Direct Comparison of Quality of Life in Patients with Allergic Rhinitis Undergoing Sublingual Versus Subcutaneous Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Sources and Measurements
2.3. Qualitative Variables and Statistical Methods
3. Results
3.1. Participants and Demographics
3.2. Associated Findings and Allergen Data
3.3. RQLQ Results and Comparison Between SLIT and SCIT
3.4. Direct Comparison of SLIT Versus SCIT
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meltzer, E.O. Allergic Rhinitis: Burden of Illness, Quality of Life, Comorbidities, and Control. Immunol. Allergy Clin. N. Am. 2016, 36, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Small, P.; Keith, P.K.; Kim, H. Allergic rhinitis. Allergy Asthma Clin. Immunol. 2018, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, A.R.; Miglani, A.; Nguyen, S.A.; Schlosser, R.J. Effect of Medical Therapy in Allergic Rhinitis: A Systematic Review and Meta-Analysis. Am. J. Rhinol. Allergy 2022, 36, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.; Løkke, A.; Hilberg, O. Compliance in subcutaneous and sublingual allergen immunotherapy: A nationwide study. Respir. Med. 2020, 170, 106039. [Google Scholar] [CrossRef]
- Tie, K.; Miller, C.; Zanation, A.M.; Ebert, C.S. Subcutaneous Versus Sublingual Immunotherapy for Adults with Allergic Rhinitis: A Systematic Review with Meta-Analyses. Laryngoscope 2022, 132, 499–508. [Google Scholar] [CrossRef]
- Wise, S.K.; Damask, C.; Roland, L.T.; Ebert, C.; Levy, J.M.; Lin, S.; Luong, A.; Rodriguez, K.; Sedaghat, A.R.; Toskala, E.; et al. International consensus statement on allergy and rhinology: Allergic rhinitis—2023. Int. Forum Allergy Rhinol. 2023, 13, 293–859. [Google Scholar] [CrossRef]
- Juniper, E.F.; Guyatt, G.H. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 1991, 21, 77–83. [Google Scholar] [CrossRef]
- Juniper, E.F.; Guyatt, G.H.; Griffith, L.E.; Ferrie, P.J. Interpretation of rhinoconjunctivitis quality of life questionnaire data. J. Allergy Clin. Immunol. 1996, 98, 843–845. [Google Scholar] [CrossRef]
- Hardin, F.M.; Eskander, P.N.; Franzese, C. Cost-effective Analysis of Subcutaneous vs Sublingual Immunotherapy From the Payor’s Perspective. OTO Open 2021, 5, 2473974X211052955. [Google Scholar] [CrossRef]
- Khinchi, M.S.; Poulsen, L.K.; Carat, F.; André, C.; Hansen, A.B.; Malling, H.J. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy: A randomized, placebo-controlled, double-blind, double-dummy study. Allergy 2004, 59, 45–53. [Google Scholar] [CrossRef]
- Tahamiler, R.; Saritzali, G.; Canakcioglu, S.; Ozcora, E.; Dirican, A. Comparison of the long-term efficacy of subcutaneous and sublingual immunotherapies in perennial rhinitis. ORL J. Oto-Rhino-Laryngol. Its Relat. Spec. 2008, 70, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Keles, S.; Karakoc-Aydiner, E.; Ozen, A.; Izgi, A.G.; Tevetoglu, A.; Akkoc, T.; Bahceciler, N.N.; Barlan, I. A novel approach in allergen-specific immunotherapy: Combination of sublingual and subcutaneous routes. J. Allergy Clin. Immunol. 2011, 128, 808–815.e7. [Google Scholar] [CrossRef] [PubMed]
- Nolte, H.; Maloney, J.; Nelson, H.S.; Bernstein, D.I.; Lu, S.; Li, Z.; Kaur, A.; Zieglmayer, P.; Zieglmayer, R.; Lemell, P.; et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J. Allergy Clin. Immunol. 2015, 135, 1494–1501.e6. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Emminger, W.; Rehm, D.; Backer, V.; Tommerup, L.; Kleine-Tebbe, J. Effective treatment of house dust mite–induced allergic rhinitis with 2 doses of the SQ HDM SLIT-tablet: Results from a randomized, double-blind, placebo-controlled phase III trial. J. Allergy Clin. Immunol. 2016, 137, 444–451.e8. [Google Scholar] [CrossRef]
- Worm, M.; Rak, S.; de Blay, F.; Malling, H.; Melac, M.; Cadic, V.; Zeldin, R.K. Sustained efficacy and safety of a 300IR daily dose of a sublingual solution of birch pollen allergen extract in adults with allergic rhinoconjunctivitis: Results of a double-blind, placebo-controlled study. Clin. Transl. Allergy 2014, 4, 7. [Google Scholar] [CrossRef]
- Mösges, R.; Bachert, C.; Panzner, P.; Calderon, M.A.; Haazen, L.; Pirotton, S.; Wathelet, N.; Durham, S.R.; Bonny, M.; Legon, T.; et al. Short course of grass allergen peptides immunotherapy over 3 weeks reduces seasonal symptoms in allergic rhinoconjunctivitis with/without asthma: A randomized, multicenter, double-blind, placebo-controlled trial. Allergy 2018, 73, 1842–1850. [Google Scholar] [CrossRef]
- Pfaar, O.; Nell, M.J.; Boot, J.D.; Versteeg, S.A.; van Ree, R.; Roger, A.; Riechelmann, H.; Sperl, A.; Elberink, J.N.G.O.; Diamant, Z.; et al. A randomized, 5-arm dose finding study with a mite allergoid SCIT in allergic rhinoconjunctivitis patients. Allergy 2016, 71, 967–976. [Google Scholar] [CrossRef]
- Schwanke, T.; Carragee, E.; Bremberg, M.; Reisacher, W.R. Quality-of-life outcomes in patients who underwent subcutaneous immunotherapy and sublingual immunotherapy in a real-world clinical setting. Am. J. Rhinol. Allergy 2017, 31, 310–316. [Google Scholar] [CrossRef]
- Chelladurai, Y.; Lin, S.Y. Effectiveness of subcutaneous versus sublingual immunotherapy for allergic rhinitis: Current update. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 211–215. [Google Scholar] [CrossRef]
- Blaiss, M.S.; Gronskyte Juhl, R.; Siew, L.Q.C.; Hammerby, E.; Devillier, P. Determining the minimal important differences in the RQLQ score with grass and tree allergy immunotherapy versus placebo in adults with moderate-to-severe allergy. Allergy 2022, 77, 1843–1851. [Google Scholar] [CrossRef]
- Tsabouri, S.; Mavroudi, A.; Feketea, G.; Guibas, G.V. Subcutaneous and Sublingual Immunotherapy in Allergic Asthma in Children. Front. Pediatr. 2017, 5, 82. [Google Scholar] [CrossRef]
- Morris, M.S.; Lowery, A.; Theodoropoulos, D.S.; Duquette, R.D.; Morris, D.L. Quality of life improvement with sublingual immunotherapy: A prospective study of efficacy. J. Allergy 2012, 2012, 253879. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Penagos, M.; Durham, S.R. Long-term efficacy of the sublingual and subcutaneous routes in allergen immunotherapy. Allergy Asthma Proc. 2022, 43, 292–298. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall n = 41 (100) | SLIT n = 21 (51.2) | SCIT n = 20 (48.8) |
|---|---|---|---|
| Mean Age at Baseline | |||
| 43 | 48 | 39 | |
| Sex | |||
| Male | 19 (46.3) | 9 (42.9) | 10 (50.0) |
| Female | 22 (53.7) | 12 (57.1) | 10 (50.0) |
| Race | |||
| White | 35 (85.4) | 20 (95.2) | 15 (75.0) |
| Black | 2 (4.9) | 0 (0.0) | 2 (10.0) |
| Asian | 2 (4.9) | 0 (0.0) | 2 (10.0) |
| Other | 2 (4.8) | 1 (4.8) | 1 (5.0) |
| Ethnicity | |||
| Hispanic/Latino | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not Hispanic/Latino | 39 (95.1) | 19 (90.5) | 20 (100) |
| Unknown/Declined to Answer | 2 (4.9) | 2 (9.5) | 0 (0.0) |
| Characteristic | Overall n = 41 (100) | SLIT n = 21 (51.2) | SCIT n = 20 (48.8) |
|---|---|---|---|
| Associated Findings | |||
| Chronic sinusitis | 20 (48.8) | 14 (66.7) | 6 (30.0) |
| Asthma | 15 (36.6) | 9 (42.9) | 6 (30.0) |
| Total IgE Assessed, n (%) | 18 (43.9) | 10 (47.6) | 8 (40.0) |
| Avg IgE (ng/mL) | 210.0 | 83.5 | 336.5 |
| Additional Therapies | |||
| Topical Steroid Use | 31 (75.6) | 17 (81.0) | 14 (70.0) |
| Nasal Irrigation Use | 26 (63.4) | 13 (61.9) | 13 (65.0) |
| Characteristic | Overall n = 41 (100) | SLIT n = 21 (51.2) | SCIT n = 20 (48.8) |
|---|---|---|---|
| Number of Positive Antigens | |||
| Mild (1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate (2–3) | 38 (92.7) | 19 (90.5) | 19 (95.0) |
| Severe (4+) | 3 (7.3) | 2 (9.5) | 1 (5.0) |
| Antigen Type * | |||
| Total Dust Allergy Assessed, n (%) | 41 (100.0) | 21 (100.0) | 20 (100.0) |
| n (%) with allergy to dust antigen | 21 (51.2) | 12 (57.1) | 9 (45.0) |
| Total Mold Allergy Assessed, n (%) | 39 (95.1) | 21 (100.0) | 18 (90.0) |
| n (%) with allergy to mold antigen | 26 (63.4) | 12 (57.1) | 14 (77.8) |
| Total Cat Dander Allergy Assessed, n (%) | 41 (100.0) | 21 (100.0) | 20 (100.0) |
| n (%) with allergy to cat dander antigen | 24 (58.5) | 12 (57.1) | 12 (60.0) |
| Total Grass Pollen Allergy Assessed, n (%) | 41 (100.0) | 21 (100.0) | 20 (100.0) |
| n (%) with allergy to grass pollen antigen | 33 (80.5) | 17 (81.0) | 16 (80.0) |
| Total Ragweed Allergy Assessed, n (%) | 40 (97.6) | 20 (95.2) | 20 (100.0) |
| n (%) with allergy to ragweed antigen | 27 (65.9) | 12 (60.0) | 15 (75.0) |
| Timepoint | Overall n = 41 (100) | SLIT n = 21 (51.2) | SCIT n = 20 (48.8) |
|---|---|---|---|
| Pre-Treatment | n = 41 (100) | n = 21 (51.2) | n = 20 (48.8) |
| Average per question, Entire Survey (max 6) | 2.69 | 2.98 | 2.38 |
| Activities | 2.87 | 3.11 | 2.62 |
| Sleep | 2.94 | 3.13 | 2.75 |
| Non-nose/eye Sx | 2.50 | 2.71 | 2.28 |
| Practical Problems | 2.88 | 3.25 | 2.48 |
| Nasal Sx | 3.40 | 3.81 | 2.98 |
| Eye Sx | 2.29 | 2.65 | 1.91 |
| Emotional Sx | 2.22 | 2.55 | 1.89 |
| Post-treatment * | n = 41 (100) | n = 21 (51.2) | n = 20 (48.8) |
| Total Assessed from 6 to 12 months after tx, n (%) | 24 (58.5) | 8 (38.1) | 16 (80.0) |
| Average per question, Entire Survey (max 6) | 1.98 | 2.68 | 1.63 |
| Activities | 2.22 | 3.21 | 1.73 |
| Sleep | 1.93 | 2.54 | 1.63 |
| Non-nose/eye Sx | 1.81 | 2.18 | 1.63 |
| Practical Problems | 2.07 | 2.88 | 1.66 |
| Nasal Sx | 2.55 | 3.38 | 2.14 |
| Eye Sx | 1.78 | 2.63 | 1.36 |
| Emotional Sx | 1.68 | 2.47 | 1.28 |
| Total Assessed from 12 to 18 months after tx, n (%) | 20 (48.8) | 11 (52.4) | 9 (45.0) |
| Average per question, Entire Survey (max 6) | 1.70 | 1.60 | 1.83 |
| Activities | 1.73 | 1.64 | 1.85 |
| Sleep | 1.65 | 1.48 | 1.85 |
| Non-nose/eye Sx | 1.66 | 1.48 | 1.89 |
| Practical Problems | 1.95 | 1.94 | 1.96 |
| Nasal Sx | 2.06 | 1.98 | 2.17 |
| Eye Sx | 1.58 | 1.59 | 1.56 |
| Emotional Sx | 1.38 | 1.25 | 1.53 |
| Total Assessed from 18 to 24 months after tx, n (%) | 13 (32.5) | 4 (19.0) | 9 (45.0) |
| Average per question, Entire Survey (max 6) | 1.98 | 1.95 | 2.00 |
| Activities | 2.08 | 2.00 | 2.11 |
| Sleep | 1.67 | 2.08 | 1.48 |
| Non-nose/eye Sx | 1.87 | 1.68 | 1.95 |
| Practical Problems | 2.05 | 2.17 | 2.00 |
| Nasal Sx | 2.44 | 2.19 | 2.56 |
| Eye Sx | 2.10 | 2.19 | 2.06 |
| Emotional Sx | 1.73 | 1.63 | 1.78 |
| Total Assessed from 24+ months after tx, n (%) | 8 (19.5) | 3 (14.3) | 5 (25.0) |
| Average per question, Entire Survey (max 6) | 2.29 | 2.42 | 2.21 |
| Activities | 2.46 | 2.56 | 2.40 |
| Sleep | 2.29 | 3.0 | 1.87 |
| Non-nose/eye Sx | 2.34 | 2.81 | 2.06 |
| Practical Problems | 2.29 | 1.44 | 2.80 |
| Nasal Sx | 2.88 | 3.00 | 2.80 |
| Eye Sx | 1.84 | 1.67 | 1.95 |
| Emotional Sx | 1.94 | 2.08 | 1.85 |
| Group | Difference in Means | Std. Error of the Mean | p = x | 95% CI | Effect Size |
|---|---|---|---|---|---|
| Total cohort n = 41 (100) | |||||
| Overall, mean change in RQLQ score | −0.63 | 0.20 | 0.004 | −0.80, −0.016 | −0.48 |
| Activities, mean change in score | −0.63 | 0.271 | 0.026 | −0.67, −0.042 | −0.36 |
| Mean, Sleep | −0.99 | 0.20 | <0.001 | −1.13, −0.43 | −0.78 |
| Mean, Non-nose/eye Sx | −0.50 | 0.20 | 0.019 | −0.70, −0.06 | −0.38 |
| Mean, Practical Problems | −0.68 | 0.22 | 0.003 | −0.82, −0.17 −0.087, −0.21 | −0.49 |
| Mean, Nasal Sx | −0.85 | 0.25 | 0.001 | −0.087, −0.21 | −0.54 |
| Mean, Eye Sx | −0.48 | 0.18 | 0.012 | −0.73, −0.09 | −0.41 |
| Mean, Emotional Sx | −0.49 | 0.190 | 0.013 | −0.72, −0.09 | −0.41 |
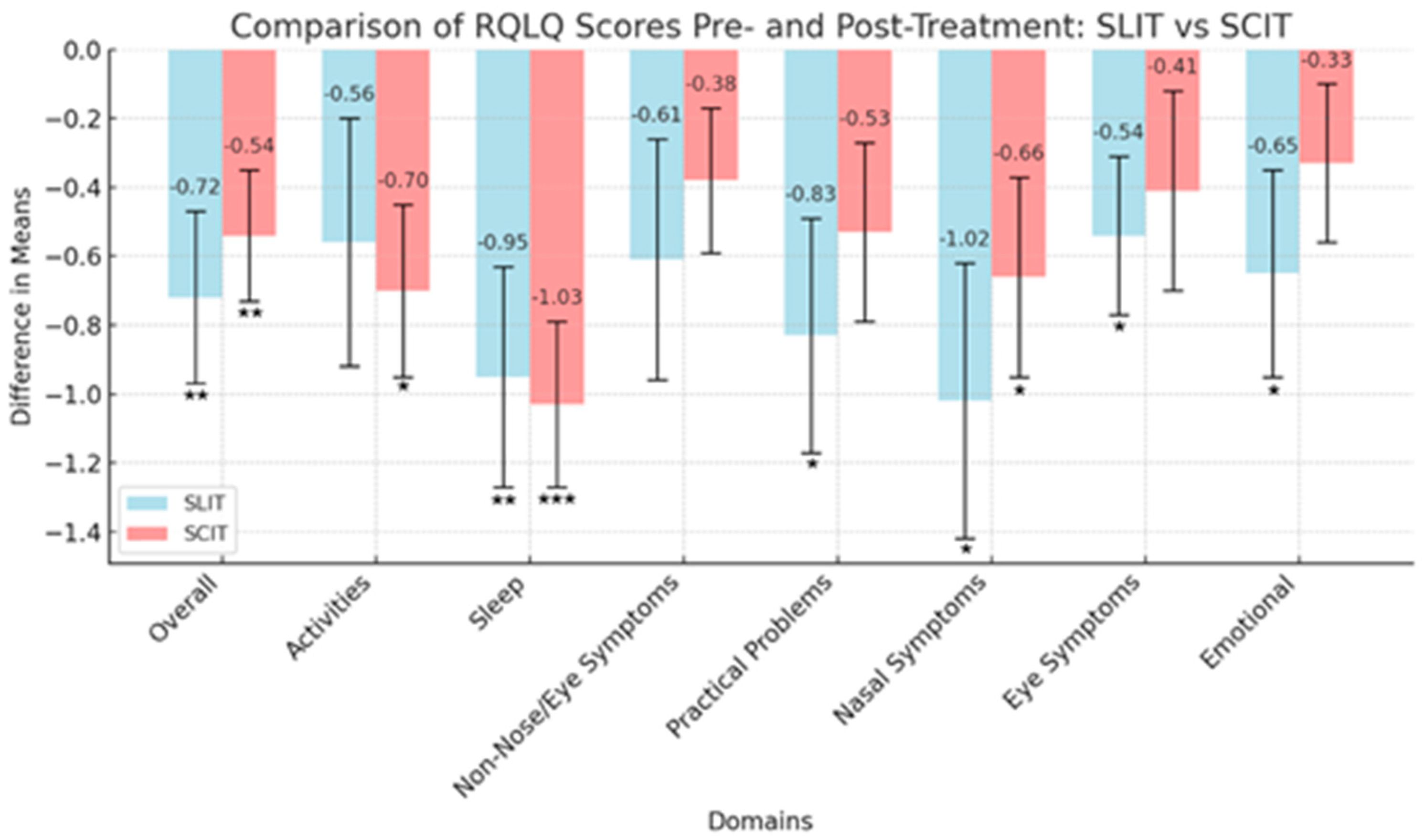
| SLIT cohort n = 21 (51.2) | |||||
| Overall, mean change in RQLQ score | −0.72 | 0.250 | 0.009 | −1.09, −0.15 | −0.63 |
| Activities, mean change in score | −0.56 | 0.360 | 0.138 | −0.77, 0.11 | −0.34 |
| Sleep | −0.95 | 0.32 | 0.007 | −1.13, −0.18 | −0.66 |
| Non-Nose/Eye Symptoms | −0.61 | 0.35 | 0.093 | −0.83, 0.063 | −0.39 |
| Practical Problems | −0.83 | 0.34 | 0.026 | −0.98, −0.06 | −0.53 |
| Nasal Symptoms | −1.02 | 0.40 | 0.018 | −1.02, −0.10 | −0.56 |
| Eye Symptoms | −0.54 | 0.23 | 0.028 | −0.97, −0.06 | −0.52 |
| Emotional | −0.65 | 0.30 | 0.043 | −0.92, −0.01 | −0.47 |
| SCIT cohort n = 20 (48.8) | |||||
| Overall, mean change in RQLQ score | −0.54 | 0.19 | 0.009 | −1.12, −0.16 | −0.65 |
| Activities, mean change in score | −0.70 | 0.25 | 0.013 | −1.09, −0.13 | −0.62 |
| Sleep | −1.03 | 0.24 | <0.001 | −1.48, −0.42 | −0.96 |
| Non-Nose/Eye Symptoms | −0.38 | 0.21 | 0.094 | −0.85, 0.07 | −0.39 |
| Practical Problems | −0.53 | 0.26 | 0.057 | −0.91, 0.01 | −0.45 |
| Nasal Symptoms | −0.66 | 0.29 | 0.032 | −0.98, −0.05 | −0.52 |
| Eye Symptoms | −0.41 | 0.29 | 0.174 | −0.76, 0.14 | −0.32 |
| Emotional | −0.33 | 0.23 | 0.168 | −0.77, 0.13 | −0.32 |
| Difference in Means | Std. Error of the Difference | p = x | 95% CI | Effect Size | |
|---|---|---|---|---|---|
| Overall, mean change in RQLQ score | −0.18 | 0.31 | 0.57 | −0.79, 0.434 | −0.18 |
| Activities, mean change in score | 0.14 | 0.44 | 0.75 | −0.51, 0.71 | 0.102 |
| Sleep | 0.08 | 0.40 | 0.84 | −0.55, 0.68 | 0.06 |
| Non-Nose/Eye Symptoms | −0.23 | 0.41 | 0.57 | −0.79, 0.44 | −0.18 |
| Practical Problems | −0.29 | 0.43 | 0.51 | −0.82, 0.41 | −0.21 |
| Nasal Symptoms | −0.36 | 0.49 | 0.47 | −0.84, 0.39 | −0.23 |
| Eye Symptoms | −0.12 | 0.37 | 0.74 | −0.72, 0.51 | −0.11 |
| Emotional | −0.33 | 0.38 | 0.39 | −0.88, 0.35 | −0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, L.M.; Longfellow, G.A.; Kessel, J.C.; Thorp, B.D.; Kimple, A.J.; Klatt-Cromwell, C.N.; Senior, B.A.; Ebert, C.S., Jr. Direct Comparison of Quality of Life in Patients with Allergic Rhinitis Undergoing Sublingual Versus Subcutaneous Immunotherapy. J. Clin. Med. 2024, 13, 6397. https://doi.org/10.3390/jcm13216397
Cook LM, Longfellow GA, Kessel JC, Thorp BD, Kimple AJ, Klatt-Cromwell CN, Senior BA, Ebert CS Jr. Direct Comparison of Quality of Life in Patients with Allergic Rhinitis Undergoing Sublingual Versus Subcutaneous Immunotherapy. Journal of Clinical Medicine. 2024; 13(21):6397. https://doi.org/10.3390/jcm13216397
Chicago/Turabian StyleCook, Lauren M., Grace A. Longfellow, Julia C. Kessel, Brian D. Thorp, Adam J. Kimple, Cristine N. Klatt-Cromwell, Brent A. Senior, and Charles S. Ebert, Jr. 2024. "Direct Comparison of Quality of Life in Patients with Allergic Rhinitis Undergoing Sublingual Versus Subcutaneous Immunotherapy" Journal of Clinical Medicine 13, no. 21: 6397. https://doi.org/10.3390/jcm13216397
APA StyleCook, L. M., Longfellow, G. A., Kessel, J. C., Thorp, B. D., Kimple, A. J., Klatt-Cromwell, C. N., Senior, B. A., & Ebert, C. S., Jr. (2024). Direct Comparison of Quality of Life in Patients with Allergic Rhinitis Undergoing Sublingual Versus Subcutaneous Immunotherapy. Journal of Clinical Medicine, 13(21), 6397. https://doi.org/10.3390/jcm13216397







