diagnostic value, compared to the UroVysion® probe set, but there are limited published studies to perform a rigorous meta-analysis [33,47]. Studies using the PB FISH probe set were excluded, as these tests are not widely available and have inherent differences in performance characteristics; therefore, their inclusion would introduce significant bias in the meta-analysis. The notable heterogeneity observed based on prediction ellipses from SROC curves (Figure 3B) were likely a result of differences in the patient population, prevalence of malignancy, and thresholds used in each study. Additionally, a comparison of FISH performance with brush cytology or newer techniques, such as NGS, was not available, as the studies lacked paired data. Additionally, due to the lack of follow-up data, whether individuals with abnormalities other than polysomy went on to develop malignancy could not be ascertained and should be studied in future studies.
In conclusion, a positive FISH definition of polysomy or 9p deletion with polysomy has the best performance characteristics, with moderate sensitivity and high specificity for detecting malignancy among patients with biliary strictures. Given the low diagnostic specificity, FISH tetrasomy or trisomy should not be routinely considered to be a positive FISH result.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13216457/s1, Supplementary Materials S1. PRISMA-DTA Checklist [48]. Supplementary Materials S2. Search Strategy. Supplementary Materials S3. Risk of Bias Assessment with the QUADAS-2 Tool. Supplementary Materials S4. Diagnostic Accuracy of FISH for detection of Malignancy. Supplementary Materials S5. Funnel plot with Deek’s Linear Test for Publication Bias (p = 0.572). Supplementary Materials S6. Summary receiver operating curves for only patients with PSC.
Author Contributions
Conceptualization, M.A., D.M.S. and V.C.; data curation, D.M.S., L.J.P. and V.C.; formal analysis, D.M.S.; methodology, M.A. and D.M.S.; project administration, V.C.; supervision, B.R.K. and V.C.; validation, E.G.B.F., A.S., M.A.C., G.J.G., J.E. and L.R.R.; writing—original draft, M.A. and D.M.S.; writing—review and editing, M.A., D.M.S., B.R.K., L.J.P., E.G.B.F., A.S., M.A.C., G.J.G., J.E., L.R.R. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
VC received funding through the NCI-funded Mayo Clinic SPORE in the Hepatobiliary Cancers (P50 CA210964) Developmental Research Program.
Institutional Review Board Statement
Institutional review board approval was not needed for this study due to the use of publicly available data.
Data Availability Statement
The data are available upon reasonable request from the first or corresponding author.
Conflicts of Interest
Vinay Chandrasekhara consults for Boston Scientific. He received grants from Micro-Tech Endoscopy and StarMed. He owns stock in Nevakar. The remaining authors have no conflicts to report.
Abbreviations
| CI | Confidence Interval |
| DOR | Diagnostic Odds Ratio |
| DTA | Diagnostic Test Accuracy |
| FISH | Fluorescence In Situ Hybridization |
| LR | Likelihood Ratio |
| PB | Pancreaticobiliary |
| PSC | Primary Sclerosing Cholangitis |
| ROC | Receiver Operating Characteristic Curve |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Studies-2 |
| SROC | Summary Receiver Operating Characteristic Curve |
References
- Dorrell, R.; Pawa, S.; Zhou, Y.; Lalwani, N.; Pawa, R. The Diagnostic Dilemma of Malignant Biliary Strictures. Diagnostics 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Tummala, P.; Munigala, S.; Eloubeidi, M.A.; Agarwal, B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: Prevalence of malignancy and potential role of EUS-FNA. J. Clin. Gastroenterol. 2013, 47, 532–537. [Google Scholar] [CrossRef]
- Burnett, A.S.; Calvert, T.J.; Chokshi, R.J. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J. Surg. Res. 2013, 184, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Elmunzer, B.J.; Maranki, J.L.; Gómez, V.; Tavakkoli, A.; Sauer, B.G.; Limketkai, B.N.; Brennan, E.A.; Attridge, E.M.; Brigham, T.J.; Wang, A.Y. ACG Clinical Guideline: Diagnosis and Management of Biliary Strictures. Off. J. Am. Coll. Gastroenterol.|ACG 2023, 118, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Kipp, B.R.; Stadheim, L.M.; Halling, S.A.; Pochron, N.L.; Harmsen, S.; Nagorney, D.M.; Sebo, T.J.; Therneau, T.M.; Gores, G.J.; de Groen, P.C.; et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am. J. Gastroenterol. 2004, 99, 1675–1681. [Google Scholar] [CrossRef]
- Han, S.; Tatman, P.; Mehrotra, S.; Wani, S.; Attwell, A.R.; Edmundowicz, S.A.; Brauer, B.C.; Wagh, M.S.; Hammad, H.T.; Shah, R.J. Combination of ERCP-Based Modalities Increases Diagnostic Yield for Biliary Strictures. Dig. Dis. Sci. 2021, 66, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.G.; Witt, B. Cytologic Diagnosis of Biliary Strictures: FISH or Cut the Sensitivity Rate? Dig. Dis. Sci. 2018, 63, 549–550. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; the PRISMA-DTA Group. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Deeks, J.J.; Bossuyt, P.M.; Leeflang, M.M.; Takwoingi, Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Cooley, M.A.; Schneider, A.R.; Barr Fritcher, E.G.; Milosevic, D.; Levy, M.J.; Bridgeman, A.R.; Martin, J.A.; Petersen, B.T.; Abu Dayyeh, B.K.; Storm, A.C.; et al. Utility of methylated DNA markers for the diagnosis of malignant biliary strictures. Hepatology 2024, 21, 21. [Google Scholar] [CrossRef]
- Barr Fritcher, E.G.; Voss, J.S.; Jenkins, S.M.; Lingineni, R.K.; Clayton, A.C.; Roberts, L.R.; Halling, K.C.; Talwalkar, J.A.; Gores, G.J.; Kipp, B.R. Primary sclerosing cholangitis with equivocal cytology: Fluorescence in situ hybridization and serum CA 19-9 predict risk of malignancy. Cancer Cytopathol. 2013, 121, 708–717. [Google Scholar] [CrossRef]
- Moreno Luna, L.E.; Kipp, B.; Halling, K.C.; Sebo, T.J.; Kremers, W.K.; Roberts, L.R.; Barr Fritcher, E.G.; Levy, M.J.; Gores, G.J. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 2006, 131, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Holmes, I.M.; Chen-Yost, H.I.; Smola, B.; Lew, M.; Betz, B.L.; Brown, N.A.; Pang, J. Performance of fluorescence in situ hybridization in biliary brushings with equivocal cytology: An institutional experience. J. Am. Soc. Cytopathol. 2024, 13, 285–290. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.W.; Choi, S.H.; Huh, J.; Park, S.H. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis. Korean J. Radiol. 2015, 16, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Barr Fritcher, E.G.; Kipp, B.R.; Halling, K.C.; Oberg, T.N.; Bryant, S.C.; Tarrell, R.F.; Gores, G.J.; Levy, M.J.; Clayton, A.C.; Sebo, T.J.; et al. A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures. Gastroenterology 2009, 136, 2180–2186. [Google Scholar] [CrossRef]
- Eaton, J.E.; Barr Fritcher, E.G.; Gores, G.J.; Atkinson, E.J.; Tabibian, J.H.; Topazian, M.D.; Gossard, A.A.; Halling, K.C.; Kipp, B.R.; Lazaridis, K.N. Biliary multifocal chromosomal polysomy and cholangiocarcinoma in primary sclerosing cholangitis. Am. J. Gastroenterol. 2015, 110, 299–309. [Google Scholar] [CrossRef]
- Nanda, A.; Brown, J.M.; Berger, S.H.; Lewis, M.M.; Barr Fritcher, E.G.; Gores, G.J.; Keilin, S.A.; Woods, K.E.; Cai, Q.; Willingham, F.F. Triple modality testing by endoscopic retrograde cholangiopancreatography for the diagnosis of cholangiocarcinoma. Ther. Adv. Gastroenterol. 2015, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.C.; Zheng, Z.; McDonald, T.; Le, L.J.P.; Dias-Santagata, D.; Borger, D.; Batten, J.; Vernovsky, K.; Sweeney, B.; Arpin, R.N.; et al. Next-Generation Sequencing and Fluorescence in Situ Hybridization Have Comparable Performance Characteristics in the Analysis of Pancreaticobiliary Brushings for Malignancy. J. Mol. Diagn. 2016, 18, 124–130. [Google Scholar] [CrossRef]
- Gonda, T.A.; Viterbo, D.; Gausman, V.; Kipp, C.; Sethi, A.; Poneros, J.M.; Gress, F.; Park, T.; Khan, A.; Jackson, S.A.; et al. Mutation Profile and Fluorescence In Situ Hybridization Analyses Increase Detection of Malignancies in Biliary Strictures. Clin. Gastroenterol. Hepatol. 2017, 15, 913–919.e911. [Google Scholar] [CrossRef]
- Brooks, C.; Gausman, V.; Kokoy-Mondragon, C.; Munot, K.; Amin, S.P.; Desai, A.; Kipp, C.; Poneros, J.; Sethi, A.; Gress, F.G.; et al. Role of Fluorescent In Situ Hybridization, Cholangioscopic Biopsies, and EUS-FNA in the Evaluation of Biliary Strictures. Dig. Dis. Sci. 2018, 63, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J. UroVysion Multi-Target Fluorescence in situ Hybridization Assay for the Detection of Malignant Bile Duct Brushing Specimens: A Comparison with Routine Cytology. Acta Cytol. 2018, 62, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, V.M.; Mullady, D.K.; Das, K.; Lang, G.; Hollander, T.G.; Murad, F.M.; Jackson, S.A.; Toney, N.A.; Finkelstein, S.D.; Edmundowicz, S.A. The Diagnostic Yield of Malignancy Comparing Cytology, FISH, and Molecular Analysis of Cell Free Cytology Brush Supernatant in Patients With Biliary Strictures Undergoing Endoscopic Retrograde Cholangiography (ERC): A Prospective Study. J. Clin. Gastroenterol. 2019, 53, 686–692. [Google Scholar] [CrossRef]
- Mettman, D.; Saeed, A.; Shold, J.; Laury, R.; Ly, A.; Khan, I.; Golem, S.; Olyaee, M.; O’Neil, M. Refined pancreatobiliary UroVysion criteria and an approach for further optimization. Cancer Med. 2021, 10, 5725–5738. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; la Sancha, C.; Saad, M.; Alkashash, A.; Ullah, A.; Alruwaii, F.; Velasquez Zarate, L.; Cramer, H.M.; Wu, H.H. The Role of Fluorescence In Situ Hybridization in Pancreatobiliary Brushing Cytology: A Large Retrospective Review with Histologic Correlation. Diagnostics 2022, 12, 2486. [Google Scholar] [CrossRef]
- Gonda, T.A.; Glick, M.P.; Sethi, A.; Poneros, J.M.; Palmas, W.; Iqbal, S.; Gonzalez, S.; Nandula, S.V.; Emond, J.C.; Brown, R.S.; et al. Polysomy and p16 deletion by fluorescence in situ hybridization in the diagnosis of indeterminate biliary strictures. Gastrointest. Endosc. 2012, 75, 74–79. [Google Scholar] [CrossRef]
- Smoczynski, M.; Jablonska, A.; Matyskiel, A.; Lakomy, J.; Dubowik, M.; Marek, I.; Biernat, W.; Limon, J. Routine brush cytology and fluorescence in situ hybridization for assessment of pancreatobiliary strictures. Gastrointest. Endosc. 2012, 75, 65–73. [Google Scholar] [CrossRef]
- Boldorini, R.; Paganotti, A.; Andorno, S.; Orlando, S.; Mercalli, F.; Orsello, M.; Ballarè, M.; Magnani, C.; Sartori, M. A multistep cytological approach for patients with jaundice and biliary strictures of indeterminate origin. J. Clin. Pathol. 2015, 68, 283–287. [Google Scholar] [CrossRef]
- Chaiteerakij, R.; Barr Fritcher, E.G.; Angsuwatcharakon, P.; Ridtitid, W.; Chaithongrat, S.; Leerapun, A.; Baron, T.H.; Kipp, B.R.; Henry, M.R.; Halling, K.C.; et al. Fluorescence in situ hybridization compared with conventional cytology for the diagnosis of malignant biliary tract strictures in Asian patients. Gastrointest. Endosc. 2016, 83, 1228–1235. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, X.; Yang, A.; Yao, F.; Guo, T.; Wu, D.; Wu, S.; Qiu, H.; Zhou, W.; Huo, Z.; et al. Fluorescence In Situ Hybridization with the UroVysion Kit for the Detection of Biliary Cancer in Chinese Patients. Clin. Lab. 2017, 63, 407–413. [Google Scholar] [CrossRef]
- Zoundjiekpon, V.D.; Falt, P.; Zapletalova, J.; Vanek, P.; Kurfurstova, D.; Slobodova, Z.; Skanderova, D.; Korinkova, G.; Skalicky, P.; Lovecek, M.; et al. Fluorescence In Situ Hybridization in Primary Diagnosis of Biliary Strictures: A Single-Center Prospective Interventional Study. Biomedicines 2023, 11, 755. [Google Scholar] [CrossRef]
- Baroud, S.; Sahakian, A.J.; Sawas, T.; Storm, A.C.; Martin, J.A.; Abu Dayyeh, B.K.; Topazian, M.D.; Levy, M.J.; Roberts, L.R.; Gores, G.J.; et al. Impact of trimodality sampling on detection of malignant biliary strictures compared with patients with primary sclerosing cholangitis. Gastrointest. Endosc. 2022, 95, 884–892. [Google Scholar] [CrossRef] [PubMed]
- DeHaan, R.D.; Kipp, B.R.; Smyrk, T.C.; Abraham, S.C.; Roberts, L.R.; Halling, K.C. An assessment of chromosomal alterations detected by fluorescence in situ hybridization and p16 expression in sporadic and primary sclerosing cholangitis-associated cholangiocarcinomas. Hum. Pathol. 2007, 38, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A. Cellular and molecular biology of biliary tract cancers. Surg. Oncol. Clin. N. Am. 2002, 11, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Njei, B.; Venkatesh, P.G.K.; Vargo, J.J.; Parsi, M.A. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: A systematic review and meta-analysis. Gastrointest. Endosc. 2014, 79, 943–950.e3. [Google Scholar] [CrossRef] [PubMed]
- Harbhajanka, A.; Michael, C.W.; Janaki, N.; Gokozan, H.N.; Wasman, J.; Bomeisl, P.; Yoest, J.; Sadri, N. Tiny but mighty: Use of next generation sequencing on discarded cytocentrifuged bile duct brushing specimens to increase sensitivity of cytological diagnosis. Mod. Pathol. 2020, 33, 2019–2025. [Google Scholar] [CrossRef]
- Singhi, A.D.; Nikiforova, M.N.; Chennat, J.; Papachristou, G.I.; Khalid, A.; Rabinovitz, M.; Das, R.; Sarkaria, S.; Ayasso, M.S.; Wald, A.I.; et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut 2020, 69, 52–61. [Google Scholar] [CrossRef]
- Arechederra, M.; Rullan, M.; Amat, I.; Oyon, D.; Zabalza, L.; Elizalde, M.; Latasa, M.U.; Mercado, M.R.; Ruiz-Clavijo, D.; Saldana, C.; et al. Next-generation sequencing of bile cell-free DNA for the early detection of patients with malignant biliary strictures. Gut 2022, 71, 1141–1151. [Google Scholar] [CrossRef]
- Nakaoka, T.; Saito, Y.; Saito, H. Aberrant DNA Methylation as a Biomarker and a Therapeutic Target of Cholangiocarcinoma. Int. J. Mol. Sci. 2017, 18, 1111. [Google Scholar] [CrossRef]
- Vedeld, H.M.; Grimsrud, M.M.; Andresen, K.; Pharo, H.D.; von Seth, E.; Karlsen, T.H.; Honne, H.; Paulsen, V.; Farkkila, M.A.; Bergquist, A.; et al. Early and accurate detection of cholangiocarcinoma in patients with primary sclerosing cholangitis by methylation markers in bile. Hepatology 2022, 75, 59–73. [Google Scholar] [CrossRef]
- Andresen, K.; Boberg, K.M.; Vedeld, H.M.; Honne, H.; Jebsen, P.; Hektoen, M.; Wadsworth, C.A.; Clausen, O.P.; Lundin, K.E.; Paulsen, V.; et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology 2015, 61, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Lapitz, A.; Fabris, L. Comparative performance of methylation DNA markers, brushing cytology, and FISH in diagnosing malignant biliary strictures. Hepatology 2024. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Tyberg, A.; Slivka, A.; Adler, D.G.; Desai, A.P.; Sejpal, D.V.; Pleskow, D.K.; Bertani, H.; Gan, S.I.; Shah, R.; et al. Digital Single-operator Cholangioscopy (DSOC) Improves Interobserver Agreement (IOA) and Accuracy for Evaluation of Indeterminate Biliary Strictures: The Monaco Classification. J. Clin. Gastroenterol. 2022, 56, e94–e97. [Google Scholar] [CrossRef]
- Sethi, A.; Chen, Y.K.; Austin, G.L.; Brown, W.R.; Brauer, B.C.; Fukami, N.N.; Khan, A.H.; Shah, R.J. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: A single-center experience. Gastrointest. Endosc. 2011, 73, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch VJ, H.W. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar]
- Ji, H.; Barr Fritcher, E.G.; Yin, J.; Bainter, T.M.; Zemla, T.J.; Gores, G.J.; Halling, K.C.; Kipp, B.R.; Roberts, L.R. Evaluating the Significance of Pancreatobiliary Fluorescence In Situ Hybridization Polysomy on Prognosis in De Novo Cholangiocarcinoma. Clin. Transl. Gastroenterol. 2022, 13, e00523. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. MetaArXiv 2020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).