Cryptosporidium spp. Infection in Adult Kidney Transplant Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Cryptosporidiosis in Adult Kidney Transplant Patients
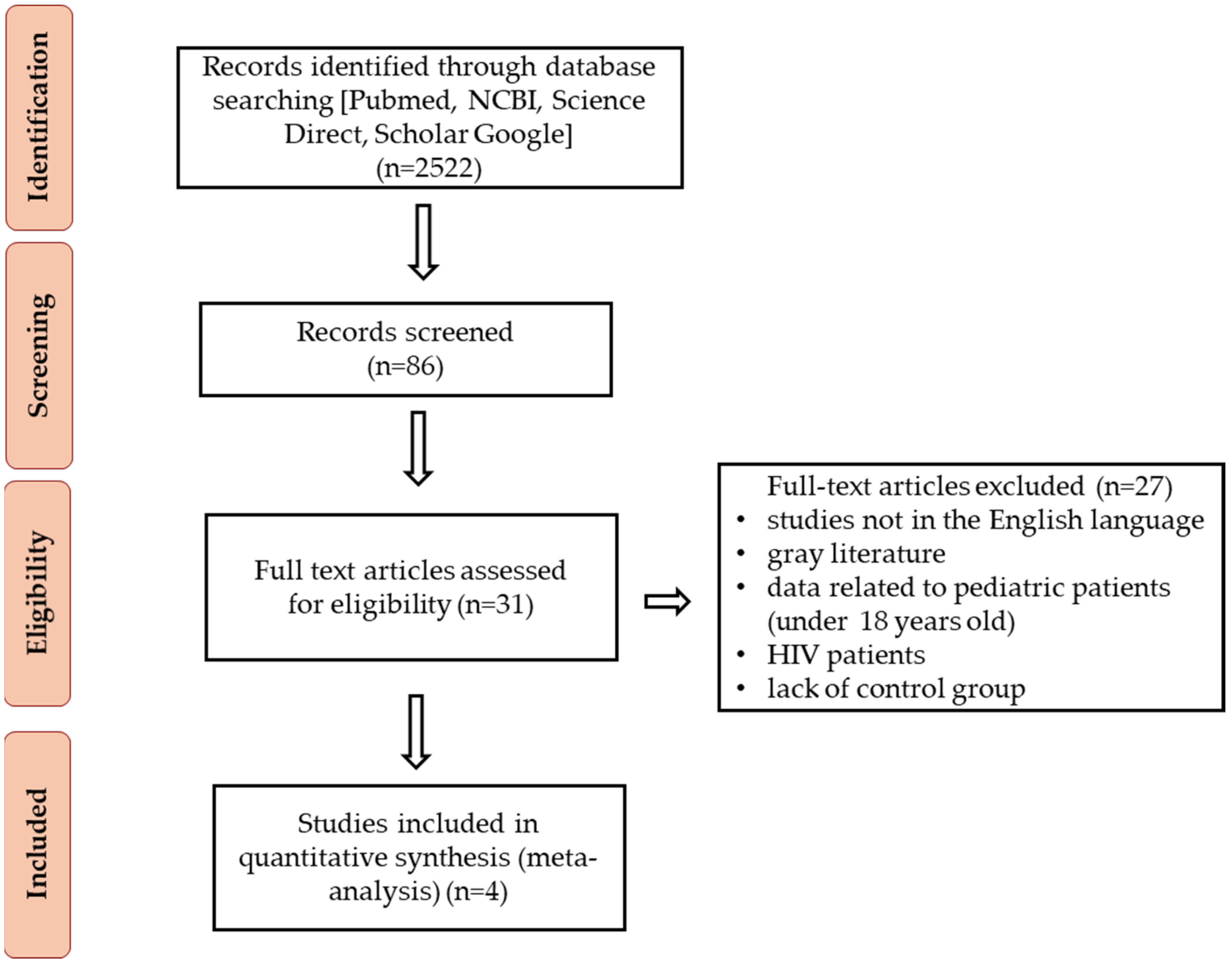
2.1. Methods
2.2. Results and Discussion
2.2.1. Epidemiological Research
2.2.2. Analysis of Case Reports
3. Limitations
4. Conclusions
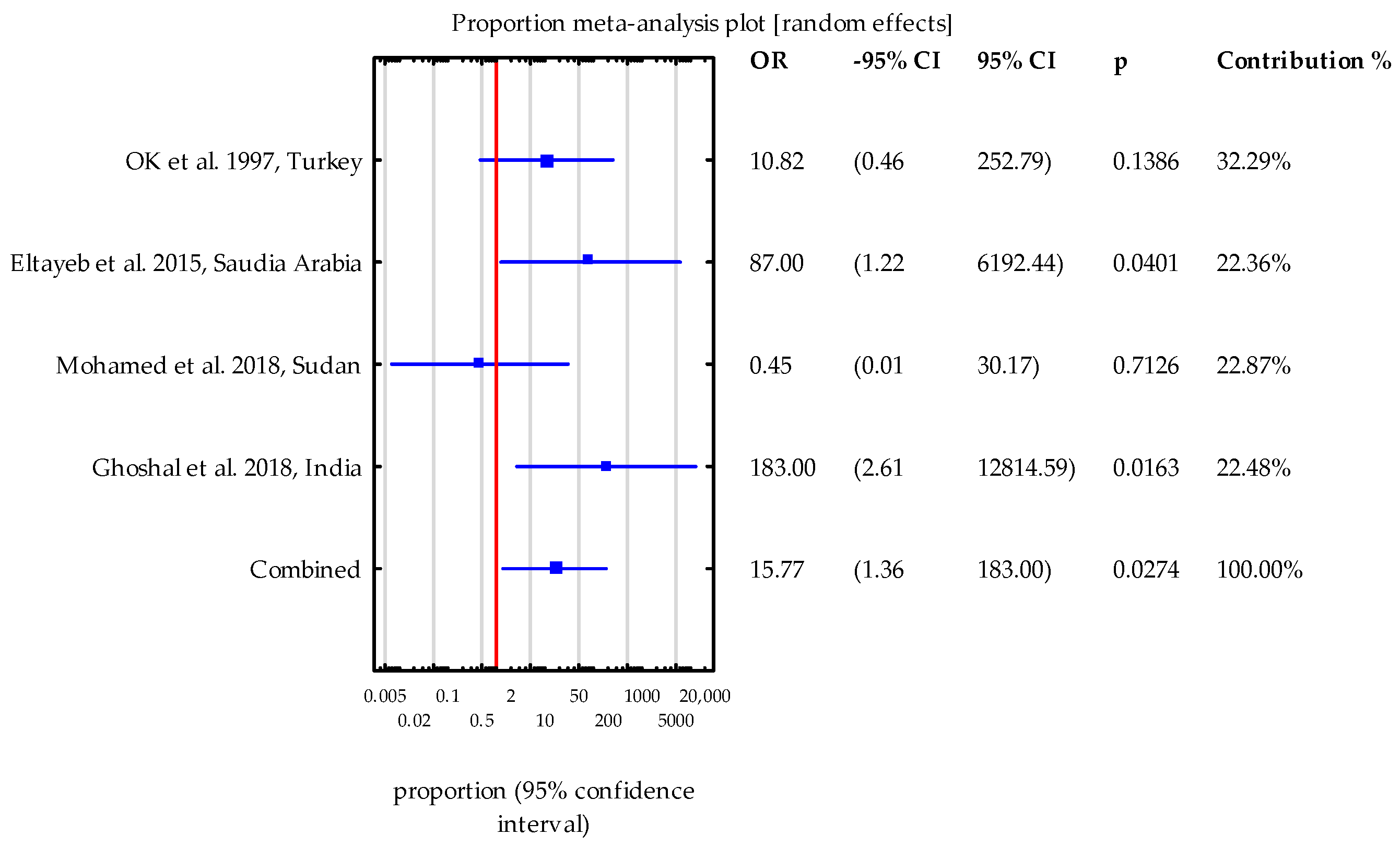
- This systematic review and meta-analysis have highlighted an often-overlooked connection between Cryptosporidium spp. infections and kidney transplantation. Our study demonstrates that adult kidney transplant patients are at a significantly higher risk of acquiring Cryptosporidium spp. compared to healthy participants.
- Cryptosporidium spp. infections can be asymptomatic, making it essential to screen both symptomatic and asymptomatic kidney transplant recipients. The clinical presentation of cryptosporidiosis typically involves digestive symptoms and can be complicated by biliary tract involvement.
- In KTR patients presenting with diarrhea, it is crucial to not only test for Cryptosporidium spp. but also to rule out bacterial and viral etiologies, including infections such as C. difficile, C. colitis, Clostridium spp., and rotavirus.
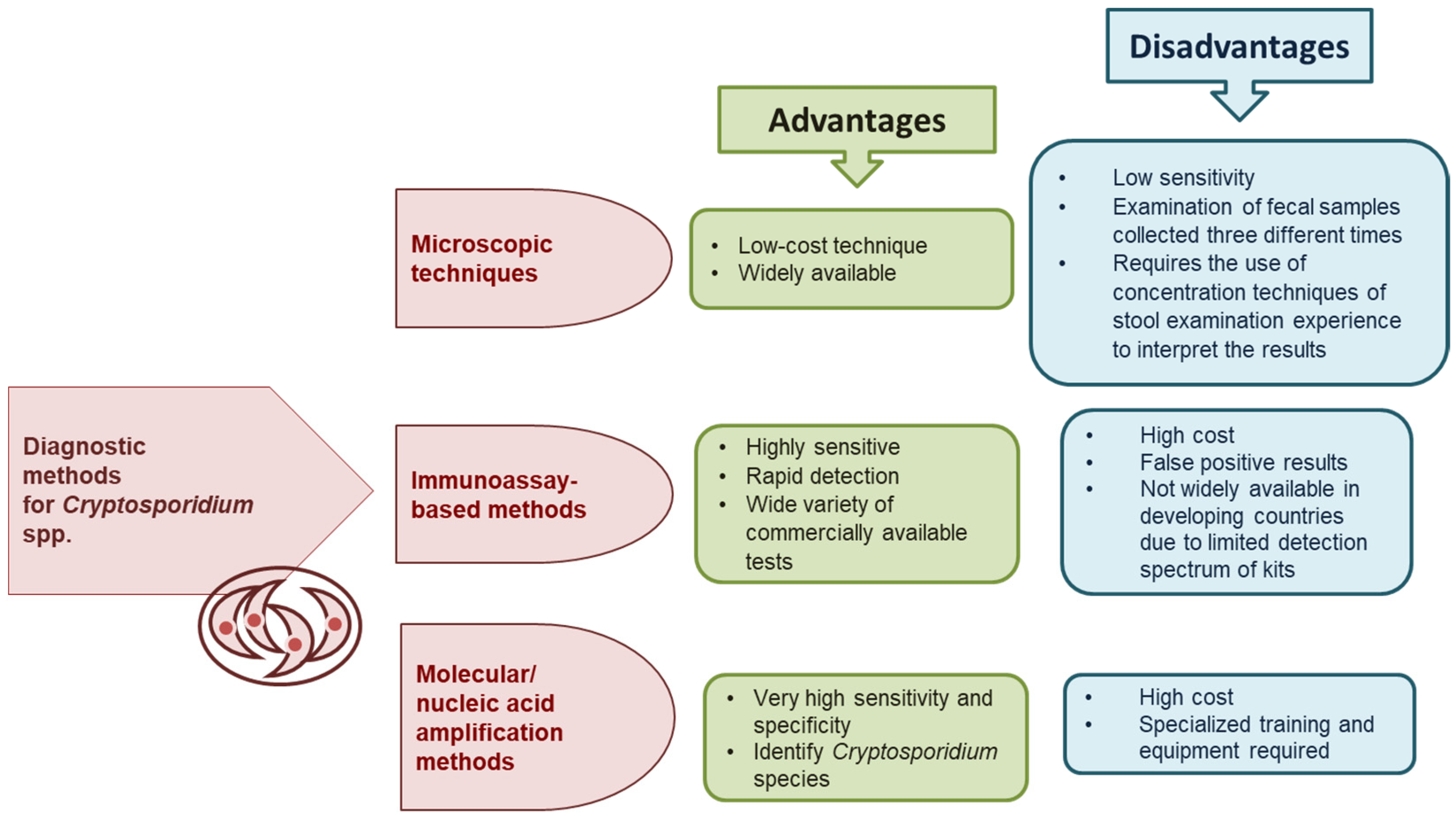
- The diagnosis of Cryptosporidium spp. infections primarily relies on microscopic methods, which are known for their low sensitivity. Therefore, diagnostic approaches should include both direct methods and, where possible, molecular techniques.
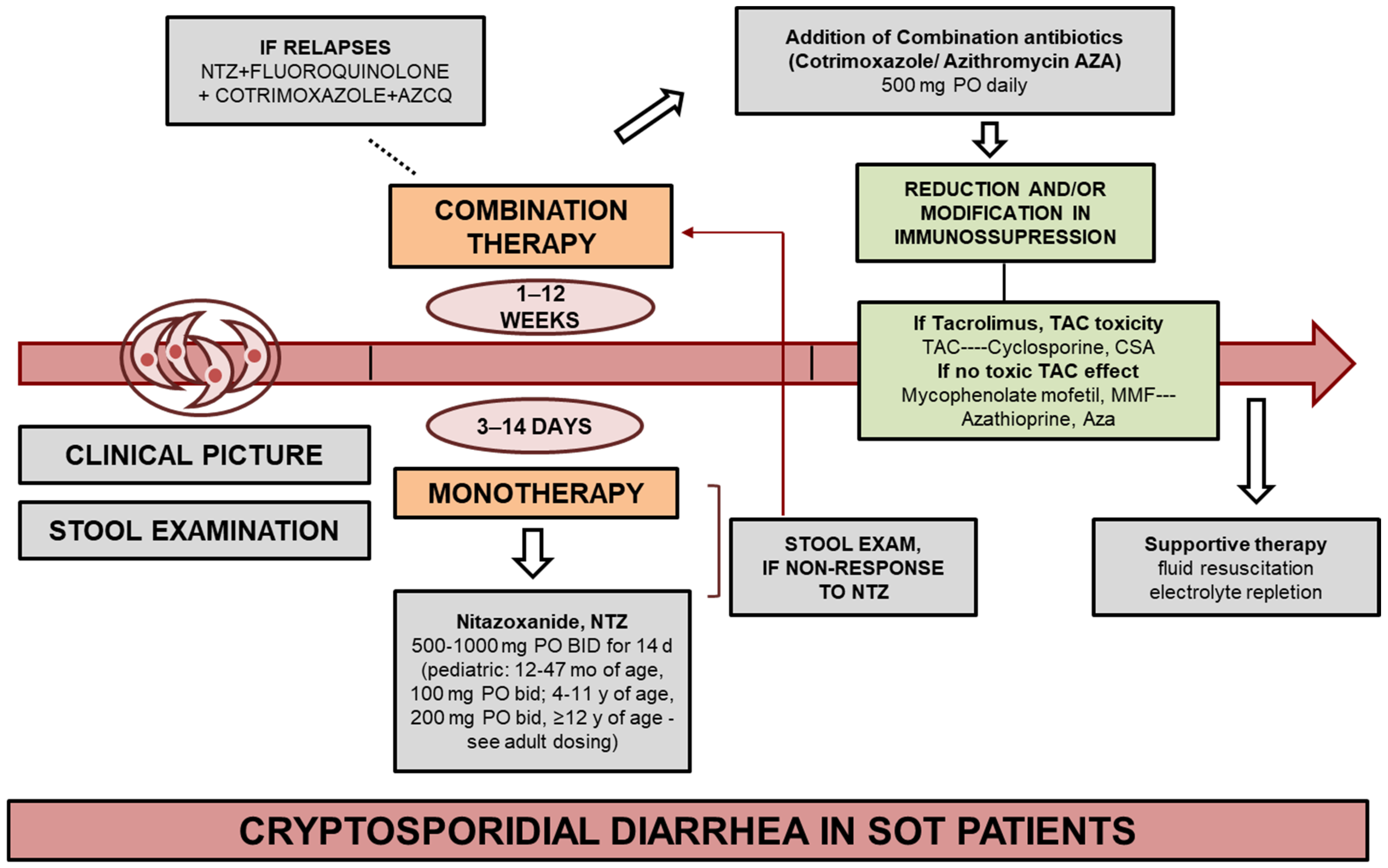
- Based on the analyzed cases, the most effective treatment results were achieved with reduction in immunosuppression if possible (strong, very low) and nitazoxanide at a dose of 500 mg twice daily for 14 days.
- Considering the public health implications of our findings, the current epidemiological data underscore the need for further research to develop effective prevention and intervention strategies against cryptosporidiosis. Preventive measures, regular screening programs, and the treatment of Cryptosporidium spp. infections should be integrated into the clinical care of transplant patients. It is also important that patients are informed about environmental risk factors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fletcher, S.M.; Stark, D.; Harkness, J.; Ellis, J. Enteric protozoa in the developed world: A public health perspective. Clin. Microbiol. Rev. 2012, 25, 420–449. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L. The burden and etiology of diarrheal illness in developing countries. Pediatr. Clin. N. Am. 2017, 64, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Plutzer, J.; Karanis, P. Genetic polymorphism in Cryptosporidium species: An update. Vet. Parasitol. 2009, 165, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Hafez, H.M. Cryptosporidiosis: From prevention to treatment, a narrative review. Microorganisms 2022, 10, 2456. [Google Scholar] [CrossRef]
- Iqbal, A.; Lim, Y.A.; Mahdy, M.A.; Dixon, B.R.; Surin, J. Epidemiology of cryptosporidiosis in HIV-infected individuals: A global perspective. Open Access Sci. Rep. 2012, 1, 431. [Google Scholar]
- Bouzid, M.; Hunter, P.R.; Chalmers, R.M.; Tyler, K.M. Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev. 2013, 26, 115–134. [Google Scholar] [CrossRef]
- Hatalova, E.; Guman, T.; Bednarova, V.; Simova, V.T.; Logoida, M.; Halanova, M. Occurrence of Cryptosporidium parvum IIaA17G1R1 in hospitalized hemato-oncological patients in Slovakia. Parasitol. Res. 2022, 121, 471–476. [Google Scholar] [CrossRef]
- Cengiz, Z.T.; Yilmaz, H.; Sahin, I.H.; Kapmaz, M.; Ekici, P. The frequency of Cryptosporidium spp. in immunocompromised patients by modified acid-fast staining, cassette Kit and ELISA methods: Comparison of the diagnostic techniques. Jundishapur J. Microbiol. 2017, 10, e36479. [Google Scholar] [CrossRef]
- Hunter, P.R.; Nichols, G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin. Microbiol. Rev. 2002, 15, 145–154. [Google Scholar] [CrossRef]
- Pumipuntu, N.; Piratae, S. Cryptosporidiosis: A zoonotic disease concern. Vet. World 2018, 11, 681. [Google Scholar] [CrossRef]
- Chalmers, R.M.; Davies, A.P. Minireview: Clinical cryptosporidiosis. Exp. Parasitol. 2010, 124, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Shirley, D.A.; Moonah, S.N.; Kotloff, K.L. Burden of disease from cryptosporidiosis. Curr. Opin. Infect. Dis. 2012, 25, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Leitch, G.J.; He, Q. Cryptosporidiosis—An overview. J. Biomed. Res. 2012, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lendner, M.; Daugschies, A. Cryptosporidium infections: Molecular advances. Parasitology 2014, 141, 1511–1532. [Google Scholar] [CrossRef]
- Hlavsa, M.C.; Watson, J.C.; Beach, M.J. Cryptosporidiosis surveillance—United States 1999–2002. MMWR Surveill. Summ. 2005, 54, 1–8. [Google Scholar]
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef]
- Bruce, B.B.; Blass, M.A.; Blumberg, H.M.; Lennox, J.L.; del Rio, C.; Horsburgh, C.R., Jr. Risk of Cryptosporidium parvum transmission between hospital roommates. Clin. Infect. Dis. 2000, 31, 947–950. [Google Scholar] [CrossRef][Green Version]
- Graczyk, T.K.; Fayer, R.; Knight, R.; Mhangami-Ruwende, B.; Trout, J.M.; Da Silva, A.J.; Pieniazek, N.J. Mechanical transport and transmission of Cryptosporidium parvum oocysts by wild filth flies. Am. J. Trop. Med. Hyg. 2000, 63, 178–183. [Google Scholar] [CrossRef]
- Korich, D.G.; Mead, J.R.; Madore, M.S.; Sinclair, N.A.; Sterling, C.R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 1990, 56, 1423–1428. [Google Scholar] [CrossRef]
- Khurana, S.; Chaudhary, P. Laboratory diagnosis of cryptosporidiosis. Trop. Parasitol. 2018, 8, 2–7. [Google Scholar] [CrossRef]
- Caccio, S.M.; Widmer, G. Cryptosporidium: Parasite and Disease; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- O’Leary, J.K.; Sleator, R.D.; Lucey, B. Cryptosporidium spp. diagnosis and research in the 21st century. Food Waterborne Parasitol. 2021, 24, e00131. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, D.; Cnops, L.; Verschueren, J.; Van Esbroeck, M. Comparison of four rapid diagnostic tests, ELISA, microscopy and PCR for the detection of Giardia lamblia, Cryptosporidium spp. and Entamoeba histolytica in feces. J. Microbiol. Methods 2015, 110, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010, 124, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Gunasekera, T.S.; Barardi, C.R.; Veal, D.; Vesey, G. Determination of Cryptosporidium parvum oocyst viability by fluorescence in situ hybridization using a ribosomal RNA-directed probe. J. Appl. Microbiol. 2004, 96, 409–417. [Google Scholar] [CrossRef]
- Cunha, F.S.; Peralta, R.H.S.; Peralta, J.M. New insights into the detection and molecular characterization of Cryptosporidium with emphasis in Brazilian studies: A review. Rev. Inst. Med. Trop. 2019, 61, e28. [Google Scholar] [CrossRef]
- Omoruyi, B.E.; Nwodo, U.U.; Udem, C.S.; Okonkwo, F.O. Comparative diagnostic techniques for Cryptosporidium infection. Molecules 2014, 19, 2674–2683. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Kumar, S.; Smith, W.A.; Sahu, P.S. Revisiting the global problem of cryptosporidiosis and recommendations. Trop. Parasitol. 2017, 7, 8–17. [Google Scholar]
- Vanathy, K.; Parija, S.C.; Mandal, J.; Hamide, A.; Krishnamurthy, S. Cryptosporidiosis: A mini review. Trop. Parasitol. 2017, 7, 72–80. [Google Scholar]
- Love, M.S.; Choy, R.K.M. Emerging treatment options for cryptosporidiosis. Curr. Opin. Infect. Dis. 2021, 34, 455–462. [Google Scholar] [CrossRef]
- Florescu, D.F.; Sandkovsky, U. Cryptosporidium infection in solid organ transplantation. World J. Transplant. 2016, 6, 460–471. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Eckmann, L. Drug development against the major diarrhea-causing parasites of the small intestine, Cryptosporidium and Giardia. Front. Microbiol. 2015, 6, 1208. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.F.; Ayoub, A.; Ayers, M.S. Treatment of diarrhea caused by Cryptosporidium parvum: A prospective randomized, double-blind, placebo-controlled study of nitazoxanide. J. Infect. Dis. 2001, 184, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.F.; Kabil, S.M.; El-Gohary, Y.; Younis, A.M. Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species. Clin. Gastroenterol. Hepatol. 2006, 4, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Berahmat, R.; Spotin, A.; Ahmadpour, E.; Mahami-Oskouei, M.; Rezamand, A.; Aminisani, N.; Ghojazadeh, M.; Ghoyounchi, R.; Mikaeili-Galeh, T. Human cryptosporidiosis in Iran: A systematic review and meta-analysis. Parasitol. Res. 2017, 116, 1111–1128. [Google Scholar] [CrossRef]
- Eltayeb, L.B.; Waggiallah, H.A. Impact of immunosuppressive drugs on sudanese renal transplant recipients infected with intestinal parasites. Int. J. Curr. Res. Rev. 2016, 8, 20–24. [Google Scholar]
- Ghoshal, U.; Ranjan, P.; Dey, A.; Ghoshal, U.C. Intestinal cryptosporidiosis in renal transplant recipients: Prevalence, species detection and comparative evaluation of SSU rRNA and Cryptosporidium oocyst wall protein genes. Indian J. Med. Microbiol. 2018, 36, 247–250. [Google Scholar] [CrossRef]
- Ishaque, M.; Rashid, R.; Mubarak, M. Gastrointestinal complications in renal transplant recipients detected by endoscopic biopsies in a developing country. Indian J. Gastroenterol. 2015, 34, 51–57. [Google Scholar] [CrossRef]
- Jafari, R.; Gharibi, Z.; Fallah, M. The Prevalence of Cryptosporidium infection among renal transplanted patients in hamadan city, west of Iran. Avicenna J. Clin. Microbiol. Infect. 2014, 1, 19570. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Siddig, E.E.; Mohamed, M.A.; Alzein, B.A.; Osman, H.H.S.; Tanyous, E.E.; Elamin, B.K.; Edris, A.M.M. Enteroparasitosis infections among renal transplant recipients in Khartoum state, Sudan 2012–2013. BMC Res. Notes 2018, 11, 621. [Google Scholar] [CrossRef]
- Ok, U.Z.; Cirit, M.; Uner, A.; Ok, E.; Akcicek, F.; Basci, A.; Ozcel, M.A. Cryptosporidiosis and blastocystosis in renal transplant recipients. Nephron 1997, 75, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Saxena, N.G.; Thakare, S.B.; Pajai, A.E.; Bajpai, D.; Jamale, T.E. Diarrhea after kidney transplantation: A study of risk factors and outcomes. J. Postgrad. Med. 2023, 69, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Shad, S.; Hanif, F.; Ul Haq, M.; Luck, N.H.; Aziz, T.; Mubarak, M. Frequencies of common infectious organisms causing chronic diarrhea in renal transplant patients. Exp. Clin. Transplant. 2019, 17, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Udgiri, N.; Minz, M.; Kashyap, R.; Heer, M.; Gupta, C.S.; Mohandas, K.; Minz, R.W.; Malla, N. Intestinal cryptosporidiasis in living related renal transplant recipients. Transplant. Proc. 2004, 36, 2128–2129. [Google Scholar] [CrossRef]
- Brunet, J.; Lemoine, J.P.; Pesson, B.; Valot, S.; Sautour, M.; Dalle, F.; Muller, C.; Borni-Duval, C.; Caillard, S.; Moulin, B.; et al. Ruling out nosocomial transmission of Cryptosporidium in a renal transplantation unit: Case report. BMC Infect. Dis. 2016, 16, 363. [Google Scholar] [CrossRef]
- Deltombe, C.; Lefebvre, M.; Morio, F.; Boutoille, D.; Imbert, B.M.; Le Pape, P.; Raffi, F.; Hourmant, M. Cryptosporidiosis and microsporidiosis as causes of diarrhea in kidney and/or pancreas transplant recipients. Med. Mal. Infect. 2020, 50, 407–413. [Google Scholar] [CrossRef]
- Burdese, M.; Veglio, V.; Consiglio, V.; Soragna, G.; Mezza, E.; Bergamo, D.; Tattoli, F.; Rossetti, M.; Jeantet, A.; Segoloni, G.P.; et al. A dance teacher with kidney-pancreas transplant and diarrhoea: What is the cause? Nephrol. Diallysis Transplant. 2005, 20, 1759–1761. [Google Scholar] [CrossRef][Green Version]
- Castellano Carrasco, R.; Torres Sanchez, M.J.; de Teresa Alguacil, F.J.; Osuna Ortega, A. Diarrhea and malnutrition in kidney transplant recipient: A case of infection by cryptosporidiosis. Nefrologia 2017, 37, 338–339. [Google Scholar] [CrossRef]
- Shrateh, O.N.; Jobran, A.; Zaid, M.A.; Saleh, M. Successful management of life-threatening post-COVID-19 cryptosporidiosis in a renal transplant patient: A case report. Pan Afr. Med. J. 2023, 45, 10. [Google Scholar]
- Khalil-ur-Rahman; Al-Amoudi, A.; Badreddine, S.; Al-Shehri, A.M.; Kanaan, H.; Al-Ghamdi, S.M. Cryptosporidiosis in a renal transplant patient treated with paromomycin. Ann. Saudi Med. 2007, 27, 373–374. [Google Scholar] [CrossRef]
- Zheng, S.; Ko, K.K.; Chan, K.S.; Venkatachalam, I. Case report: Diagnosis of cryptosporidiosis in renal transplantation in a low-prevalence setting. Am. J. Trop. Med. Hyg. 2019, 100, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, P.S.; Parameswaran, S.; Morkhandikar, S.; Shankar, V. Successful eradication of Cryptosporidium in kidney transplant recipients—Two case reports. Indian J. Transplant. 2014, 8, 22–24. [Google Scholar] [CrossRef]
- Abdo, A.; Klassen, J.; Urbanski, S.; Raber, E.; Swain, M.G. Reversible sclerosing cholangitis secondary to cryptosporidiosis in a renal transplant patient. J. Hepatol. 2003, 38, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.Q.; Gohh, R.Y.; Morrissey, P.E.; Dworkin, L.D.; Gautam, A.; Monaco, A.P.; Yango, A.F., Jr. Cryptosporidium infection in renal transplant patients. Clin. Nephrol. 2005, 63, 305–309. [Google Scholar] [CrossRef]
- Tomczak, E.; McDougal, A.N.; White, A.C., Jr. resolution of cryptosporidiosis in transplant recipients: Review of the literature and presentation of a renal transplant patient treated with nitazoxanide, azithromycin, and rifaximin. Open Forum Infect. Dis. 2021, 9, ofab610. [Google Scholar] [CrossRef]
- Bonatti, H.; Barroso, L.F., 2nd; Sawyer, R.G.; Kotton, C.N.; Sifri, C.D. Cryptosporidium enteritis in solid organ transplant recipients: Multicenter retrospective evaluation of 10 cases reveals an association with elevated tacrolimus concentrations. Transplant. Infect. Dis. 2012, 14, 635–648. [Google Scholar] [CrossRef]
- Kotton, C.N.; Lattes, R.; AST Infectious Diseases Community of Practice. Parasitic infections in solid organ transplant recipients. Am. J. Transplant. 2009, 9, S234–S251. [Google Scholar] [CrossRef]
- Piva, S.; Caffara, M.; Pasquali, F.; Castagnetti, C.; Iacono, E.; Massella, E.; Zanoni, R.G.; Galuppi, R. Detection and quantification of Cryptosporidium oocysts in environmental surfaces of an Equine Perinatology Unit. Prev. Vet. Med. 2016, 131, 67–74. [Google Scholar] [CrossRef]
- Bhadauria, D.; Goel, A.; Kaul, A.; Sharma, R.K.; Gupta, A.; Ruhela, V.; Gupta, A.; Vardhan, H.; Prasad, N. Cryptosporidium infection after renal transplantation in an endemic area. Transpl. Infect. Dis. 2015, 17, 48–55. [Google Scholar] [CrossRef]
- Rosser, J.I.; Blackburn, B.G. Pathogenic intestinal parasites in transplant recipients emerging transplant infections. In Emerging Transplant Infections; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1397–1450. [Google Scholar]
- Cheng, F.; Li, Q.; Cui, Z.; Wang, Z.; Zeng, F.; Zhang, Y. Tacrolimus concentration is effectively predicted using combined clinical and genetic factors in the perioperative period of kidney transplantation and associated with acute rejection. J. Immunol. Res. 2022, 2022, 3129389. [Google Scholar] [CrossRef]
- Dey, A.; Ghoshal, U.; Agarwal, V.; Ghoshal, U.C. Genotyping of Cryptosporidium species and their clinical manifestations in patients with renal transplantation and human immunodeficiency virus infection. J. Pathog. 2016, 2016, 2623602. [Google Scholar] [CrossRef] [PubMed]
- Sonambekar, A.; Mehta, V.; Desai, D.; Abraham, P.; Almeida, A.; Joshi, A.; Gupta, T.; Sirsat, R.; Kothari, J. Diarrhea in kidney transplant recipients: Etiology and outcome. Indian J. Gastroenterol. 2020, 39, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Calmet, F.H.; Yarur, A.J.; Pukazhendhi, G.; Ahmad, J.; Bhamidimarri, K.R. Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Ann. Gastroenterol. 2015, 28, 366–373. [Google Scholar] [PubMed]
- Farthing, M.J. Clinical aspects of human cryptosporidiosis. Contrib. Microbiol. 2000, 6, 50–74. [Google Scholar]
- Hunter, P.R.; Hadfield, S.J.; Wilkinson, D.; Lake, I.R.; Harrison, F.C.; Chalmers, R.M. Correlation between subtypes of Cryptosporidium parvum in humans and risk. Emerg. Infect. Dis. 2007, 13, 82–88. [Google Scholar] [CrossRef]
- Robertson, B.; Sinclair, M.I.; Forbes, A.B.; Veitch, M.; Kirk, M.; Cunliffe, D.; Willis, J.; Fairley, C.K. Case-control studies of sporadic cryptosporidiosis in Melbourne and Adelaide, Australia. Epidemiol. Infect. 2002, 128, 419–431. [Google Scholar] [CrossRef]
- Bahdi, F.; Jain, S.; Agarwal, S.K. Cryptosporidiosis in an immunosuppressed patient with persistent diarrhea. Clevel. Clin. J. Med. 2021, 88, 24–26. [Google Scholar] [CrossRef]
- Aulagnon, F.; Scemla, A.; DeWolf, S.; Legendre, C.; Zuber, J. Diarrhea after kidney transplantation: A new look at a frequent symptom. Transplantation 2014, 98, 806–816. [Google Scholar] [CrossRef]
- Clifford, C.P.; Crook, D.W.; Conlon, C.P.; Fraise, A.P.; Day, D.G.; Peto, T.E. Impact of waterborne outbreak of cryptosporidiosis on AIDS and renal transplant patients. Lancet 1990, 335, 1455–1456. [Google Scholar] [CrossRef]
- Roncoroni, A.J.; Gomez, M.A.; Mera, J.; Cagnoni, P.; Michel, M.D. Cryptosporidium infection in renal transplant patients. J. Infect. Dis. 1989, 160, 559. [Google Scholar] [CrossRef]
- Arslan, H.; Inci, E.K.; Azap, O.K.; Karakayali, H.; Torgay, A.; Haberal, M. Etiologic agents of diarrhea in solid organ recipients. Transpl. Infect. Dis. 2007, 9, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, N.; Gorgani-Firouzjaee, T.; Ghaffari, S.; Bayani, M.; Ghaffari, T.; Chehrazi, M. Association between Cryptosporidium infection and cancer: A systematic review and meta-analysis. Parasitol. Int. 2020, 74, 101979. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Khalil, S.; Mirdha, B. Molecular appraisal of intestinal parasitic infection in transplant recipients. Indian J. Med. Res. 2016, 144, 258–263. [Google Scholar] [PubMed]
- Manser, M.; Granlund, M.; Edwards, H.; Saez, A.; Petersen, E.; Evengard, B.; Chiodini, P.; European Society of Clinical Microbiology and Infectious Diseases Study Group on Clinical Parasitology. Detection of Cryptosporidium and Giardia in clinical laboratories in Europe—A comparative study. Clin. Microbiol. Infect. 2014, 20, O65–O71. [Google Scholar] [CrossRef] [PubMed]
- Casemore, D.P. ACP Broadsheet 128: June 1991. Laboratory methods for diagnosing cryptosporidiosis. J. Clin. Pathol. 1991, 44, 445–451. [Google Scholar] [CrossRef]
- Goñi, P.; Martín, B.; Villacampa, M.; García, A.; Seral, C.; Castillo, F.J.; Clavel, A. Evaluation of an immunochromatographic dip strip test for simultaneous detection of Cryptosporidium spp., Giardia duodenalis, and Entamoeba histolytica antigens in human faecal samples. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2077–2082. [Google Scholar] [CrossRef]
- Conteas, C.N.; Sowerby, T.; Berlin, G.W.; Dahlan, F.; Nguyen, A.; Porschen, R.; Donovan, J.; LaRiviere, M.; Orenstein, J.M. Fluorescence techniques for diagnosing intestinal microsporidiosis in stool, enteric fluid, and biopsy specimens from acquired immunodeficiency syndrome patients with chronic diarrhea. Arch. Pathol. Lab. Med. 1996, 120, 847–853. [Google Scholar]
- Vohra, P.; Sharma, M.; Chaudhary, U. A comprehensive review of diagnostic techniques for detection of Cryptosporidium parvum in stool samples. IOSR J. Pharm. 2012, 13, 15–26. [Google Scholar] [CrossRef]
- Meurs, L.; Polderman, A.M.; Vinkeles Melchers, N.V.; Brienen, E.A.; Verweij, J.J.; Groosjohan, B.; Mendes, F.; Mechendura, M.; Hepp, D.H.; Langenberg, M.C.; et al. Diagnosing polyparasitism in a high-prevalence setting in Beira, Mozambique: Detection of intestinal parasites in fecal samples by microscopy and real-time PCR. PLoS Negl. Trop. Dis. 2017, 11, e0005310. [Google Scholar] [CrossRef]
- Shafiekhani, M.; Nikoupour, H.; Mirjalili, M. The experience and outcomes of multidisciplinary clinical pharmacist-led parenteral nutrition service for individuals with intestinal failure in a center without home parenteral nutrition. Eur. J. Clin. Nutr. 2022, 76, 841–847. [Google Scholar] [CrossRef]
- Karuthu, S.; Blumberg, E.A. Common infections in kidney transplant recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Dungca, L.B.P.; Lin, C.C.; Yong, C.C.; Chen, C.L. Epidemiology and risk factors of early bacterial infections after pediatric living donor liver transplantation. Transplant. Proc. 2024, 56, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Dharnidharka, V.R.; Agodoa, L.Y.; Abbott, K.C. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients—An analysis of USRDS data. Am. J. Transplant. 2007, 7, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.S.; Mawhorter, S.D. Parasitic infections in solid organ transplantation. Am. J. Transplant. 2013, 13, 280–303. [Google Scholar] [CrossRef]
- Tie, X.; Zhang, Z.; Zhou, R.; Li, Y.; Xu, J.; Yin, W. A case of septic shock due to delayed diagnosis of Cryptosporidium infection after liver transplantation. BMC Infect. Dis. 2023, 23, 260. [Google Scholar] [CrossRef]
- Krause, I.; Amir, J.; Cleper, R.; Dagan, A.; Behor, J.; Samra, Z.; Davidovits, M. Cryptosporidiosis in children following solid organ transplantation. Pediatr. Infect. Dis. J. 2012, 31, 1135–1138. [Google Scholar] [CrossRef]
- Baishanbo, A.; Gargala, G.; Duclos, C.; François, A.; Rossignol, J.F.; Ballet, J.J.; Favennec, L. Efficacy of nitazoxanide and paromomycin in biliary tract cryptosporidiosis in an immunosuppressed gerbil model. J. Antimicrob. Chemother. 2006, 57, 353–355. [Google Scholar] [CrossRef]

| Country/ Year | Patient Age/Sex | Kidney Transplantation | Cryptosporidium spp. Infection | Treatment of Cryptosporidiosis | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time After/Number of Grafts | Immunosuppressive Treatment | Diagnostic Methods | Species/Intensity | Coinfection | Symptoms | Risk Factor | Drugs | Time | |||
| France, 2016 | 60/M | 8 years/1st graft | TAC (4 mg/day) + MMF) (1 g × 2/day) + PRED (7.5 mg/day) | mZN stain, PCR | C. felis/5–10 oocysts/slide | No | watery diarrhea (2 weeks), nausea, vomiting, weight loss (6 kg) | contact with dog | NTZ 500 mg b.i.d. × 14 d | 2 weeks | [46] |
| 64/M | 2 years/1st graft | TAC (7 mg × 2/day) + MMF (750 mg × 2/day) + PRED (10 mg/day) | mZN stain, PCR | C. hominis/>100 oocysts/slide | ND | watery diarrhea, abdominal pain, weight loss (13 kg) | travel to Mali | 1. Reduction in TAC | 4 weeks | ||
| 2. NTZ 500 b.i.d. × 14 d | |||||||||||
| 34/M | 10 days/2st graft | TAC (6 mg × 2/day) + MMF (750 mg × 2/day) + PRED (25 mg/day) | mZN stain, PCR | C. parvum/1–5 oocysts/slide | ND | watery diarrhea, abdominal pain weight loss (10 kg) | travel to Kosovo | 1. Reduction in TAC | 4 months | ||
| 2. NTZ 500 mg b.i.d × 14 d | |||||||||||
| France, 2014–2015 | 68/M | 56 months/1st graft | Anti-IL2r CNI + MMF | mZN stain, PCR | C. parvum | No | diarrhea, vomiting, dehydration, weight loss (8 kg), acute kidney injury, acidosis | contact with animals and children | 1. Reduction in MMF until diarrhea resolved (6 patients) 2. 500 mg NTZ b.i.d. × 4 weeks (3 patients) | 2 weeks—3 patients, 4 weeks—3 patients | [47] |
| 42/F | 25 months/1st graft | Anti-IL2r CNI + MMF | mZN stain and PCR | ND | No | fever, abdominal pain, diarrhea, vomiting, dehydration, weight loss (4 kg) | previous antibiotic therapy | ||||
| 77/M | 14 days/1st graft | Anti-IL2r CNI + MMF | C. parvum | No | severe diarrhea, dehydration, weight loss (3 kg), acute kidney injury | contact with untreated water | |||||
| 53/M | 2 days/1st graft | Anti-IL2r CNI + MMF | C. felis | diarrhea, vomiting, dehydration, weight loss (4 kg) | contact with cat | ||||||
| 64/F | 65 months/1st graft | Depleting therapy CNI + MMF | C. parvum | No | fever, abdominal pain, diarrhea, vomiting, dehydration, weight loss (2 kg) | none | |||||
| 37/F | 57 months/3st graft | Desensitization, depleting therapy CNI + MMF | C. parvum | Norovirus | fever, abdominal pain, diarrhea, vomiting, dehydration, weight loss (3 kg) | work as a nurse, contact with recreative water, treated with phenoxymethylpenicillin/F | |||||
| Italy, 2005 | 42/F | 1 year/1st graft | TAC (present levels 8–10 ng/mL) + MMF (1250 mg/day) + PRED (5 mg/day) | MAFST | No | ND | abdominal pain, diarrhea (1 week) | traveled to Cuba | Rifaximin 600 mg t.i.d. | 1 year | [48] |
| Spain, 2017 | 57/M | 11 months/1 st graft | PRED (5 mg daily) + CNIs + MMF | ND | ND | No | watery diarrhea (8–10 times per day), abdominal discomfort, and weight loss | ND | Paromomycin 700 mg t.i.d. | 2 weeks | [49] |
| Turkey, 1997 | 38/M | 2 years/1st graft | ND | MAFST | ND | ND | diarrhea (10 days) | ND | Spiramycin 2 g q.d. × 10 d | 3 months | [42] |
| 42/M | 1 year/1st graft | ND | ND | ND | abdominal pain, distention | ND | Spiramycin 2 g q.d. × 10 d | 4 months | |||
| Palestine, 2022 | 41/M | 2 years/1st graft | ND | ND | ND | COVID-19 | weakness, fever of 39 °C, yellowish diarrhea occurring 4–5 times daily without blood | ND | NTZ 500 mg mg b.i.d × 14 d | ND | [50] |
| Saudi Arabia, 2007 | 60/F | 4 months/1st graft | TAC (2 mg b.l.d.) + PRED (10 mg q.d.) + TMP-SMX | Biopsis, PAS-stain | ND | No | watery diarrhea (6–7 times per day), colicky abdominal pain | ND | paramomycin 500 mg b.l.d × 1 months | 1 months | [51] |
| Singapore, 2019 | 37/M | 2 years/1st graft | PRED + MMF + TAC | MAFST | ND | No | acute diarrhea, up to 10 times daily (2 weeks), abdominal discomfort, coryzal symptoms | ND | paromomycin 1 g b.i.d. + AZM 500 mg q.d. | 4 weeks | [52] |
| 2. MMF reduction to 1 g/day | |||||||||||
| India, 2014 | 35/F | 4 months | TAC 3 mg/d + MMF 2 g/d + PRED 10 mg | MAFST | ND | No | watery diarrhea | ND | 1. NTZ 500 mg mg b.i.d. × 3 d, TAC reduction (2 mg/d) | 4 weeks | [53] |
| more than 2 weeks of profuse watery diarrhea, abdominal cramps, dehydration | ND | 3. TAC reduction (1 mg/d), MMF reduction (1 g/d0, NTZ 500 mg mg b.i.d. and AZA 500 mg q.d | |||||||||
| ND | 3. NTZ 500 mg mg b.i.d. and AZA 500 mg q.d., MMF replaced AZA 100 mg | ||||||||||
| 30/M | 4 months | RIX | MAFST | ND | ND | diarrhea | ND | NTZ 500 mg mg d.d., AZA 500 mg q.d., TAC reduction (1.5 g/d) | 4 weeks | ||
| Canada, 2003 | 40/F | 9 years/1st graft | TAC 3 mg b.i.d. + PRED 15 mg q.o.d. + AZA (50 mg d.) | ND | Cryptosporidium-induced sclerosing cholangitis | ND | diarrhea (4–6 watery stools per day), weight loss, marked itching, fatigue | ND | 1. UDCA 15 mg/kg d., reduction in TAC to 2.5 mg b.i.d., AZA to 25 mg d. | 3 months | [54] |
| 2. After 2 weeks, UDCA 15 mg/kg d, RIF 300 mg d × 3 weeks, after 2 weeks TAC increase to 10 mg b.i.d | |||||||||||
| USA, 2005 | 59/F | 2 weeks | TAC + SLM + PRED | Biopsies, MAFST | ND | ND | nausea, vomiting, cramps, abdominal pain, profuse diarrhea | ND | Paromomycin 1 g b.i.d. × 4 weeks, reduced immunosuppression | 2 weeks | [55] |
| USA, 2020 | 24/M | 1 month/2nd graft | TAC + MMF | Biopsies, EIA | ND | No | chronic watery diarrhea, fevers, chills, nausea | ND | 1. NTZ 500 mg b.i.d × 7 d, exchange of MMF for AZA | 10 days | [56] |
| 2. NTZ 1 g b.i.d. + AZM 600 mg d + rifaximin 550 mg b.i.d. + intravenous fluids + diphenoxylate-atropine | |||||||||||
| USA, 2004–2010 | 51/M | 48.3 months/1st graft | TAC + MMF + PRED | mZN stain | ND | No | diarrhea | travel | AZM 250 mg q.d. × 21 d | ND | [57] |
| 53/M | 151 months/1st graft | CYA + AZA + PRED | EIA | ND | Staphylococcus epidermidis | diarrhea, malaise, vomiting | ND | None | ND | ||
| 36/M | 53.1 months | TAC + NNF + PRED | mZN stain | ND | No | diarrhea | travel | AZM 600 mg q.d. and NTZ 500 mg b.i.d. × 18 d; MMF was discontinued | ND | ||
| 52/M | 3.4 months | TAC + MMF + PRED | EIA | ND | No | diarrhea, malaise, abdominal pain, vomiting | restaurant | AZM 600 mg q.d. × 2 d and NTZ 500 mg b.i.d. × 6 d; MMF was discontinued | ND | ||
| 36/M | 34.8 months | TAC + MMF + PRED | EIA | ND | diarrhea, vomiting | well water, farm animals | AZM 250 mg q.d. × 14 d; MMF was discontinued | ND | |||
| 57/M | 22.1 months | TAC + MMF + PRED | mZN stain | ND | Campylobacter jejuni | diarrhea, malaise, abdominal pain, vomiting | travel | MMF dose reduction | ND | ||
| 34/M | 66.0 months | TAC + MMF + PRED | EIA | ND | ND | diarrhea, malaise | travel | AZM 600 mg q.d. × 5 d., NTZ 500 mg b.i.d. × 14 d., TMP-SMX × 14 d | ND | ||
| Characteristics | Value (AM ± SD) | |
|---|---|---|
| age of patients | total (n = 28) | 47.25 ± 13.19 |
| male (n = 20) | 47.20 ± 14.05 | |
| female (n = 8) | 47.38 ± 11.61 | |
| time to onset of diarrhea after kidney transplantation (in months) | total (n = 28) | 28.69 ± 35.27 |
| male (n = 20) | 31.64 ± 38.61 | |
| female (n = 8) | 21.30 ± 25.78 | |
| duration of treatment (days) | total (n = 12) | 72.00 ± 101.03 |
| male (n = 9) | 50.77 ± 47.02 | |
| female (n = 3) | 135.67 ± 198.73 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Korzeniewski, K.; Mularczyk, M.; Kabat-Koperska, J.; Ziętek, P.; Marchelek-Myśliwiec, M. Cryptosporidium spp. Infection in Adult Kidney Transplant Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 6395. https://doi.org/10.3390/jcm13216395
Kosik-Bogacka D, Łanocha-Arendarczyk N, Korzeniewski K, Mularczyk M, Kabat-Koperska J, Ziętek P, Marchelek-Myśliwiec M. Cryptosporidium spp. Infection in Adult Kidney Transplant Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(21):6395. https://doi.org/10.3390/jcm13216395
Chicago/Turabian StyleKosik-Bogacka, Danuta, Natalia Łanocha-Arendarczyk, Krzysztof Korzeniewski, Maciej Mularczyk, Joanna Kabat-Koperska, Paweł Ziętek, and Małgorzata Marchelek-Myśliwiec. 2024. "Cryptosporidium spp. Infection in Adult Kidney Transplant Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 21: 6395. https://doi.org/10.3390/jcm13216395
APA StyleKosik-Bogacka, D., Łanocha-Arendarczyk, N., Korzeniewski, K., Mularczyk, M., Kabat-Koperska, J., Ziętek, P., & Marchelek-Myśliwiec, M. (2024). Cryptosporidium spp. Infection in Adult Kidney Transplant Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(21), 6395. https://doi.org/10.3390/jcm13216395







