Oldie but Goldie: The Fundamental Role of Radioiodine in the Management of Thyroid Cancer
Abstract
1. Introduction
2. Methods
3. Diagnostic Functional Imaging
4. Postoperative Iodine-131 Therapy (p-RIT)
- Tumor diameter greater than 20 mm: The risk of locoregional and/or distant metastatic disease at diagnosis/initial treatment increases with tumor size, becoming significant for lesions > 2 cm [8,51,52,53,54,55]. The ATA guidelines also report that, in these patients, the risk of structural disease persistence/recurrence (and therefore a more severe prognosis) increases significantly, rising from 1 to 2% in unifocal microcarcinomas to 5% for tumors measuring between 2 and 4 cm [10].
- Presence of thyroid residue and/or locoregional metastases on postoperative ultrasound and/or functional imaging: Thyroid residue visible on morphological and/or functional imaging (indicating a suboptimal surgery) increases the risk of disease persistence/recurrence, which grows as its size increases, and also limits the diagnostic value of thyroglobulin (Tg) during follow-up. Additionally, the identification of locoregional lymph node metastases on ultrasound imaging indicates radioiodine therapy (with a therapeutic intent) [37].
- Measurable postoperative basal and/or stimulated thyroglobulin; positive anti-thyroglobulin antibodies: RIT should also be considered for patients with basal and/or stimulated Tg (endogenous or exogenous stimulation using recombinant TSH, rhTSH) above the local institutional cut-off and, in any case, above 2 and 5 ng/mL, respectively [10,16,58,59]. Moreover, RIT should always be considered in patients with positive anti-thyroglobulin antibodies (AbTg). In fact, the positivity of AbTg makes the follow-up of patients more difficult and less reliable, limiting both the diagnostic accuracy and the clinical significance of Tg measurements [10,16,58,59]. The AbTg value is also important since the commonly reported reference cut-offs refer to the adult population with an intact thyroid. Therefore, the use of the Limit of Quantification (LoQ) or the Limit of Detection (LoD) has been proposed to exclude the presence of potentially interfering AbTg [60,61].
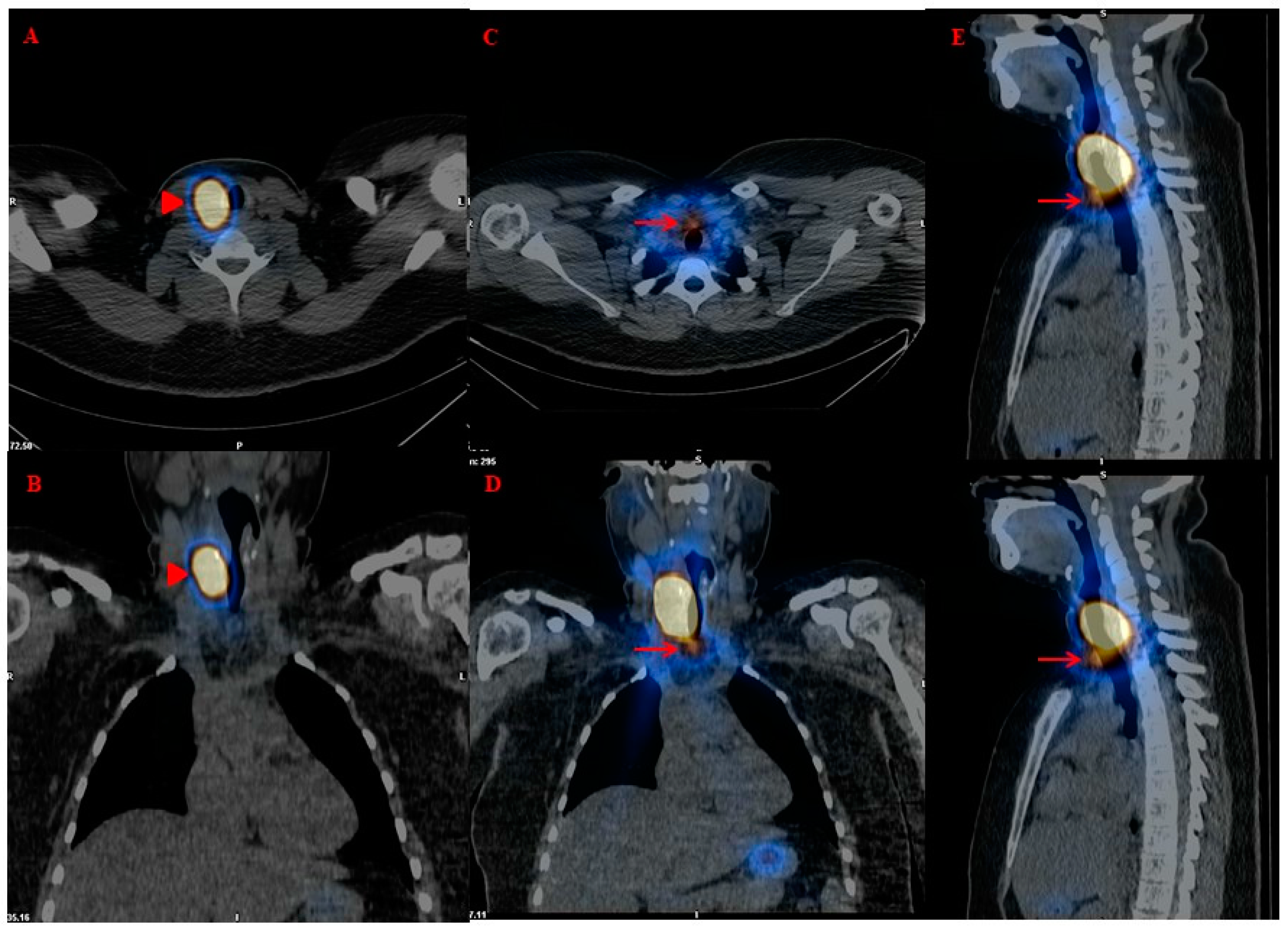
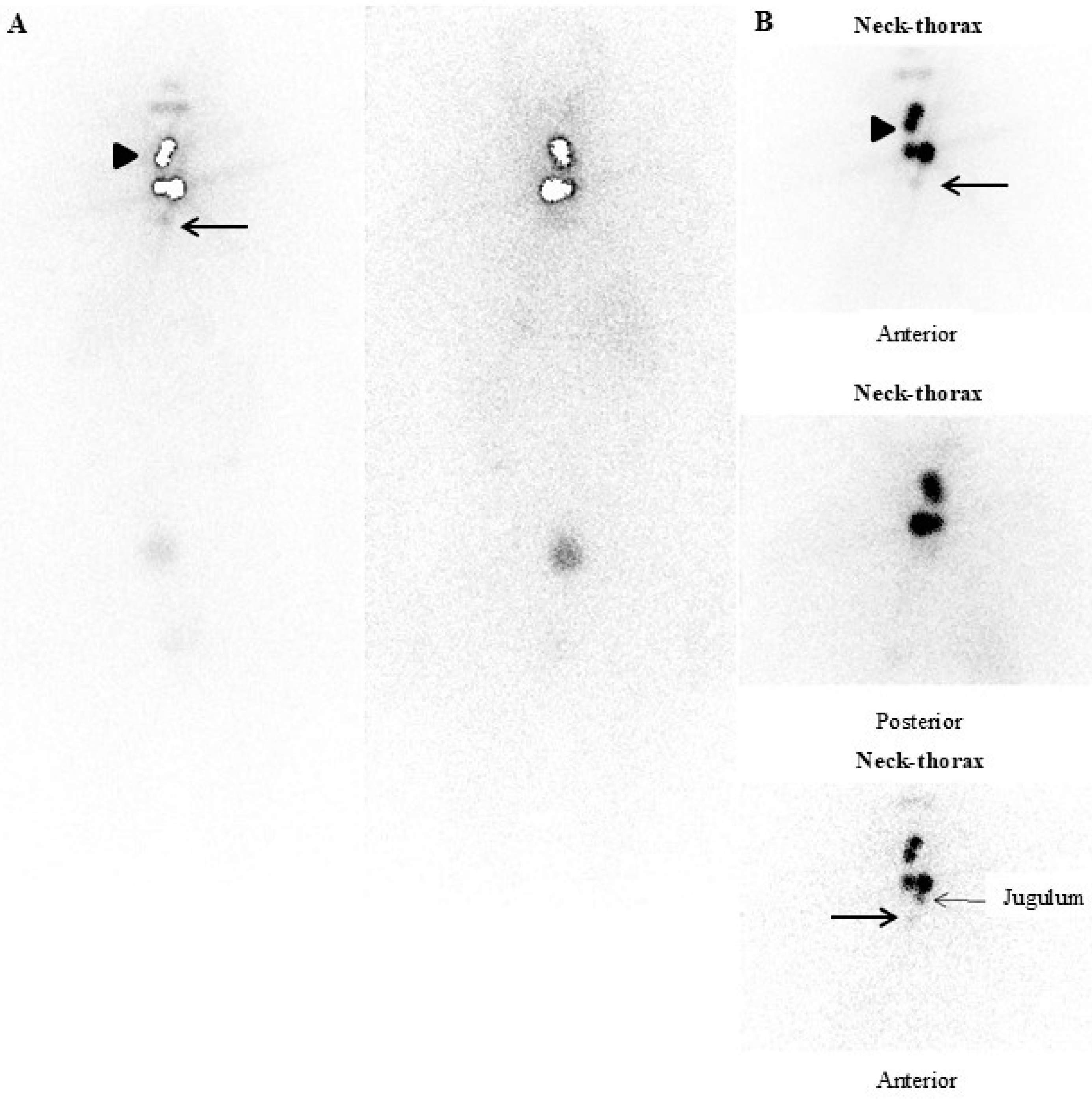
- Presence of locoregional and/or distant metastases on postoperative functional whole-body imaging and “hybrid” imaging (131-123I-Dx-imaging): Postoperative diagnostic functional imaging (123/131I-Dx-imaging) provides information that changes the histopathological risk class in 15% of DTC patients. The use of 123/131I-Dx-imaging, by revealing the presence of locoregional and/or distant metastases, could modify the clinical management of a significant number of patients, indicating the need for 131I therapy with therapeutic intent [16].
- Less than optimal pre- and postoperative neck-US evaluation;
- Less than optimal thyroid surgery (i.e., low-volume thyroid surgeon);
- Poorly informative histological report (i.e., not a standardized report);
- Positive anti-thyroglobulin antibody;
- Limited access to referral centers;
- Patient’s will to maximize the therapeutic process to reduce anxiety related to disease recurrence, considering that the goal of cancer treatment is not only to prolong survival but also to maintain and improve the quality of life.
4.1. Postoperative Iodine-131 Therapy (p-RIT) Planning (Empiric Approach)
4.2. Postoperative Iodine-131 Therapy (p-RIT) Planning (Dosimetry Approach)
5. General Considerations on Patients’ Preparation and Strategy to Deliver Postoperative Iodine-131 Therapy (p-RIT)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campennì, A.; Giovanella, L. Nuclear medicine therapy of thyroid cancer post-thyroidectomy. In Nuclear Medicine and Molecular Imaging; Sathekge, M., Luster, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 4, pp. 42–55. [Google Scholar] [CrossRef]
- Chen, A.Y.; Jemal, A.; Ward, E.M. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009, 115, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2014; based on November 2016 SEER data submission, posted to the SEER web site, April 2017; National Cancer Institute: Bethesda, MD, USA; 2017. Available online: https://seer.cancer.gov/csr/1975_2014/ (accessed on 2 April 2018).
- Gallicchio, R.; Giacomobono, S.; Capacchione, D.; Nardelli, A.; Barbato, F.; Nappi, A.; Pellegrino, T.; Storto, G. Should patients with remnants from thyroid microcarcinoma really not be treated with iodine-131 ablation? Endocrine 2013, 44, 426–433. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Tala, H.; Shah, J.; Leboeuf, R.; Ghossein, R.; Gonen, M.; Brokhin, M.; Omry, G.; Fagin, J.A.; Shaha, A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid Off. J. Am. Thyroid Assoc. 2010, 20, 1341–1349. [Google Scholar] [CrossRef]
- Lamartina, L.; Grani, G.; Durante, C.; Borget, I.; Filetti, S.; Schlumberger, M. Follow-up of differentiated thyroid cancer—What should (and what should not) be done. Nat. Rev. Endocrinol. 2018, 14, 538–551. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1. [Google Scholar] [CrossRef]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.G.; Tennvall, J.; Bombardieri, E.; European Association of Nuclear Medicine (EANM). Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef]
- Giovanella, L.; Tuncel, M.; Aghaee, A.; Campenni, A.; De Virgilio, A.; Ovčariček, P.P. Theranostics of Thyroid Cancer. Semin. Nucl. Med. 2024, 54, 470–487. [Google Scholar] [CrossRef]
- Cooper, D.S.; Specker, B.; Ho, M.; Sperling, M.; Ladenson, P.W.; Ross, D.S.; Ain, K.B.; Bigos, S.T.; Brierley, J.D.; Haugen, B.R.; et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid Off. J. Am. Thyroid Assoc. 1998, 8, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S.; Sturgeon, C. Extent of surgery affects survival for papillary thyroid cancer. Ann. Surg. 2007, 246, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Bergstralh, E.J.; Grant, C.S.; McIver, B.; Thompson, G.B.; van Heerden, J.A.; Goellner, J.R. Impact of primary surgery on outcome in 300 patients with pathologic tumor-node-metastasis stage III papillary thyroid carcinoma treated at one institution from 1940 through 1989. Surgery 1999, 126, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.M.; Giovanella, L.; Greenspan, B.; Lawson, S.A.; Luster, M.; Van Nostrand, D.; Peacock, J.G.; Ovčariček, P.P.; Silberstein, E.; Tulchinsky, M.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Nuclear Medicine Evaluation and Therapy of Differentiated Thyroid Cancer: Abbreviated Version. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 15N–35N. [Google Scholar]
- Avram, A.M. Radioiodine scintigraphy with SPECT/CT: An important diagnostic tool for thyroid cancer staging and risk stratification. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2012, 53, 754–764. [Google Scholar] [CrossRef]
- Atkins, F.B.; Van Nostrand, D.; Wartofsky, L. Dosimetrically Determined Prescribed Activity of 131I for the Treatment of Metastatic Differentiated Thyroid Carcinoma; Springer Science + Business Media: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Campennì, A.; Ruggeri, R.M.; Siracusa, M.; Comis, A.D.; Romano, D.; Vento, A.; Lanzafame, H.; Capoccetti, F.; Alibrandi, A.; Baldari, S.; et al. Early preablation rhTSH-stimulated thyroglobulin predicts outcome of differentiated thyroid cancer (DTC) patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2466–2475. [Google Scholar] [CrossRef]
- Campennì, A.; Vrachimis, A.; Siracusa, M.; Baldari, S.; Giovanella, L. Usefulness of 123I-spect/ct to assess the response to initial therapy in differentiated thyroid cancer patients. Endocrine 2021, 74, 193–196. [Google Scholar] [CrossRef]
- Barwick, T.; Murray, I.; Megadmi, H.; Drake, W.M.; Plowman, P.N.; Akker, S.A.; Chew, S.L.; Grossman, A.B.; Avril, N. Single photon emission computed tomography (SPECT)/computed tomography using Iodine-123 in patients with differentiated thyroid cancer: Additional value over whole body planar imaging and SPECT. Eur. J. Endocrinol. 2010, 162, 1131–1139. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; AlShaikh, O.; Tuli, M.; Al-Sugair, A.; Alamawi, R.; Al-Rasheed, M.M. Diagnostic value of recombinant human thyrotropin-stimulated 123I whole-body scintigraphy in the follow-up of patients with differentiated thyroid cancer. Clin. Nucl. Med. 2012, 37, 229–234. [Google Scholar] [CrossRef]
- Siddiqi, A.; Foley, R.R.; Britton, K.E.; Sibtain, A.; Plowman, P.N.; Grossman, A.B.; Monson, J.P.; Besser, G.M. The role of 123I-diagnostic imaging in the follow-up of patients with differentiated thyroid carcinoma as compared to 131I-scanning: Avoidance of negative therapeutic uptake due to stunning. Clin. Endocrinol. 2001, 55, 515–521. [Google Scholar] [CrossRef]
- Avram, A.M.; Fig, L.M.; Frey, K.A.; Gross, M.D.; Wong, K.K. Preablation 131-I scans with SPECT/CT in postoperative thyroid cancer patients: What is the impact on staging? J. Clin. Endocrinol. Metab. 2013, 98, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Sisson, J.C.; Avram, A.M.; Lawson, S.A.; Gauger, P.G.; Doherty, G. The so-called stunning of thyroid tissue. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2006, 47, 1406–1412. [Google Scholar]
- Campennì, A.; Ruggeri, R.M.; Siracusa, M.; Giacoppo, G.; La Torre, F.; Saccomanno, A.; Alibrandi, A.; Dionigi, G.; Tuccari, G.; Baldari, S.; et al. Isthmus topography is a risk factor for persistent disease in patients with differentiated thyroid cancer. Eur. J. Endocrinol. 2021, 185, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadis, I.; Boutzios, G.; Karalaki, M.; Misiakos, E.; Karatzas, T. Papillary thyroid carcinoma of the isthmus: Total thyroidectomy or isthmusectomy? Am. J. Surg. 2018, 216, 135–139. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Gao, L.; Xie, L.; Cai, X. Evaluation of thyroid isthmusectomy as a potential treatment for papillary thyroid carcinoma limited to the isthmus: A clinical study of 73 patients. Head Neck 2016, 38 (Suppl. S1), E1510–E1514. [Google Scholar] [CrossRef]
- Karatzas, T.; Charitoudis, G.; Vasileiadis, D.; Kapetanakis, S.; Vasileiadis, I. Surgical treatment for dominant malignant nodules of the isthmus of the thyroid gland: A case control study. Int. J. Surg. 2015, 18, 64–68. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeong, J.J.; Nam, K.; Chung, W.Y.; Chang, H.; Park, C.S. Papillary carcinoma located in the thyroid isthmus. World J. Surg. 2010, 34, 36–39. [Google Scholar] [CrossRef]
- Song, C.M.; Lee, D.W.; Ji, Y.B.; Jeong, J.H.; Park, J.H.; Tae, K. Frequency and pattern of central lymph node metastasis in papillary carcinoma of the thyroid isthmus. Head Neck 2016, 38 (Suppl. S1), E412–E416. [Google Scholar] [CrossRef]
- Chai, Y.J.; Kim, S.; Choi, J.Y.; Koo, D.H.; Lee, K.E.; Youn, Y. Papillary thyroid carcinoma located in the isthmus or upper third is associated with Delphian lymph node metastasis. World J. Surg. 2014, 38, 1306–1311. [Google Scholar] [CrossRef]
- Van Nostrand, D. Surveillance radioiodine whole body scans. In Thyroid Cancer: A Comprehensive Guide to Clinical Management, 3rd ed.; Wartofsky, L., Van Nostrand, D., Eds.; Springer: New York, NY, USA, 2016; pp. 471–474. [Google Scholar]
- Ovčariček, P.P.; Campenni, A.; de Keizer, B.; Deandreis, D.; Kreissl, M.C.; Vrachimis, A.; Tuncel, M.; Giovanella, L. Molecular Theranostics in Radioiodine-Refractory Differentiated Thyroid Cancer. Cancers 2023, 15, 4290. [Google Scholar] [CrossRef]
- Campennì, A.; Ruggeri, R.M.; Siracusa, M.; Romano, D.; Giacoppo, G.; Crocè, L.; Rosarno, H.; Russo, S.; Cardile, D.; Capoccetti, F.; et al. Thyroglobulin Value Predict Iodine-123 Imaging Result in Differentiated Thyroid Cancer Patients. Cancers 2023, 15, 2242. [Google Scholar] [CrossRef] [PubMed]
- Spanu, A.; Solinas, M.E.; Chessa, F.; Sanna, D.; Nuvoli, S.; Madeddu, G. 131I SPECT/CT in the follow-up of differentiated thyroid carcinoma: Incremental value versus planar imaging. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2009, 50, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Fuehrer, D.; et al. Controversies, Consensus, and Collaboration in the Use of 131I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid Off. J. Am. Thyroid Assoc. 2019, 29, 461–470. [Google Scholar] [CrossRef]
- Giovanella, L.; D’aurizio, F.; Algeciras-Schimnich, A.; Görges, R.; Ovcaricek, P.P.; Tuttle, R.M.; Visser, W.E.; Verburg, F.A.; hsTg&TgAb Consensus Working Group; Borowczyk, M.; et al. Thyroglobulin and thyroglobulin antibody: An updated clinical and laboratory expert consensus. Eur. J. Endocrinol. 2023, 189, R11–R27. [Google Scholar] [CrossRef]
- Rosario, P.W.; Xavier, A.C.M.; Calsolari, M.R. Value of postoperative thyroglobulin and ultrasonography for the indication of ablation and 131 activity in patients with thyroid cancer and low risk of recurrence. Thyroid Off. J. Am. Thyroid Assoc. 2011, 21, 49–53. [Google Scholar] [CrossRef]
- Park, E.-K.; Chung, J.-K.; Lim, I.H.; Park, D.J.; Lee, D.S.; Lee, M.C.; Cho, B.Y. Recurrent/metastatic thyroid carcinomas false negative for serum thyroglobulin but positive by posttherapy I-131 whole body scans. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 172–179. [Google Scholar] [CrossRef]
- RRobenshtok, E.; Grewal, R.K.; Fish, S.; Sabra, M.; Tuttle, R.M. A low postoperative nonstimulated serum thyroglobulin level does not exclude the presence of radioactive iodine avid metastatic foci in intermediate-risk differentiated thyroid cancer patients. Thyroid Off. J. Am. Thyroid Assoc. 2013, 23, 436–442. [Google Scholar] [CrossRef]
- Mertens, I.J.R.; DE Klerk, J.M.H.; Zelissen, P.M.J.; Thijssen, J.H.H.; Sie-Go, D.M.D.S.; Han, S.H.; VAN Rijk, P.P. Undetectable serum thyroglobulin in a patient with metastatic follicular thyroid cancer. Clin. Nucl. Med. 1999, 24, 346–349. [Google Scholar] [CrossRef]
- Brendel, A.J.; Lambert, B.; Guyot, M.; Jeandot, R.; Dubourg, H.; Roger, P.; Wynchauk, S.; Manciet, G.; Lefort, G. Low levels of serum thyroglobulin after withdrawal of thyroid suppression therapy in the follow up of differentiated thyroid carcinoma. Eur. J. Nucl. Med. 1990, 16, 35–38. [Google Scholar] [CrossRef]
- Grant, S.; Luttrell, B.; Reeve, T.; Wiseman, J.; Wilmshurst, E.; Stiel, J.; Donohoe, D.; Cooper, R.; Bridgman, M. Thyroglobulin may be undetectable in the serum of patients with metastatic disease secondary to differentiated thyroid carcinoma. Follow-up of differentiated thyroid carcinoma. Cancer 1984, 54, 1625–1628. [Google Scholar] [CrossRef]
- Rosário, P.W.S.; Guimarães, V.C.; Maia, F.F.R.; Fagundes, T.A.; Purisch, S.; Padrao, E.L.; Rezende, L.L.; Barroso, A.L. Thyroglobulin before ablation and correlation with posttreatment scanning. Laryngoscope 2005, 115, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.T.; Jager, P.L.; van der Wal, J.E.; Sluiter, W.J.; Plukker, J.T.M.; Dierckx, R.A.J.O.; Wolffenbuttel, B.H.R.; Links, T.P. The follow-up of patients with differentiated thyroid cancer and undetectable thyroglobulin (Tg) and Tg antibodies during ablation. Eur. J. Endocrinol. 2008, 158, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Müller-Gärtner, H.-W.; Schneider, C. Clinical evaluation of tumor characteristics predisposing serum thyroglobulin to be undetectable in patients with differentiated thyroid cancer. Cancer 1988, 61, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, D.; Campennì, A.; Forleo, R.; Lapi, P. False-positive radioiodine uptake after radioiodine treatment in differentiated thyroid cancer. Endocrine 2023, 81, 30–35. [Google Scholar] [CrossRef]
- Triggiani, V.; Giagulli, V.A.; Iovino, M.; De Pergola, G.; Licchelli, B.; Varraso, A.; Dicembrino, F.; Valle, G.; Guastamacchia, E. False positive diagnosis on (131)iodine whole-body scintigraphy of differentiated thyroid cancers. Endocrine 2016, 53, 626–635. [Google Scholar] [CrossRef]
- Gonzalez Carvalho, J.M.; Görlich, D.; Schober, O.; Wenning, C.; Riemann, B.; Verburg, F.A.; Vrachimis, A. Evaluation of 131I scintigraphy and stimulated thyroglobulin levels in the follow up of patients with DTC: A retrospective analysis of 1420 patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 744–756. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.; Newbold, K.; Papotti, M.; Berruti, A.; ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Leboulleux, S.; Bournaud, C.; Chougnet, C.N.; Zerdoud, S.; Al Ghuzlan, A.; Catargi, B.; Do Cao, C.; Kelly, A.; Barge, M.-L.; Lacroix, L.; et al. Thyroidectomy without Radioiodine in Patients with Low-Risk Thyroid Cancer. N. Engl. J. Med. 2022, 386, 923–932. [Google Scholar] [CrossRef]
- Schvartz, C.; Bonnetain, F.; Dabakuyo, S.; Gauthier, M.; Cueff, A.; Fieffé, S.; Pochart, J.-M.; Cochet, I.; Crevisy, E.; Dalac, A.; et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J. Clin. Endocrinol. Metab. 2012, 97, 1526–1535. [Google Scholar] [CrossRef]
- Padovani, R.D.P.; Chablani, S.V.; Tuttle, R.M. Radioactive iodine therapy: Multiple faces of the same polyhedron. Arch. Endocrinol. Metab. 2022, 66, 393–406, Advance online publication. [Google Scholar] [CrossRef]
- Campennì, A.; Barbaro, D.; Guzzo, M.; Capoccetti, F.; Giovanella, L. Personalized management of differentiated thyroid cancer in real life—Practical guidance from a multidisciplinary panel of experts. Endocrine 2020, 70, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Flux, G.; Giovanella, L.; van Nostrand, D.; Muylle, K.; Luster, M. Differentiated thyroid cancer patients potentially benefitting from postoperative I-131 therapy: A review of the literature of the past decade. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Campennì, A.; Giovanella, L.; Siracusa, M.; Stipo, M.E.; Alibrandi, A.; Cucinotta, M.; Ruggeri, R.M.; Baldari, S. Is malignant nodule topography an additional risk factor for metastatic disease in low-risk differentiated thyroid cancer? Thyroid Off. J. Am. Thyroid Assoc. 2014, 24, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur. Thyroid J. 2022, 11, e210046. [Google Scholar] [CrossRef] [PubMed]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.; Newbold, K.; Papotti, M.; Berruti, A.; ESMO Guidelines Committee. ESMO Clinical Practice Guideline update on the use of systemic therapy in advanced thyroid cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 674–684. [Google Scholar] [CrossRef]
- Verburg, F.A.; Luster, M.; Cupini, C.; Chiovato, L.; Duntas, L.; Elisei, R.; Feldt-Rasmussen, U.; Rimmele, H.; Seregni, E.; Smit, J.W.; et al. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: A clinical position statement. Thyroid Off. J. Am. Thyroid Assoc. 2013, 23, 1211–1225. [Google Scholar] [CrossRef]
- D’Aurizio, F.; Metus, P.; Ferrari, A.; Caruso, B.; Castello, R.; Villalta, D.; Steffan, A.; Gaspardo, K.; Pesente, F.; Bizzaro, N.; et al. Definition of the upper reference limit for thyroglobulin antibodies according to the National Academy of Clinical Biochemistry guidelines: Comparison of eleven different automated methods. Autoimmun. Highlights 2017, 8, 8. [Google Scholar] [CrossRef]
- Hänscheid, H.; Lassmann, M.; Luster, M.; Thomas, S.R.; Pacini, F.; Ceccarelli, C.; Ladenson, P.W.; Wahl, R.L.; Schlumberger, M.; Ricard, M.; et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: Procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2006, 47, 648–654. [Google Scholar]
- Ruel, E.; Thomas, S.; Dinan, M.; Perkins, J.M.; Roman, S.A.; Sosa, J.A. Adjuvant radioactive iodine therapy is associated with improved survival for patients with intermediate-risk papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2015, 100, 1529–1536. [Google Scholar] [CrossRef]
- Reiners, C.; Biko, J.; Haenscheid, H.; Hebestreit, H.; Kirinjuk, S.; Baranowski, O.; Marlowe, R.J.; Demidchik, E.; Drozd, V.; Demidchik, Y. Twenty-five years after Chernobyl: Outcome of radioiodine treatment in children and adolescents with very high-risk radiation-induced differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 3039–3048. [Google Scholar] [CrossRef]
- Tang, J.; Kong, D.; Cui, Q.; Wang, K.; Zhang, D.; Liao, X.; Gong, Y.; Wu, G. The role of radioactive iodine therapy in papillary thyroid cancer: An observational study based on SEER. OncoTargets Ther. 2018, 11, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Deandreis, D.; Vrachimis, A.; Campenni, A.; Ovcaricek, P.P. Molecular Imaging and Theragnostics of Thyroid Cancers. Cancers 2022, 14, 1272. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhu, R.; Liu, Y.; Hong, Y.; Ge, J.; Xuan, J.; Niu, W.; Yu, X.; Qin, J.-J.; Li, Q. Radioiodine-refractory differentiated thyroid cancer: Molecular mechanisms and therapeutic strategies for radioiodine resistance. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2024, 72, 101013. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.M.; Dewaraja, Y.K. Thyroid Cancer Radiotheragnostics: The case for activity adjusted 131I therapy. Clin. Transl. Imaging 2018, 6, 335–346. [Google Scholar] [CrossRef]
- Chen, M.-K.; Yasrebi, M.; Samii, J.; Staib, L.H.; Doddamane, I.; Cheng, D.W. The utility of I-123 pretherapy scan in I-131 radioiodine therapy for thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2012, 22, 304–309. [Google Scholar] [CrossRef]
- Gulec, S.A.; Kuker, R.A.; Goryawala, M.; Fernandez, C.; Perez, R.; Khan-Ghany, A.; Apaza, A.; Harja, E.; Harrell, M. 124I PET/CT in Patients with Differentiated Thyroid Cancer: Clinical and Quantitative Image Analysis. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 441–448. [Google Scholar] [CrossRef]
- Santhanam, P.; Taieb, D.; Solnes, L.; Marashdeh, W.; Ladenson, P.W. Utility of I-124 PET/CT in identifying radioiodine avid lesions in differentiated thyroid cancer: A systematic review and meta-analysis. Clin. Endocrinol. 2017, 86, 645–651. [Google Scholar] [CrossRef]
- Schneider, D.F.; Ojomo, K.A.; Chen, H.; Sippel, R.S. Remnant uptake as a postoperative oncologic quality indicator. Thyroid Off. J. Am. Thyroid Assoc. 2013, 23, 1269–1276. [Google Scholar] [CrossRef]
- Adam, M.A.; Thomas, S.; Youngwirth, L.; Hyslop, T.; Reed, S.D.; Scheri, R.P.; Roman, S.A.; Sosa, J.A. Is There a Minimum Number of Thyroidectomies a Surgeon Should Perform to Optimize Patient Outcomes? Ann. Surg. 2017, 265, 402–407. [Google Scholar] [CrossRef]
- Krajewska, J.; Chmielik, E.; Jarząb, B. Dynamic risk stratification in the follow-up of thyroid cancer: What is still to be discovered in 2017? Endocr.-Relat. Cancer 2017, 24, R387–R402. [Google Scholar] [CrossRef]
- Cohen, O.; Blank, A.; Meiersdorf, S.; Hod, K.; Gabay, S.; Guindy, M.; Khafif, A. Impact of high-quality ultrasound following community ultrasound on surgical planning and active surveillance in patients with thyroid cancer. Clin. Endocrinol. 2021, 94, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Thyrogen Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020898s054lbl.pdf (accessed on 21 April 2022).
- Pacini, F.; Ladenson, P.W.; Schlumberger, M.; Driedger, A.; Luster, M.; Kloos, R.T.; Sherman, S.; Haugen, B.; Corone, C.; Molinaro, E.; et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: Results of an international, randomized, controlled study. J. Clin. Endocrinol. Metab. 2006, 91, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.C.; Marchal, F.; Angelos, P.; Bernet, V.; Boucai, L.; Buchholzer, S.; Burkey, B.; Eisele, D.; Erkul, E.; Faure, F.; et al. Salivary and lacrimal dysfunction after radioactive iodine for differentiated thyroid cancer: American Head and Neck Society Endocrine Surgery Section and Salivary Gland Section joint multidisciplinary clinical consensus statement of otolaryngology, ophthalmology, nuclear medicine and endocrinology. Head Neck 2020, 42, 3446–3459. [Google Scholar] [CrossRef]
- Diessl, S.; Holzberger, B.; Mäder, U.; Grelle, I.; Smit, J.W.A.; Buck, A.K.; Reiners, C.; Verburg, F.A. Impact of moderate vs stringent TSH suppression on survival in advanced differentiated thyroid carcinoma. Clin. Endocrinol. 2012, 76, 586–592. [Google Scholar] [CrossRef]
- Brose, M.S.; Smit, J.; Capdevila, J.; Elisei, R.; Nutting, C.; Pitoia, F.; Robinson, B.; Schlumberger, M.; Shong, Y.K.; Takami, H. Regional approaches to the management of patients with advanced, radioactive iodine-refractory differentiated thyroid carcinoma. Expert Rev. Anticancer Ther. 2012, 12, 1137–1147. [Google Scholar] [CrossRef]
- Schlumberger, M.; Brose, M.; Elisei, R.; Leboulleux, S.; Luster, M.; Pitoia, F.; Pacini, F. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014, 2, 356–358. [Google Scholar] [CrossRef]
- Sacks, W.; Braunstein, G.D. Evolving approaches in managing radioactive iodine-refractory differentiated thyroid cancer. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2014, 20, 263–275. [Google Scholar] [CrossRef]
- Kiyota, N.; Robinson, B.; Shah, M.; Hoff, A.O.; Taylor, M.H.; Li, D.; Dutcus, C.E.; Lee, E.K.; Kim, S.-B.; Tahara, M. Defining Radioiodine-Refractory Differentiated Thyroid Cancer: Efficacy and Safety of Lenvatinib by Radioiodine-Refractory Criteria in the SELECT Trial. Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 1135–1141. [Google Scholar] [CrossRef]
- Chung, J.H. Low iodine diet for preparation for radioactive iodine therapy in differentiated thyroid carcinoma in Korea. Endocrinol. Metab. 2013, 28, 157–163. [Google Scholar] [CrossRef]
- Maxon, H.R.; Thomas, S.R.; Boehringer, A.; Drilling, J.; Sperling, M.I.; Sparks, J.C.; Chen, I.W. Low iodine diet in I-131 ablation of thyroid remnants. Clin. Nucl. Med. 1983, 8, 123–126. [Google Scholar] [CrossRef]
- Park, J.T., 2nd; Hennessey, J.V. Two-week low iodine diet is necessary for adequate outpatient preparation for radioiodine rhTSH scanning in patients taking levothyroxine. Thyroid Off. J. Am. Thyroid Assoc. 2004, 14, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, C.; Uruno, T.; Takamura, Y.; Ito, Y.; Miya, A.; Kobayashi, K.; Matsuzuka, F.; Amino, N.; Kuma, K.; Miyauchi, A. Reevaluation of stringent low iodine diet in outpatient preparation for radioiodine examination and therapy. Endocr. J. 2005, 52, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, D.; Verburg, F.A.; Luster, M.; Reiners, C.; Rubello, D. ALARA in rhTSH-stimulated post-surgical thyroid remnant ablation: What is the lowest reasonably achievable activity? Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1251–1254. [Google Scholar] [CrossRef][Green Version]
- Sohn, S.Y.; Choi, J.Y.; Jang, H.W.; Kim, H.J.; Jin, S.M.; Kim, S.W.; Suh, S.; Hur, K.Y.; Kim, J.H.; Chung, J.H.; et al. Association between excessive urinary iodine excretion and failure of radioactive iodine thyroid ablation in patients with papillary thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2013, 23, 741–747. [Google Scholar] [CrossRef]
- Bartel, T.B.; Magerefteh, S.; Avram, A.M.; Balon, H.R.; De Blanche, L.E.; Dadparvar, S.; Johnston, M.; Moreau, S. SNMMI Procedure Standard for Scintigraphy for Differentiated Thyroid Cancer. J. Nucl. Med. Technol. 2020, 48, 202–209. [Google Scholar] [CrossRef]
- Schlumberger, M.; Catargi, B.; Borget, I.; Deandreis, D.; Zerdoud, S.; Bridji, B.; Bardet, S.; Leenhardt, L.; Bastie, D.; Schvartz, C.; et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N. Engl. J. Med. 2012, 366, 1663–1673. [Google Scholar] [CrossRef]
- Mallick, U.; Harmer, C.; Yap, B.; Wadsley, J.; Clarke, S.; Moss, L.; Nicol, A.; Clark, P.M.; Farnell, K.; McCready, R.; et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N. Engl. J. Med. 2012, 366, 1674–1685. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.; Driedger, A.; Reiners, C.; Kloos, R.T.; Sherman, S.I.; Haugen, B.; Corone, C.; Molinaro, E.; Grasso, L.; et al. Follow-up of low-risk differentiated thyroid cancer patients who underwent radioiodine ablation of postsurgical thyroid remnants after either recombinant human thyrotropin or thyroid hormone withdrawal. J. Clin. Endocrinol. Metab. 2009, 94, 4171–4179. [Google Scholar] [CrossRef]
- Schroeder, P.R.; Haugen, B.R.; Pacini, F.; Reiners, C.; Schlumberger, M.; Sherman, S.I.; Cooper, D.S.; Schuff, K.G.; Braverman, L.E.; Skarulis, M.C.; et al. A comparison of short-term changes in health-related quality of life in thyroid carcinoma patients undergoing diagnostic evaluation with recombinant human thyrotropin compared with thyroid hormone withdrawal. J. Clin. Endocrinol. Metab. 2006, 91, 878–884. [Google Scholar] [CrossRef]
- Dueren, C.; Dietlein, M.; Luster, M.; Plenzig, F.; Steinke, R.; Grimm, J.; Groth, P.; Eichhorn, W.; Reiners, C. The use of thyrogen in the treatment of differentiated thyroid carcinoma: An intraindividual comparison of clinical effects and implications of daily life. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2010, 118, 513–519. [Google Scholar] [CrossRef]
- Tagay, S.; Herpertz, S.; Langkafel, M.; Erim, Y.; Bockisch, A.; Senf, W.; Görges, R. Health-related Quality of Life, depression and anxiety in thyroid cancer patients. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2006, 15, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Ovčariček, P.P.; Kreissl, M.C.; Campenni, A.; de Keizer, B.; Tuncel, M.; Vrachimis, A.; Deandreis, D.; Giovanella, L. SNMMI/EANM practice guideline vs. ETA Consensus Statement: Differences and similarities in approaching differentiated thyroid cancer management-the EANM perspective. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Zanotti-Fregonara, P.; Hindié, E. On the effectiveness of recombinant human TSH as a stimulating agent for 131I treatment of metastatic differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2264–2266. [Google Scholar] [CrossRef] [PubMed]
- Plyku, D.; Hobbs, R.F.; Huang, K.; Atkins, F.; Garcia, C.; Sgouros, G.; Van Nostrand, D. Recombinant Human Thyroid-Stimulating Hormone Versus Thyroid Hormone Withdrawal in 124I PET/CT-Based Dosimetry for 131I Therapy of Metastatic Differentiated Thyroid Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 1146–1154. [Google Scholar] [CrossRef]
- Botella-Carretero, J.I.; Galán, J.M.; Caballero, C.; Sancho, J.; Escobar-Morreale, H.F. Quality of life and psychometric functionality in patients with differentiated thyroid carcinoma. Endocr.-Relat. Cancer 2003, 10, 601–610. [Google Scholar] [CrossRef]
- Tagay, S.; Herpertz, S.; Langkafel, M.; Erim, Y.; Freudenberg, L.; Schöpper, N.; Bockisch, A.; Senf, W.; Görges, R. Health-related quality of life, anxiety and depression in thyroid cancer patients under short-term hypothyroidism and TSH-suppressive levothyroxine treatment. Eur. J. Endocrinol. 2005, 153, 755–763. [Google Scholar] [CrossRef]
- Taïeb, D.; Sebag, F.; Cherenko, M.; Baumstarck-Barrau, K.; Fortanier, C.; Farman-Ara, B.; De Micco, C.; Vaillant, J.; Thomas, S.; Conte-Devolx, B.; et al. Quality of life changes and clinical outcomes in thyroid cancer patients undergoing radioiodine remnant ablation (RRA) with recombinant human TSH (rhTSH): A randomized controlled study. Clin. Endocrinol. 2009, 71, 115–123. [Google Scholar] [CrossRef]
- Badihian, S.; Jalalpour, P.; Mirdamadi, M.; Moslehi, M. Quality of Life, Anxiety and Depression in Patients with Differentiated Thyroid Cancer under Short Term Hypothyroidism Induced by Levothyroxine Withdrawal. Kvalita života, úzkost a deprese u pacientů s diferencovaným karcinomem štítné žlázy během krátkodobé hypotyreózy indukované vysazením levothyroxinu. Klin. Onkol. Cas. Ceske A Slov. Onkol. Spol. 2016, 29, 439–444. [Google Scholar]
- Smith, C.D.; Grondin, R.; LeMaster, W.; Martin, B.; Gold, B.T.; Ain, K.B. Reversible cognitive, motor, and driving impairments in severe hypothyroidism. Thyroid Off. J. Am. Thyroid Assoc. 2015, 25, 28–36. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Feng, F.; Zou, R.-J.; Fu, H.-L.; Sun, J.-W.; Jia, X.-Z.; Yin, Y.-F.; Wang, H. Abnormal Brain Glucose Metabolism in Papillary Thyroid Cancer Patients 4 Weeks After Withdrawal of Levothyroxine: A Cross-Sectional Study Using 18F-FDG PET/CT. Front. Endocrinol. 2021, 12, 595933. [Google Scholar] [CrossRef]
- Duntas, L.H.; Biondi, B. Short-term hypothyroidism after Levothyroxine-withdrawal in patients with differentiated thyroid cancer: Clinical and quality of life consequences. Eur. J. Endocrinol. 2007, 156, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Münte, T.F.; Lill, C.; Ötting, G.; Brabant, G. Cognitive changes in short-term hypothyroidism assessed with event-related brain potentials. Psychoneuroendocrinology 2004, 29, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Pötzi, C.; Moameni, A.; Karanikas, G.; Preitfellner, J.; Becherer, A.; Pirich, C.; Dudczak, R. Comparison of iodine uptake in tumour and nontumour tissue under thyroid hormone deprivation and with recombinant human thyrotropin in thyroid cancer patients. Clin. Endocrinol. 2006, 65, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, L.S.; Jentzen, W.; Petrich, T.; Frömke, C.; Marlowe, R.J.; Heusner, T.; Brandau, W.; Knapp, W.H.; Bockisch, A. Lesion dose in differentiated thyroid carcinoma metastases after rhTSH or thyroid hormone withdrawal: 124I PET/CT dosimetric comparisons. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Lima, C.J.; Chittimoju, S.; Wehbeh, L.; Dia, S.; Pagadala, P.; Al-Jundi, M.; Jhawar, S.; Tefera, E.; Mete, M.; Klubo-Gwiezdzinska, J.; et al. Metastatic Differentiated Thyroid Cancer Survival Is Unaffected by Mode of Preparation for 131I Administration. J. Endocr. Soc. 2022, 6, bvac032. [Google Scholar] [CrossRef]
- Giovanella, L.; Garo, M.L.; Campenní, A.; Ovčariček, P.P.; Görges, R. Thyroid Hormone Withdrawal versus Recombinant Human TSH as Preparation for I-131 Therapy in Patients with Metastatic Thyroid Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 2510. [Google Scholar] [CrossRef]
| Clinical/Pathologic Context | Prescribed 131I Activity | Strategy |
|---|---|---|
| Remnant ablation | 1.11–1.85 GBq (30–50 mCi) 131I * | Risk-adapted 131I therapy |
| Adjuvant treatment | 1.85–3.7 GBq (50–100 mCi) 131I ** | Risk-adapted 131I therapy |
| Treatment of small-volume locoregional disease | 3.7–5.6 GBq (100–150 mCi) 131I | Risk-adapted 131I therapy |
| Treatment of advanced locoregional disease and/or small-volume distant metastatic disease | 5.6–7.4 GBq (150–200 mCi) 131I | Risk-adapted 131I therapy |
| Food and Products That Should Be Avoided or Limited | Type of Medication | Recommended Time of Withdrawal |
|---|---|---|
| Iodized salt (avoided) | Water-soluble intravenous radiographic contrast agents | 6–8 wk *, assuming normal renal function |
| Any vitamins or supplements that contain iodine (avoided) | Lipophilic intravenous radiographic contrast agents | 3–6 mo # |
| Foods from the sea (avoided) | Thyroxine | 3–4 wk * |
| Herbal supplements (avoided) | Triiodothyronine | 10–14 d § |
| Eggs (avoided) | Methimazole | 2–5 d § before RAI therapy |
| Milk or other dairy products, including ice cream, cheese, yogurt, and butter (avoided) | Propylthiouracil | 2–8 wk * if RAI therapy is performed by fixed-activity method (4–7 d § if RAI therapy is performed after personalized dosimetric approach) |
| Soy products (avoided) | Lugol solution | 2–3 wk *, depending on iodide content |
| Grain products (limited) | Saturated solution of potassium iodide | 2–3 wk * |
| Beef, chicken, and turkey (limited) | Topical iodine (e.g., surgical skin preparation) | 2–3 wk * |
| Amiodarone | 3–6 mo # or longer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campennì, A.; Siracusa, M.; Ruggeri, R.M. Oldie but Goldie: The Fundamental Role of Radioiodine in the Management of Thyroid Cancer. J. Clin. Med. 2024, 13, 6400. https://doi.org/10.3390/jcm13216400
Campennì A, Siracusa M, Ruggeri RM. Oldie but Goldie: The Fundamental Role of Radioiodine in the Management of Thyroid Cancer. Journal of Clinical Medicine. 2024; 13(21):6400. https://doi.org/10.3390/jcm13216400
Chicago/Turabian StyleCampennì, Alfredo, Massimiliano Siracusa, and Rosaria Maddalena Ruggeri. 2024. "Oldie but Goldie: The Fundamental Role of Radioiodine in the Management of Thyroid Cancer" Journal of Clinical Medicine 13, no. 21: 6400. https://doi.org/10.3390/jcm13216400
APA StyleCampennì, A., Siracusa, M., & Ruggeri, R. M. (2024). Oldie but Goldie: The Fundamental Role of Radioiodine in the Management of Thyroid Cancer. Journal of Clinical Medicine, 13(21), 6400. https://doi.org/10.3390/jcm13216400






