Effect of Glucagon-like Peptide-1 Receptor Agonism on Aortic Valve Stenosis Risk: A Mendelian Randomization Analysis
Abstract
1. Introduction
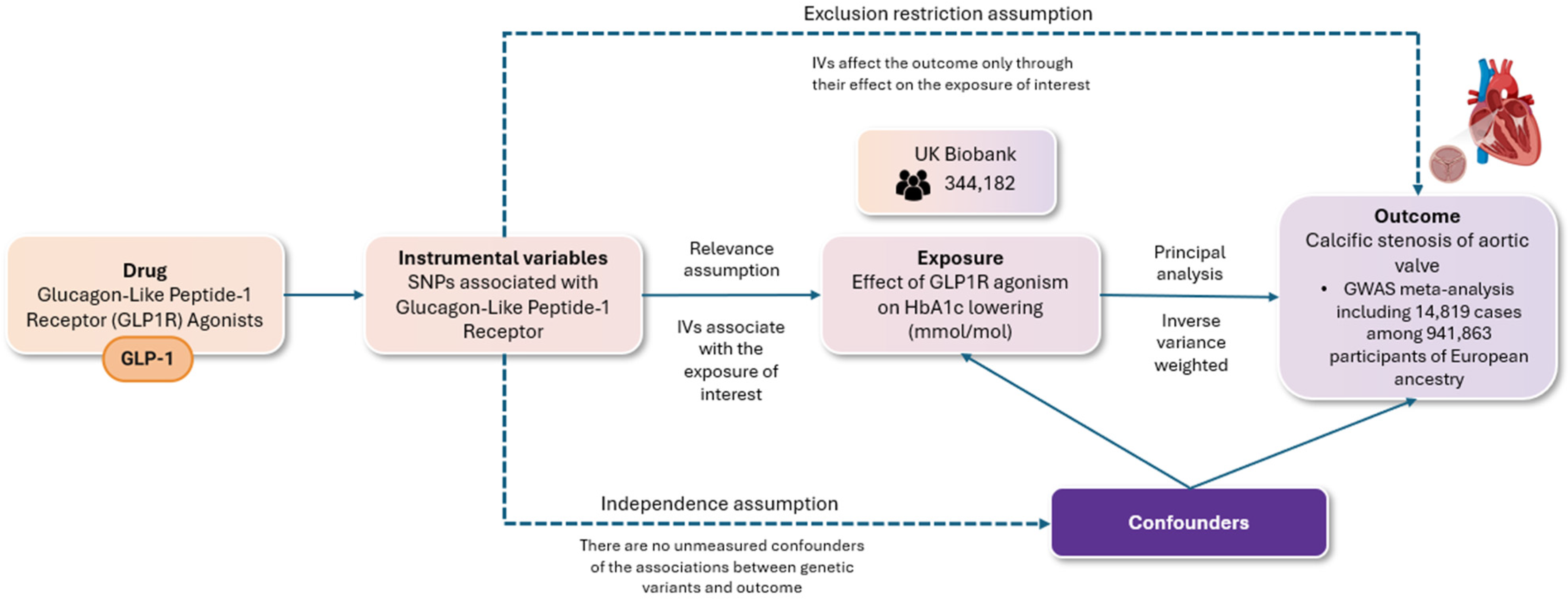
2. Materials and Methods
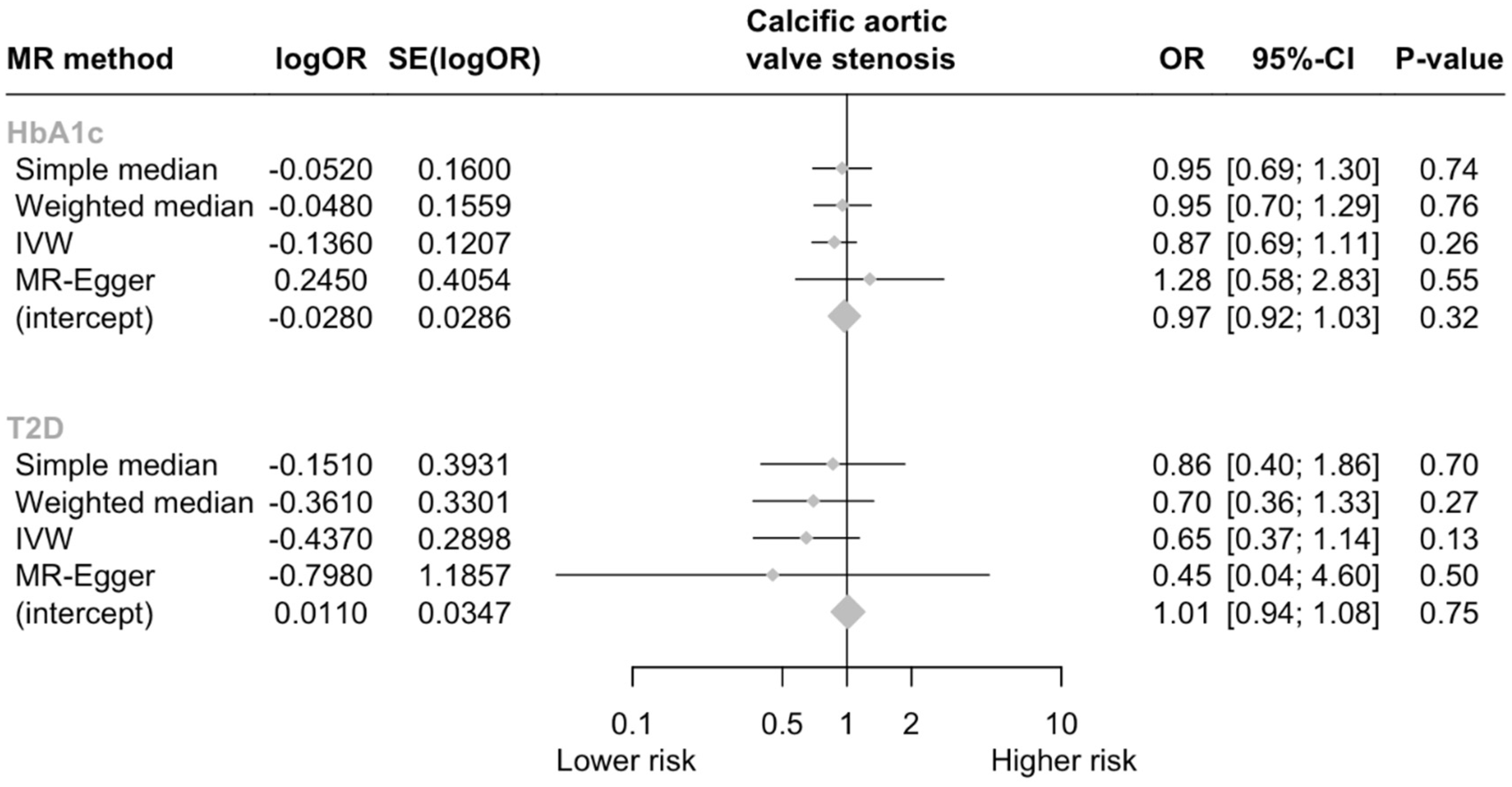
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andell, P.; Li, X.; Martinsson, A.; Andersson, C.; Stagmo, M.; Zöller, B.; Sundquist, K.; Smith, J.G. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart 2017, 103, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, R.; Aspelund, T.; Harris, T.B.; Gudnason, V. The prevalence of aortic stenosis in the elderly in Iceland and predictions for the coming decades: The AGES-Reykjavík study. Int. J. Cardiol. 2014, 176, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Martinsson, A.; Li, X.; Zöller, B.; Andell, P.; Andersson, C.; Sundquist, K.; Smith, J.G. Familial Aggregation of Aortic Valvular Stenosis: A Nationwide Study of Sibling Risk. Circ. Cardiovasc. Genet. 2017, 10, e001742. [Google Scholar] [CrossRef]
- Thanassoulis, G.; Campbell, C.Y.; Owens, D.S.; Smith, J.G.; Smith, A.V.; Peloso, G.M.; Kerr, K.F.; Pechlivanis, S.; Budoff, M.J.; Harris, T.B.; et al. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 2013, 368, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Thériault, S.; Dina, C.; Messika-Zeitoun, D.; Le Scouarnec, S.; Capoulade, R.; Gaudreault, N.; Rigade, S.; Li, Z.; Simonet, F.; Lamontagne, M.; et al. Genetic Association Analyses Highlight IL6, ALPL, and NAV1 As 3 New Susceptibility Genes Underlying Calcific Aortic Valve Stenosis. Circ. Genom. Precis. Med. 2019, 12, e002617. [Google Scholar] [CrossRef]
- Smith, J.G.; Luk, K.; Schulz, C.-A.; Engert, J.C.; Do, R.; Hindy, G.; Rukh, G.; Dufresne, L.; Almgren, P.; Owens, D.S.; et al. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA 2014, 312, 1764–1771. [Google Scholar] [CrossRef]
- Arsenault, B.J.; Boekholdt, S.M.; Dubé, M.-P.; Rhéaume, E.; Wareham, N.J.; Khaw, K.-T.; Sandhu, M.S.; Tardif, J.-C. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: A prospective Mendelian randomization study and replication in a case-control cohort. Circ. Cardiovasc. Genet. 2014, 7, 304–310. [Google Scholar] [CrossRef]
- Kaltoft, M.; Langsted, A.; Nordestgaard, B.G. Obesity as a Causal Risk Factor for Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2020, 75, 163–176. [Google Scholar] [CrossRef]
- D’Alessio, D. Is GLP-1 a hormone: Whether and When? J. Diabetes Investig. 2016, 7 (Suppl. 1), 50–55. [Google Scholar] [CrossRef]
- Deacon, C.F.; Holst, J.J. Immunoassays for the incretin hormones GIP and GLP-1. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Roohi, N. GLP-1, a powerful physiological incretin: An update. J. Biol. Regul. Homeost. Agents 2018, 32, 1171–1176. [Google Scholar] [PubMed]
- Nadkarni, P.; Chepurny, O.G.; Holz, G.G. Regulation of glucose homeostasis by GLP-1. Prog. Mol. Biol. Transl. Sci. 2014, 121, 23–65. [Google Scholar] [CrossRef] [PubMed]
- Mentlein, R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best. Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 443–452. [Google Scholar] [CrossRef]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Pyke, C.; Heller, R.S.; Kirk, R.K.; Ørskov, C.; Reedtz-Runge, S.; Kaastrup, P.; Hvelplund, A.; Bardram, L.; Calatayud, D.; Knudsen, L.B. GLP-1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014, 155, 1280–1290. [Google Scholar] [CrossRef]
- Marzook, A.; Tomas, A.; Jones, B. The Interplay of Glucagon-Like Peptide-1 Receptor Trafficking and Signalling in Pancreatic Beta Cells. Front. Endocrinol. 2021, 12, 678055. [Google Scholar] [CrossRef]
- Ahrén, B.; Yamada, Y.; Seino, Y. The Insulin Response to Oral Glucose in GIP and GLP-1 Receptor Knockout Mice: Review of the Literature and Stepwise Glucose Dose Response Studies in Female Mice. Front. Endocrinol. 2021, 12, 665537. [Google Scholar] [CrossRef]
- Sheikh, A. Direct cardiovascular effects of glucagon like peptide-1. Diabetol. Metab. Syndr. 2013, 5, 47. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 Receptor Agonists for the Reduction of Atherosclerotic Cardiovascular Risk in Patients with Type 2 Diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Bernal-López, M.R.; Gómez-Huelgas, R. Glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors combination therapy versus monotherapy and major adverse cardiovascular events: Do the benefits add up? Eur. J. Intern. Med. 2024, 96, 26–33. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Bernal-López, M.R.; Gómez-Huelgas, R. Therapeutic Potential of Sodium-glucose Co-transporter-2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists for Patients with Acute Coronary Syndrome: A Review of Clinical Evidence. Curr. Pharm. Des. 2024, 30, 2109–2119. [Google Scholar] [CrossRef]
- de Graaf, C.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.-M.J.-M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-like peptide-1 and its class B G protein-coupled receptors: A long march to therapeutic successes. Pharmacol. Rev. 2016, 68, 954–1013. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Sagris, M.; Koufakis, T.; Vlachakis, P.K.; Rangraze, I.R.; El Tanani, M.; Tsioufis, K.; et al. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure? Biomedicines 2024, 12, 2112. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Tzeis, S.; Fragakis, N. Glucagon-Like Peptide-1 Receptor Agonists and Atrial Fibrillation Recurrence After Ablation: A Fire Without the Smoke? Clin. Electrophysiol. 2024, 10, 1940–1941. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef]
- Xiao, F.; Zha, Q.; Zhang, Q.; Wu, Q.; Chen, Z.; Yang, Y.; Yang, K.; Liu, Y. Decreased Glucagon-Like Peptide-1 Is Associated With Calcific Aortic Valve Disease: GLP-1 Suppresses the Calcification of Aortic Valve Interstitial Cells. Front. Cardiovasc. Med. 2021, 8, 709741. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, Z.; Wang, M.; Zhang, Z.; Tan, C.; Yu, J.; Bi, Y.; Liao, X.; Zhou, X.; Ali Sheikh, M.S.; et al. Liraglutide Attenuates Aortic Valve Calcification in a High-Cholesterol-Diet-Induced Experimental Calcific Aortic Valve Disease Model in Apolipoprotein E-Deficient Mice. J. Cardiovasc. Dev. Dis. 2023, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Daghlas, I.; Karhunen, V.; Ray, D.; Zuber, V.; Burgess, S.; Tsao, P.S.; Lynch, J.A.; Lee, K.M.; Voight, B.F.; Chang, K.-M.; et al. Genetic evidence for repurposing of GLP1R (Glucagon-like peptide-1 receptor) agonists to prevent heart failure. J. Am. Heart Assoc. 2021, 10, e020331. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic, M.; Keaton, J.M.; Lynch, J.A.; Miller, D.R.; Zhou, J.; Tcheandjieu, C.; Huffman, J.E.; Assimes, T.L.; Lorenz, K.; Zhu, X.; et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 2020, 52, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Rapid GWAS of Thousands of Phenotypes for 337,000 Samples in the UK Biobank—Neale Lab n.d. Available online: https://www.nealelab.is/blog/2017/7/19/rapid-gwas-of-thousands-of-phenotypes-for-337000-samples-in-the-uk-biobank (accessed on 19 May 2024).
- Yu Chen, H.; Dina, C.; Small, A.M.; Shaffer, C.M.; Levinson, R.T.; Helgadóttir, A.; Capoulade, R.; Munter, H.M.; Martinsson, A.; Cairns, B.J.; et al. Dyslipidemia, inflammation, calcification, and adiposity in aortic stenosis: A genome-wide study. Eur. Heart J. 2023, 44, 1927–1939. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res 2019, 4, 186. [Google Scholar] [CrossRef]
- Blaser, M.C.; Kraler, S.; Lüscher, T.F.; Aikawa, E. Multi-Omics Approaches to Define Calcific Aortic Valve Disease Pathogenesis. Circ. Res. 2021, 128, 1371–1397. [Google Scholar] [CrossRef]
- Goody, P.R.; Hosen, M.R.; Christmann, D.; Niepmann, S.T.; Zietzer, A.; Adam, M.; Bönner, F.; Zimmer, S.; Nickenig, G.; Jansen, F. Aortic Valve Stenosis: From Basic Mechanisms to Novel Therapeutic Targets. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 885–900. [Google Scholar] [CrossRef]
- Zheng, K.H.; Tzolos, E.; Dweck, M.R. Pathophysiology of Aortic Stenosis and Future Perspectives for Medical Therapy. Cardiol. Clin. 2020, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tessler, I.; Albuisson, J.; Piñeiro-Sabarís, R.; Verstraeten, A.; Kamber Kaya, H.E.; Siguero-Álvarez, M.; Goudot, G.; MacGrogan, D.; Luyckx, I.; Shpitzen, S.; et al. Novel Association of the NOTCH Pathway Regulator MIB1 Gene With the Development of Bicuspid Aortic Valve. JAMA Cardiol. 2023, 8, 721–731. [Google Scholar] [CrossRef]
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005, 437, 270–274. [Google Scholar] [CrossRef]
- Majumdar, U.; Manivannan, S.; Basu, M.; Ueyama, Y.; Blaser, M.C.; Cameron, E.; McDermott, M.R.; Lincoln, J.; Cole, S.E.; Wood, S.; et al. Nitric oxide prevents aortic valve calcification by S-nitrosylation of USP9X to activate NOTCH signaling. Sci. Adv. 2021, 7, eabe3706. [Google Scholar] [CrossRef] [PubMed]
- Gee, T.; Farrar, E.; Wang, Y.; Wu, B.; Hsu, K.; Zhou, B.; Butcher, J. NFκB (Nuclear Factor κ-Light-Chain Enhancer of Activated B Cells) Activity Regulates Cell-Type-Specific and Context-Specific Susceptibility to Calcification in the Aortic Valve. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 638–655. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Merryman, W.D. Unloading the Stenotic Path to Identifying Medical Therapy for Calcific Aortic Valve Disease: Barriers and Opportunities. Circulation 2021, 143, 1455–1457. [Google Scholar] [CrossRef]
- Rodriguez-Valadez, J.M.; Tahsin, M.; Fleischmann, K.E.; Masharani, U.; Yeboah, J.; Park, M.; Li, L.; Weber, E.; Li, Y.; Berkalieva, A.; et al. Cardiovascular and Renal Benefits of Novel Diabetes Drugs by Baseline Cardiovascular Risk: A Systematic Review, Meta-analysis, and Meta-regression. Diabetes Care 2023, 46, 1300–1310. [Google Scholar] [CrossRef]
- Neves, J.S.; Packer, M.; Ferreira, J.P. Increased Risk of Heart Failure Hospitalization With GLP-1 Receptor Agonists in Patients With Reduced Ejection Fraction: A Meta-Analysis of the EXSCEL and FIGHT Trials. J. Card. Fail. 2023, 29, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE(-/-) and LDLr(-/-) Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef]
- Scisciola, L.; Rizzo, M.R.; Cataldo, V.; Fontanella, R.A.; Balestrieri, M.L.; D’Onofrio, N.; Marfella, R.; Paolisso, G.; Barbieri, M. Incretin drugs effect on epigenetic machinery: New potential therapeutic implications in preventing vascular diabetic complications. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 16489–16503. [Google Scholar] [CrossRef]
- Krasner, N.M.; Ido, Y.; Ruderman, N.B.; Cacicedo, J.M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE 2014, 9, e97554. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Bu, R.; Yang, Q.; Jia, J.; Li, T.; Wang, Q.; Chen, Y. Exendin-4 Protects against Hyperglycemia-Induced Cardiomyocyte Pyroptosis via the AMPK-TXNIP Pathway. J. Diabetes Res. 2019, 2019, 8905917. [Google Scholar] [CrossRef]
- Yue, W.; Li, Y.; Ou, D.; Yang, Q. The GLP-1 receptor agonist liraglutide protects against oxidized LDL-induced endothelial inflammation and dysfunction via KLF2. IUBMB Life 2019, 71, 1347–1354. [Google Scholar] [CrossRef]
- Ridker, P.M.; Thuren, T.; Zalewski, A.; Libby, P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 2011, 162, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, L.A.; Doverspike, A.; Hentosz, T.; Zourelias, L.; Shen, Y.-T.; Elahi, D.; Shannon, R.P. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J. Pharmacol. Exp. Ther. 2005, 312, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Younce, C.W.; Burmeister, M.A.; Ayala, J.E. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a. Am. J. Physiol.-Cell Physiol. 2013, 304, C508–C5018. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Jensen, J.K.; Binderup, T.; Grandjean, C.E.; Bentsen, S.; Ripa, R.S.; Kjaer, A. Semaglutide reduces vascular inflammation investigated by PET in a rabbit model of advanced atherosclerosis. Atherosclerosis 2022, 352, 88–95. [Google Scholar] [CrossRef]
- Ripa, R.S.; Zobel, E.H.; von Scholten, B.J.; Jensen, J.K.; Binderup, T.; Diaz, L.J.; Curovic, V.R.; Hansen, T.W.; Rossing, P.; Kjaer, A. Effect of Liraglutide on Arterial Inflammation Assessed as [(18)F]FDG Uptake in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Circ. Cardiovasc. Imaging 2021, 14, e012174. [Google Scholar] [CrossRef]
- Ray, K.K.; Ference, B.A.; Séverin, T.; Blom, D.; Nicholls, S.J.; Shiba, M.H.; Almahmeed, W.; Alonso, R.; Daccord, M.; Ezhov, M.; et al. World Heart Federation Cholesterol Roadmap 2022. Glob. Heart 2022, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- El Bekay, R.; Coín-Aragüez, L.; Fernández-García, D.; Oliva-Olivera, W.; Bernal-López, R.; Clemente-Postigo, M.; Delgado-Lista, J.; Diaz-Ruiz, A.; Guzman-Ruiz, R.; Vázquez-Martínez, R.; et al. Effects of glucagon-like peptide-1 on the differentiation and metabolism of human adipocytes. Br. J. Pharmacol. 2016, 173, 1820–1834. [Google Scholar] [CrossRef]
- Hu, Y.-W.; Yang, J.-Y.; Ma, X.; Chen, Z.-P.; Hu, Y.-R.; Zhao, J.-Y.; Li, S.-F.; Qiu, Y.-R.; Lu, J.-B.; Wang, Y.-C.; et al. A lincRNA-DYNLRB2-2/GPR119/GLP-1R/ABCA1-dependent signal transduction pathway is essential for the regulation of cholesterol homeostasis. J. Lipid Res. 2014, 55, 681–697. [Google Scholar] [CrossRef]
- Tashiro, Y.; Sato, K.; Watanabe, T.; Nohtomi, K.; Terasaki, M.; Nagashima, M.; Hirano, T. A glucagon-like peptide-1 analog liraglutide suppresses macrophage foam cell formation and atherosclerosis. Peptides 2014, 54, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Marfella, R.; Esposito, A.; Rizzo, M.R.; Angellotti, E.; Mauro, C.; Siniscalchi, M.; Chirico, F.; Caiazzo, P.; Furbatto, F.; et al. Incretin treatment and atherosclerotic plaque stability: Role of adiponectin/APPL1 signaling pathway. J. Diabetes Complicat. 2017, 31, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Joharapurkar, A.; Kshirsagar, S.; Sutariya, B.; Patel, M.; Pandey, D.; Patel, H.; Ranvir, R.; Kadam, S.; Patel, D.; et al. Coagonist of GLP-1 and glucagon decreases liver inflammation and atherosclerosis in dyslipidemic condition. Chem. Biol. Interact. 2018, 282, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Drucker, D.J. Glucagon-like Peptide-1 Receptor-based Therapeutics for Metabolic Liver Disease. Endocr. Rev. 2023, 44, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Kopytek, M.; Mazur, P.; Ząbczyk, M.; Undas, A.; Natorska, J. Diabetes concomitant to aortic stenosis is associated with increased expression of NF-κB and more pronounced valve calcification. Diabetologia 2021, 64, 2562–2574. [Google Scholar] [CrossRef]
- Ko, T.-Y.; Lin, T.-T.; Hsu, J.C.; Yang, Y.-Y.; Chuang, S.-L.; Lin, L.-Y.; Kao, H.-L.; Ho, Y.-L. Incidence, risk factors and predictors of cardiovascular mortality for aortic stenosis among patients with diabetes mellitus. Diabetes Res. Clin. Pract. 2022, 191, 110050. [Google Scholar] [CrossRef]
- Gupta, R.; Mahmoudi, E.; Behnoush, A.H.; Khalaji, A.; Malik, A.H.; Sood, A.; Bandyopadhyay, D.; Zaid, S.; Goel, A.; Sreenivasan, J.; et al. Effect of BMI on patients undergoing transcatheter aortic valve implantation: A systematic review and meta-analysis. Prog. Cardiovasc. Dis. 2023, 78, 58–66. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization (STROBE-MR) Statement. JAMA 2021. under review. [Google Scholar]
| Glycated Hemoglobin | Type 2 Diabetes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Chr | Position | EA | OA | EAF | Beta | SE | p | Beta | SE | p |
| rs10305420 | 6 | 39016636 | T | C | 0.39 | −0.051 | 0.016 | 1.30 × 10−3 | −0.032 | 0.004 | 5.11 × 10−14 |
| rs75151020 | 6 | 39031592 | C | A | 0.09 | 0.119 | 0.026 | 7.08 × 10−6 | 0.041 | 0.007 | 1.37 × 10−9 |
| rs2268647 | 6 | 39043178 | T | C | 0.52 | 0.066 | 0.015 | 1.51 × 10−5 | 0.021 | 0.004 | 4.95 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakasis, P.; Patoulias, D.; Giannakoulas, G.; Sagris, M.; Theofilis, P.; Fragakis, N.; Biondi-Zoccai, G. Effect of Glucagon-like Peptide-1 Receptor Agonism on Aortic Valve Stenosis Risk: A Mendelian Randomization Analysis. J. Clin. Med. 2024, 13, 6411. https://doi.org/10.3390/jcm13216411
Karakasis P, Patoulias D, Giannakoulas G, Sagris M, Theofilis P, Fragakis N, Biondi-Zoccai G. Effect of Glucagon-like Peptide-1 Receptor Agonism on Aortic Valve Stenosis Risk: A Mendelian Randomization Analysis. Journal of Clinical Medicine. 2024; 13(21):6411. https://doi.org/10.3390/jcm13216411
Chicago/Turabian StyleKarakasis, Paschalis, Dimitrios Patoulias, George Giannakoulas, Marios Sagris, Panagiotis Theofilis, Nikolaos Fragakis, and Giuseppe Biondi-Zoccai. 2024. "Effect of Glucagon-like Peptide-1 Receptor Agonism on Aortic Valve Stenosis Risk: A Mendelian Randomization Analysis" Journal of Clinical Medicine 13, no. 21: 6411. https://doi.org/10.3390/jcm13216411
APA StyleKarakasis, P., Patoulias, D., Giannakoulas, G., Sagris, M., Theofilis, P., Fragakis, N., & Biondi-Zoccai, G. (2024). Effect of Glucagon-like Peptide-1 Receptor Agonism on Aortic Valve Stenosis Risk: A Mendelian Randomization Analysis. Journal of Clinical Medicine, 13(21), 6411. https://doi.org/10.3390/jcm13216411









