Burden of Mitral Regurgitation in Spain from 2016–2021: An Analysis by Aetiology and Sex
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Statistical Approach
3. Results
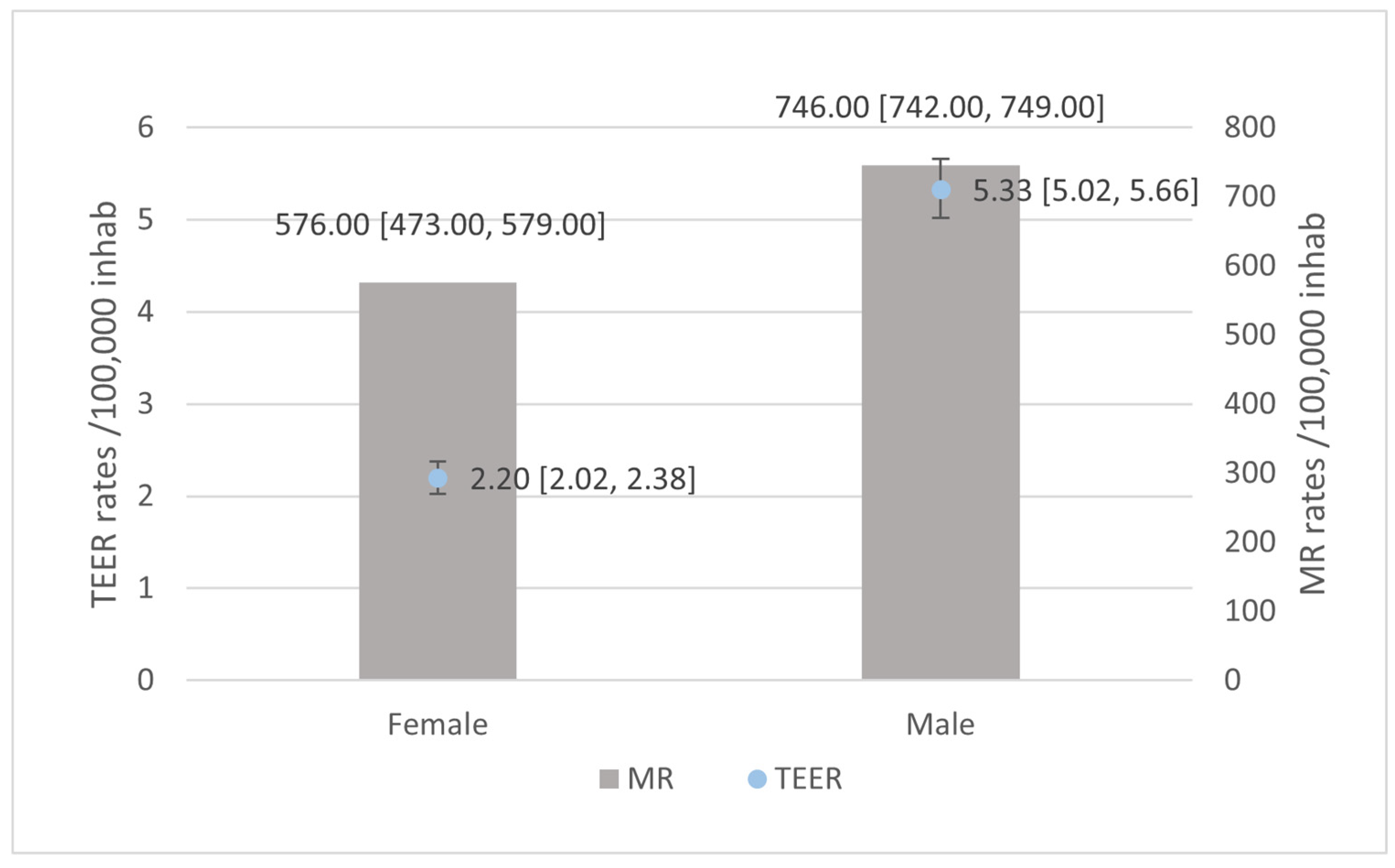
3.1. Patients’ Hospitalisations with an MR Diagnosis
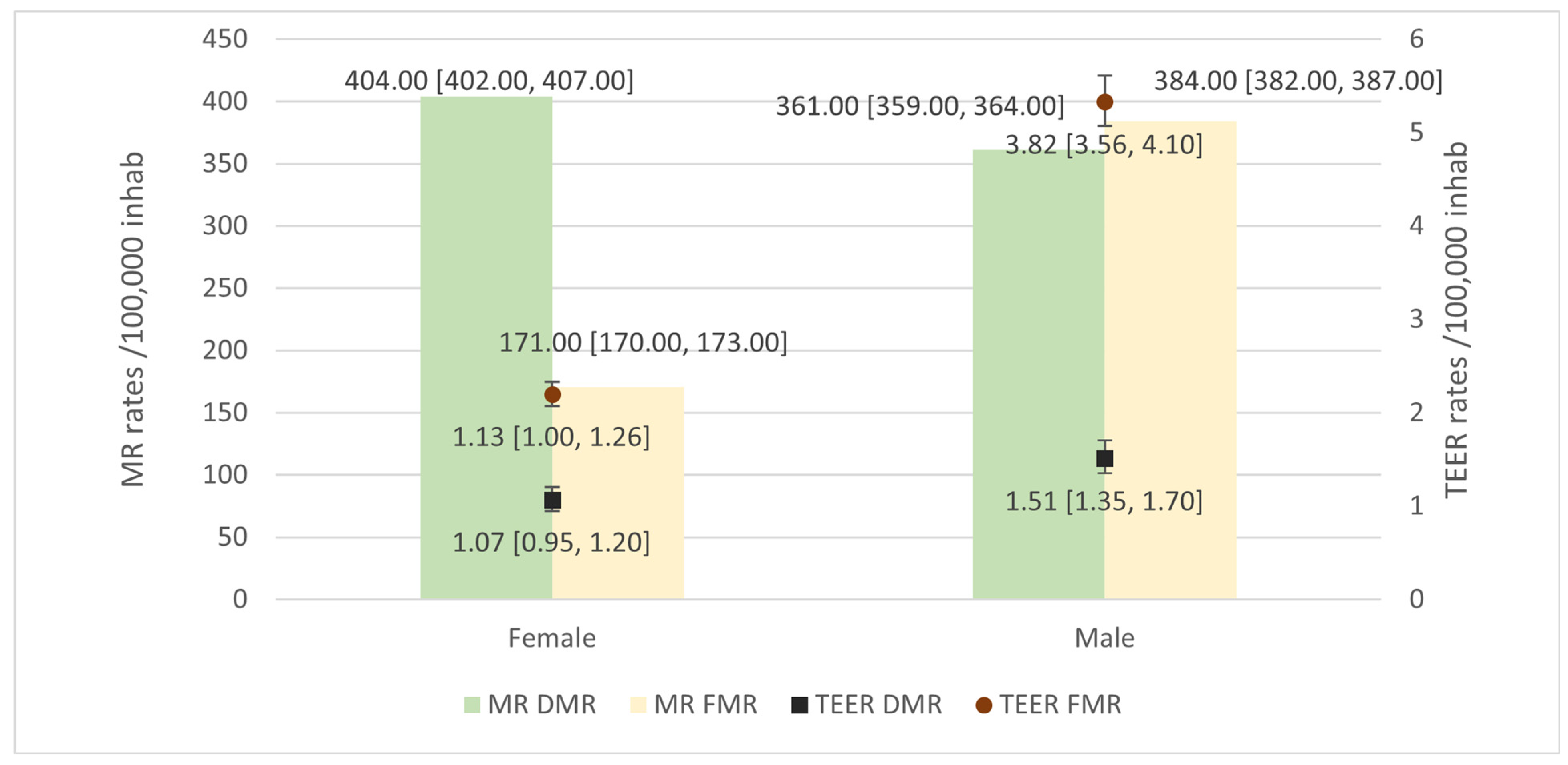
3.1.1. DMR
3.1.2. FMR
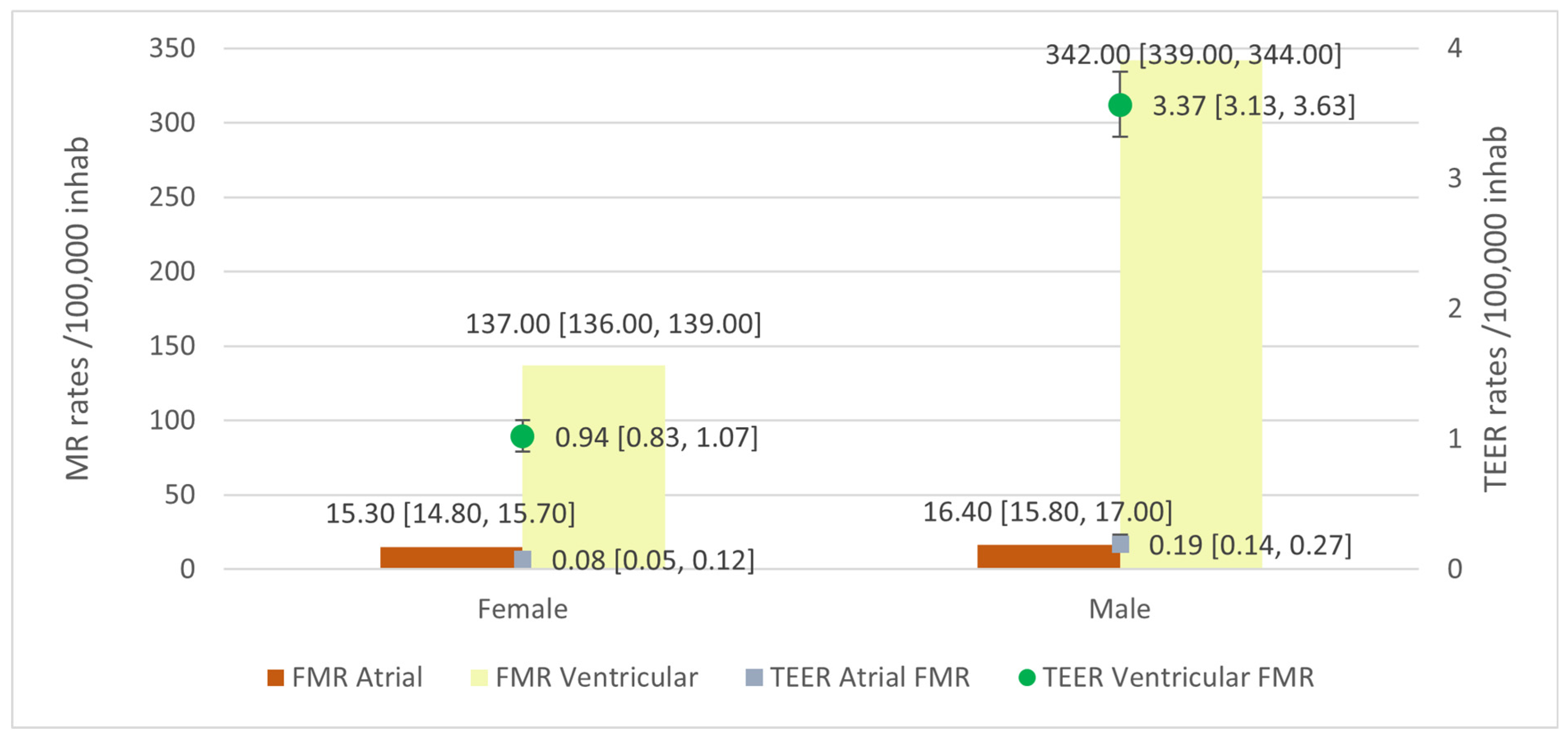
Atrial FMR
Ventricular FMR
3.2. TEER Procedures
- DMR: 595 interventions in patients with the following characteristics: mean age 74.20 years (SD 12.31) and mean aCCI of 4.40 (SD 2.05). The mean LoS of these patients for the procedure was 8.37 days (SD 11.13), with 53.54% of patients admitted to the ICU with a mean LoS of 0.68 days (SD 2.41). A total of 13 patients died in hospital (2.18%). The mean hospitalisation cost for DMR patients undergoing TEER was EUR 17,941.91 (SD EUR 8409.22). Complications were balanced between sexes.
- FMR: 1094 hospitalisations undergoing TEER with a mean age of 70.64 years (SD 10.25), which is statistically lower than that of DMR patients undergoing TEER. The mean aCCI was 4.72 (SD 2.00). In total, 73.49% of the cases were male. The mean LoS for these patients was 9.58 days (SD 12.72) and 52.83% required ICU admission with a mean LoS of 1.24 days (SD 5.40). These patients had a mean cost of EUR 17,210.43 (SD EUR 9383.85). There were no statistically significant differences between sexes regarding the prevalence of complications.
- ○
- aFMR: 59 TEER procedures were performed. All the patients were older than 71 years old and their mean age was 77.90 years (SD 4.29). In total, 64.41% of them were male and the mean aCCI was 5.10 (SD 2.29). The mean LoS was 10.95 days (SD 13.15), and 22 were admitted to the ICU with a mean LoS of 1.15 days (SD 2.53). There were no deaths. The mean cost of patients suffering from aFMR who underwent a TEER was EUR 18,055.27 (SD EUR 8606.49).
- ○
- There were 954 hospitalisations with a vFMR diagnosis undergoing a TEER. The mean age was 70.46 (SD 10.34) and 74.53% were male. The mean aCCI was 4.75 (SD 1.96). Mean LoS was 9.55 days (SD 12.91) and mean ICU LoS was 1.25 days (SD 5.55), with 52.52% requiring an ICU admission. The mean cost of these patients was EUR 17,073.91 (SD EUR 9404.06).
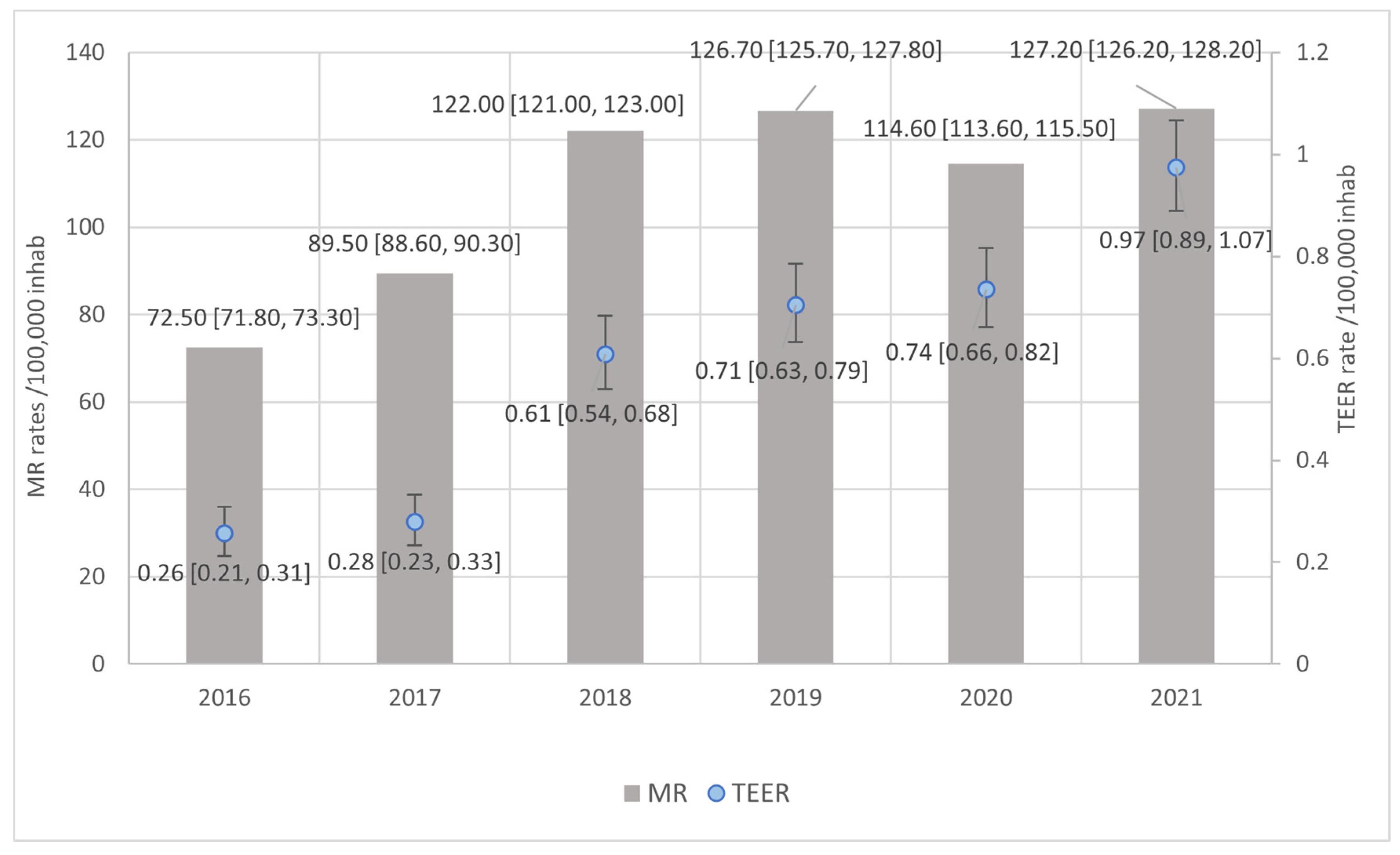
3.3. Rates of MR and TEER
4. Discussion
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of Valvular Heart Diseases: A Population-Based Study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo Ruiz, J.M.; Galderisi, M.; Buonauro, A.; Badano, L.; Aruta, P.; Swaans, M.J.; Sanchis, L.; Saraste, A.; Monaghan, M.; Theodoropoulos, K.C.; et al. Overview of Mitral Regurgitation in Europe: Results from the European Registry of Mitral Regurgitation (EuMiClip). Eur. Heart J. Cardiovasc. Imaging 2018, 19, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Alperi, A.; Pascual, I.; Avanzas, P.; Moris, C. Atrial-FMR: No Longer the Forgotten Mechanism of Functional Mitral Regurgitation. Int. J. Cardiol. 2022, 348, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Hirji, S.A.; Cote, C.L.; Javadikasgari, H.; Malarczyk, A.; McGurk, S.; Kaneko, T. Atrial Functional versus Ventricular Functional Mitral Regurgitation: Prognostic Implications. J. Thorac. Cardiovasc. Surg. 2022, 164, 1808–1815.e4. [Google Scholar] [CrossRef]
- Okamoto, C.; Okada, A.; Nishimura, K.; Moriuchi, K.; Amano, M.; Takahama, H.; Amaki, M.; Hasegawa, T.; Kanzaki, H.; Fujita, T.; et al. Prognostic Comparison of Atrial and Ventricular Functional Mitral Regurgitation. Open Heart 2021, 8, e001574. [Google Scholar] [CrossRef]
- Deng, M.X.; Barodi, B.; Elbatarny, M.; Yau, T.M. Considerations & Challenges of Mitral Valve Repair in Females: Diagnosis, Pathology, and Intervention. Curr. Opin. Cardiol. 2024, 39, 86–91. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Estrategia en Salud Cardiovascular del Sistema Nacional de Salud (ESCAV); Ministerio de Sanidad: Madrid, Spain, 2022. [Google Scholar]
- Deharo, P.; Obadia, J.F.; Guerin, P.; Cuisset, T.; Avierinos, J.F.; Habib, G.; Torras, O.; Bisson, A.; Vigny, P.; Etienne, C.S.; et al. Mitral Transcatheter Edge-to-Edge Repair vs. Isolated Mitral Surgery for Severe Mitral Regurgitation: A French Nationwide Study. Eur. Heart J. 2024, 45, 940–949. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Cruz-González, I.; Estévez-Loureiro, R.; Barreiro-Pérez, M.; Aguilera-Saborido, A.; Olmos-Blanco, C.; Rincón, L.M.; Gómez-Polo, J.C.; Arzamendi, D.; Borreguero, L.; Vilacosta, I.; et al. Mitral and Tricuspid Valve Disease: Diagnosis and Management. Consensus Document of the Section on Valvular Heart Disease and the Cardiovascular Imaging, Clinical Cardiology, and Interventional Cardiology Associations of the Spanish Society of Cardiology. Rev. Española Cardiol. 2022, 75, 911–922. [Google Scholar] [CrossRef]
- Cascos, E.; Sitges, M. Insuficiencia Mitral: Magnitud Del Problema y Opciones de Mejora. Cir. Cardiovasc. 2022, 29, S26–S31. [Google Scholar] [CrossRef]
- Zinoviev, R.; Hasan, R.K.; Gammie, J.S.; Resar, J.R.; Czarny, M.J. Economic Burden of Inpatient Care for Mitral Regurgitation in Maryland. J. Am. Heart Assoc. 2024, 13, 29875. [Google Scholar] [CrossRef]
- Charlson, M.E.; Charlson, R.E.; Peterson, J.C.; Marinopoulos, S.S.; Briggs, W.M.; Hollenberg, J.P. The Charlson Comorbidity Index Is Adapted to Predict Costs of Chronic Disease in Primary Care Patients. J. Clin. Epidemiol. 2008, 61, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a Combined Comorbidity Index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data from 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Ministerio de Sanidad Registro de Actividad de Atención Especializada. Conjunto Mínimo Básico de Datos. RAE-CMBD. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/cmbdhome.htm (accessed on 10 September 2024).
- Iung, B.; Delgado, V.; Rosenhek, R.; Price, S.; Prendergast, B.; Wendler, O.; De Bonis, M.; Tribouilloy, C.; Evangelista, A.; Bogachev-Prokophiev, A.; et al. Contemporary Presentation and Management of Valvular Heart Disease: The EUrobservational Research Programme Valvular Heart Disease II Survey. Circulation 2019, 140, 1156–1169. [Google Scholar] [CrossRef]
- Biasco, L.; Tersalvi, G.; Klersy, C.; Benfari, G.; Biaggi, P.; Corti, R.; Curti, M.; Gaemperli, O.; Jeger, R.; Maisano, F.; et al. Technical and Clinical Outcomes after Transcatheter Edge-to-Edge Repair of Mitral Regurgitation in Male and Female Patients: Is Equality Achieved? J. Am. Heart Assoc. 2024, 13, 32706. [Google Scholar] [CrossRef]
- Stehli, J.; Zaman, S.; Stähli, B.E. Sex Discrepancies in Pathophysiology, Presentation, Treatment, and Outcomes of Severe Aortic Stenosis. Front. Cardiovasc. Med. 2023, 10, 1256970. [Google Scholar] [CrossRef]
- Li, C.-H.P.; Estévez-Loureiro, R.; Freixa, X.; Teles, R.; Molina-Ramos, A.I.; Pan, M.; Nombela-Franco, L.; Melica, B.; Amat-Santos, I.J.; Cruz-González, I.; et al. Iberian Experience with PASCAL Transcatheter Edge-to-Edge Repair for Mitral Valve Regurgitation. Rev. Esp. Cardiol. 2023, 76, 25–31. [Google Scholar] [CrossRef]
- Grayburn, P.A.; MacK, M.J.; Manandhar, P.; Kosinski, A.S.; Sannino, A.; Smith, R.L.; Szerlip, M.; Vemulapalli, S. Comparison of Transcatheter Edge-to-Edge Mitral Valve Repair for Primary Mitral Regurgitation Outcomes to Hospital Volumes of Surgical Mitral Valve Repair. Circ. Cardiovasc. Interv. 2024, 17, E013581. [Google Scholar] [CrossRef] [PubMed]
- Pascual, I.; Arzamendi, D.; Carrasco-Chinchilla, F.; Fernández-Vázquez, F.; Freixa, X.; Nombela-Franco, L.; Avanzas, P.; Serrador-Frutos, A.M.; Pan, M.; Cid Álvarez, A.B.; et al. Reparación Mitral Transcatéter Según La Etiología de La Insuficiencia Mitral: Datos de La Vida Real Procedentes del Registro Español de MitraClip. Rev. Esp. Cardiol. 2020, 73, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T. Sex-Related Differences in Degenerative Mitral Regurgitation: A Call to Action. Eur. Heart J. 2024, 45, 2317–2319. [Google Scholar] [CrossRef]
- Íñiguez-Romo, A.; Zueco-Gil, J.J.; Álvarez-Bartolomé, M.; Antonio, J.; Alonso, B.; Alfonso Jiménez Díaz, V.; Loureiro, R.E.; Veiga Fernández, G.; Hwang, D.-H.L.; Martí-Sánchez, B.; et al. Resultados Del Implante Percutáneo de Válvula Aórtica En España Mediante El Registro de Actividad de Atención Especializada. REC Interv. Cardiol. 2022, 4, 123–131. [Google Scholar] [CrossRef]
- Liu, K.; Ye, Q.; Zhao, Y.; Zhao, C.; Song, L.; Wang, J. Sex Differences in the Outcomes of Degenerative Mitral Valve Repair. Ann. Thorac. Cardiovasc. Surg. 2023, 29, oa.22-00210. [Google Scholar] [CrossRef] [PubMed]
- Seeburger, J.; Eifert, S.; Pfannmüller, B.; Garbade, J.; Vollroth, M.; Misfeld, M.; Borger, M.; Mohr, F. Gender Differences in Mitral Valve Surgery. Thorac. Cardiovasc. Surg. 2012, 61, 042–046. [Google Scholar] [CrossRef]
- Yi, K.; Gao, J.; Wang, W.-X.; Ma, Y.-H.; Wang, W.; He, S.E.; Xu, X.-M.; Li, P.-F.; You, T. Gender-Related Differences on Outcome Following Transcatheter Mitral Valve Repair (TMVR): A Systematic Review and Meta-Analysis. J. Cardiothorac. Surg. 2023, 18, 31. [Google Scholar] [CrossRef]
- Mustafa, U.; Mina, G.; Gill, P.; Sheth, A.; Helmy, T. Impact of Gender on Outcomes of Transcatheter Edge to Edge Mitral Valve Repair: A Meta-Analysis. Med. Res. Arch (Online) 2023, 11. [Google Scholar] [CrossRef]
| Level | MR | MR Type | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | DMR | FMR | ||||||||||||||
| Total | Total | Atrial | Ventricular | |||||||||||||
| F | M | F | M | F | M | F | M | F | M | |||||||
| n | 159,686 | 147,597 | 112,650 | 70,355 | 47,036 | 77,242 | 4358 | 3036 | 37,583 | 68,872 | ||||||
| Age (mean (SD)) | 79.76 (11.29) | 74.62 (12.23) | *** | 80.23 (11.32) | 75.89 (12.61) | *** | 78.64 (11.14) | 73.46 (11.74) | *** | 83.44 (5.91) | 81.66 (6.06) | *** | 78.34 (11.21) | 73.31 (11.66) | *** | |
| Age (median [IQR]) | 82.00 [75.00, 87.00] | 77.00 [67.00, 84.00] | *** | 83.00 [76.00, 88.00] | 79.00 [69.00, 85.00] | *** | 81.00 [73.00, 86.00] | 75.00 [66.00, 83.00] | *** | 84.00 [79.00, 88.00] | 82.00 [77.00, 86.00] | *** | 81.00 [73.00, 86.00] | 75.00 [66.00, 82.00] | *** | |
| Charlson (mean (SD)) | 5.80 (2.29) | 5.67 (2.62) | *** | 5.74 (2.31) | 5.63 (2.71) | *** | 5.93 (2.24) | 5.70 (2.53) | *** | 6.32 (1.90) | 6.58 (2.17) | *** | 5.95 (2.23) | 5.71 (2.52) | *** | |
| Charlson (median [IQR]) | 6.00 [4.00, 7.00] | 5.00 [4.00, 7.00] | *** | 6.00 [4.00, 7.00] | 5.00 [4.00, 7.00] | *** | 6.00 [4.00, 7.00] | 6.00 [4.00, 7.00] | *** | 6.00 [5.00, 7.00] | 6.00 [5.00, 8.00] | *** | 6.00 [4.00, 7.00] | 6.00 [4.00, 7.00] | *** | |
| FA, n (%) | 1605 (1.01) | 1,954 (1.32) | *** | 993 (0.88) | 861 (1.22) | *** | 612 (1.30) | 1,093 (1.42) | *** | 76 (1.74) | 69 (2.27) | 516 (1.37) | 983 (1.43) | |||
| AMI, n (%) | 309 (0.19) | 447 (0.30) | *** | 2 (0.00) | 0 (0.00) | 307 (0.65) | 447 (0.58) | • | 60 (1.38) | 41 (1.35) | 167 (0.44) | 313 (0.45) | ||||
| Prot, n (%) | 117 (0.07) | 181 (0.12) | *** | 63 (0.06) | 67 (0.10) | ** | 54 (0.11) | 114 (0.15) | 2 (0.05) | 2 (0.07) | 49 (0.13) | 102 (0.15) | ||||
| Cereb, n (%) | TIA | 110 (0.07) | 81 (0.05) | • | 67 (0.06) | 36 (0.05) | 43 (0.09) | 45 (0.06) | 4 (0.09) | 1 (0.03) | 37 (0.10) | 42 (0.06) | • | |||
| Other | 260 (0.16) | 275 (0.19) | 151 (0.13) | 111 (0.16) | 109 (0.23) | 164 (0.21) | • | 7 (0.16) | 4 (0.13) | 85 (0.23) | 145 (0.21) | |||||
| ARF, n (%) | 158 (0.10) | 258 (0.17) | *** | 92 (0.08) | 99 (0.14) | *** | 66 (0.14) | 159 (0.21) | 1 (0.02) | 7 (0.23) | * | 53 (0.14) | 135 (0.20) | * | ||
| PPI, n (%) | 892 (0.56) | 999 (0.68) | *** | 591 (0.52) | 439 (0.62) | ** | 301 (0.64) | 560 (0.72) | * | 21 (0.48) | 14 (0.46) | 244 (0.65) | 502 (0.73) | |||
| IntubTraq, n (%) | 227 (0.14) | 406 (0.28) | *** | 150 (0.13) | 190 (0.27) | *** | 77 (0.16) | 216 (0.28) | • | 0 (0.00) | 11 (0.36) | *** | 65 (0.17) | 187 (0.27) | ** | |
| Endo, n (%) | 296 (0.19) | 328 (0.22) | * | 213 (0.19) | 211 (0.30) | *** | 83 (0.18) | 117 (0.15) | *** | 12 (0.28) | 8 (0.26) | 53 (0.14) | 88 (0.13) | |||
| LoS -days- (mean (SD)) | 8.78 (8.99) | 9.17 (10.64) | *** | 8.72 (8.85) | 9.12 (10.12) | *** | 8.95 (9.33) | 9.22 (11.10) | 8.92 (7.42) | 9.52 (10.03) | ** | 8.96 (9.46) | 9.21 (11.15) | *** | ||
| LoS -days- (median [IQR]) | 7.00 [4.00, 11.00] | 7.00 [4.00, 11.00] | • | 7.00 [4.00, 11.00] | 7.00 [4.00, 11.00] | * | 7.00 [4.00, 11.00] | 7.00 [4.00, 11.00] | 7.00 [4.00, 11.00] | 7.00 [4.00, 11.00] | 7.00 [4.00, 11.00] | 7.00 [4.00, 11.00] | ||||
| ICU, n (%) | 146,366 (91.86) | 127,750 (86.75) | *** | 105,426 (93.77) | 63,454 (90.37) | *** | 40,940 (87.28) | 64,296 (83.45) | *** | 4024 (92.51) | 2693 (88.94) | *** | 32,598 (86.99) | 57,363 (83.51) | *** | |
| ICU LoS -days- (mean (SD)) | 0.38 (2.69) | 0.64 (3.44) | *** | 0.30 (2.49) | 0.50 (3.22) | *** | 0.55 (3.11) | 0.77 (3.62) | *** | 0.24 (1.47) | 0.55 (2.83) | *** | 0.57 (3.23) | 0.77 (3.65) | *** | |
| ICU LoS -days- (median [IQR]) | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | *** | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | *** | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | *** | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | *** | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | *** | |
| Death, n (%) | 14,777 (9.25) | 12,560 (8.51) | *** | 10,330 (9.17) | 6187 (8.79) | ** | 4447 (9.45) | 6373 (8.25) | *** | 486 (11.15) | 402 (13.24) | ** | 3434 (9.14) | 5467 (7.94) | *** | |
| Urgent, n (%) | 137,498 (86.11) | 119,365 (80.87) | *** | 96,712 (85.85) | 56,497 (80.30) | *** | 40,786 (86.71) | 62,868 (81.39) | *** | 4053 (93.00) | 2696 (88.80) | *** | 32,217 (85.72) | 55,735 (80.93) | *** | |
| Cost (mean (SD)) | 5821.89 (6152.99) | 6963.11 (7861.74) | *** | 5545.49 (5748.42) | 6431.05 (7281.98) | *** | 6483.85 (6983.60) | 7447.73 (8325.38) | *** | 5123.02 (4373.58) | 6014.34 (7070.13) | *** | 6681.84 (7149.07) | 7524.27 (8319.10) | *** | |
| Cost (median [IQR]) | 3800.06 [3009.44, 5938.61] | 4,270.57 [3220.05, 6985.21] | *** | 3800.06 [3009.44, 5609.57] | 3,953.46 [3058.76, 6044.43] | *** | 4167.33 [3243.38, 6604.27] | 4521.54 [3399.29, 7741.21 | *** | 3800.06 [3013.75, 5211.42] | 3814.45 [3130.35, 5880.25] | *** | 4337.93 [3298.43, 6999.38] | 4531.38 [3408.79, 8041.93] | *** | |
| TEER, n (%) | 580 (0.36) | 1109 (0.75) | *** | 290 (0.26) | 305 (0.43) | *** | 290 (0.62) | 804 (1.04) | *** | 21 (0.48) | 38 (1.25) | *** | 243 (0.65) | 711 (1.03) | *** | |
| MR | MR Type | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | DMR | FMR | ||||||||||||||
| Total | Total | Atrial | Ventricular | |||||||||||||
| Level | F | M | F | M | F | M | F | M | F | M | ||||||
| n | 580 | 1109 | 290 | 305 | 290 | 804 | 21 | 38 | 243 | 711 | ||||||
| Age (mean (SD)) | 73.78 (11.74) | 70.90 (10.70) | *** | 76.25 (11.74) | 72.25 (12.53) | *** | 71.30 (11.22) | 70.40 (9.87) | 78.14 (4.28) | 77.76 (4.34) | 70.99 (11.64) | 70.28 (9.86) | ||||
| Age (median [IQR]) | 77.00 [68.00, 82.00] | 73.00 [65.00, 79.00] | *** | 80.00 [73.00, 84.00] | 75.00 [64.00, 82.00] | *** | 74.00 [65.00, 80.00] | 72.00 [65.00, 77.00] | * | 77.00 [75.00, 81.00] | 78.00 [74.00, 81.75] | 74.00 [65.00, 80.00] | 72.00 [64.50, 77.00] | * | ||
| Charlson (mean (SD)) | 4.48 (1.84) | 4.67 (2.11) | • | 4.42 (1.75) | 4.37 (2.30) | 4.55 (1.93) | 4.78 (2.02) | • | 4.95 (1.66) | 5.18 (2.60) | 4.57 (1.90) | 4.81 (1.99) | ||||
| Charlson (median [IQR]) | 4.00 [3.00, 6.00] | 4.00 [3.00, 6.00] | 4.00 [4.00, 5.00] | 4.00 [3.00, 6.00] | 4.00 [3.00, 6.00] | 5.00 [3.00, 6.00] | 4.00 [4.00, 6.00] | 5.00 [4.00, 5.75] | 4.00 [3.00, 6.00] | 5.00 [3.00, 6.00] | ||||||
| FA, n (%) | 19 (3.28) | 22 (1.98) | 8 (2.76) | 9 (2.95) | 11 (3.79) | 13 (1.62) | • | 1 (4.76) | 3 (7.89) | 10 (4.12) | 9 (1.27) | * | ||||
| AMI, n (%) | 1 (0.17) | 2 (0.18) | 0 (0.00) | 0 (0.00) | 1 (0.34) | 2 (0.25) | 0 (0.00) | 1 (2.63) | 1 (0.41) | 1 (0.14) | ||||||
| Prot, n (%) | 7 (1.21) | 8 (0.72) | 5 (1.72) | 1 (0.33) | 2 (0.69) | 7 (0.87) | 0 (0.00) | 1 (2.63) | 2 (0.82) | 5 (0.70) | ||||||
| Cereb, n (%) | TIA | 4 (0.69) | 1 (0.09) | • | 2 (0.69) | 0 (0.00) | 2 (0.69) | 1 (0.12) | 0 (0.00) | 0 (0.00) | 2 (0.82) | 1 (0.14) | ||||
| Other | 2 (0.34) | 4 (0.36) | 0 (0.00) | 1 (0.33) | 2 (0.69) | 3 (0.37) | 0 (0.00) | 0 (0.00) | 1 (0.41) | 2 (0.28) | ||||||
| ARF, n (%) | 5 (0.86) | 4 (0.36) | 3 (1.03) | 0 (0.00) | 2 (0.69) | 4 (0.50) | 0 (0.00) | 1 (2.63) | 2 (0.82) | 3 (0.42) | ||||||
| PPI, n (%) | 1 (0.17) | 4 (0.36) | 4 (1.38) | 3 (0.98) | 1 (0.34) | 3 (0.37) | 0 (0.00) | 0 (0.00) | 1 (0.41) | 3 (0.42) | ||||||
| IntubTraq, n (%) | 1 (0.17) | 4 (0.36) | 1 (0.34) | 1 (0.33) | 0 (0.00) | 3 (0.37) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (0.42) | ||||||
| Endo, n (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||||||
| LoS -days- (mean (SD)) | 10.02 (13.49) | 8.70 (11.44) | * | 9.47 (13.26) | 7.32 (8.53) | * | 10.57 (13.72) | 9.23 (12.32) | 8.62 (9.60) | 12.24 (14.71) | 10.74 (14.39) | 9.14 (12.35) | ||||
| LoS -days- (median [IQR]) | 5.00 [3.00, 10.00] | 5.00 [3.00, 8.00] | 5.00 [3.00, 10.00] | 5.00 [3.00, 8.00] | 5.50 [3.00, 11.00] | 5.00 [3.00, 9.00] | • | 5.00 [3.00, 11.00] | 5.00 [4.00, 13.75] | 6.00 [3.00, 10.00] | 5.00 [3.00, 9.00] | • | ||||
| ICU, n (%) | 306 (52.76) | 590 (53.25) | 152 (52.41) | 166 (54.61) | 154 (53.10) | 424 (52.74) | 7 (33.33) | 15 (39.47) | 130 (53.50) | 371 (52.18) | ||||||
| ICU LoS -days- (mean (SD)) | 0.96 (3.22) | 1.09 (5.16) | 0.67 (2.02) | 0.68 (2.73) | 1.26 (4.06) | 1.24 (5.81) | 0.76 (1.37) | 1.37 (2.98) | 1.16 (3.53) | 1.28 (6.09) | ||||||
| ICU LoS -days- (median [IQR]) | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | ||||||
| Death, n (%) | 18 (3.10) | 19 (1.71) | • | 8 (2.76) | 5 (1.64) | 10 (3.45) | 14 (1.74) | 0 (0.00) | 0 (0.00) | 8 (3.29) | 12 (1.69) | |||||
| Urgent, n (%) | 147 (25.34) | 247 (22.27) | 71 (24.48) | 53 (17.38) | * | 76 (26.21) | 194 (24.13) | 8 (38.10) | 11 (28.95) | 62 (25.51) | 171 (24.05) | |||||
| Cost (mean (SD)) | 18,014.36 (8500.58) | 17,182.44 (9325.39) | • | 18,089.21 (8603.36) | 17,801.85 (8232.10) | 17,939.50 (8410.75) | 16,947.46 (9702.43) | 19,707.61 (9696.67) | 17,142.13 (7929.80) | 17,853.98 (8362.71) | 16,807.31 (9725.61) | |||||
| Cost (median [IQR]) | 17,378.92 [13,044.88, 18,880.02] | 17,378.92 [11,994.50, 18,880.02] | ** | 17,378.92 [13,044.88, 18,880.02] | 17,378.92 [13,044.88, 23,229.36] | 17,378.92 [13,044.88, 18,880.02] | 17,378.92 [11,107.70, 18,880.02] | ** | 17,378.92 [13,044.88, 18,880.02] | 16,610.06 [13,044.88, 18,504.75] | 17,378.92 [13,044.88, 18,880.02] | 17,378.92 [11,107.70, 18,273.07] | * | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamorano, J.L.; Álvarez-Bartolomé, M.; Arzamendi, D.; Carnero-Alcázar, M.; Cruz-González, I.; Li, C.-H.P.; Pardo-Sanz, A.; Martínez-Pérez, Ó.; Cerezales, M.; Cuervo, J.; et al. Burden of Mitral Regurgitation in Spain from 2016–2021: An Analysis by Aetiology and Sex. J. Clin. Med. 2024, 13, 6372. https://doi.org/10.3390/jcm13216372
Zamorano JL, Álvarez-Bartolomé M, Arzamendi D, Carnero-Alcázar M, Cruz-González I, Li C-HP, Pardo-Sanz A, Martínez-Pérez Ó, Cerezales M, Cuervo J, et al. Burden of Mitral Regurgitation in Spain from 2016–2021: An Analysis by Aetiology and Sex. Journal of Clinical Medicine. 2024; 13(21):6372. https://doi.org/10.3390/jcm13216372
Chicago/Turabian StyleZamorano, José Luis, Mercedes Álvarez-Bartolomé, Dabit Arzamendi, Manuel Carnero-Alcázar, Ignacio Cruz-González, Chi-Hion Pedro Li, Ana Pardo-Sanz, Óscar Martínez-Pérez, Mónica Cerezales, Jesús Cuervo, and et al. 2024. "Burden of Mitral Regurgitation in Spain from 2016–2021: An Analysis by Aetiology and Sex" Journal of Clinical Medicine 13, no. 21: 6372. https://doi.org/10.3390/jcm13216372
APA StyleZamorano, J. L., Álvarez-Bartolomé, M., Arzamendi, D., Carnero-Alcázar, M., Cruz-González, I., Li, C.-H. P., Pardo-Sanz, A., Martínez-Pérez, Ó., Cerezales, M., Cuervo, J., Vernia, M., González, P., & Martí-Sánchez, B. (2024). Burden of Mitral Regurgitation in Spain from 2016–2021: An Analysis by Aetiology and Sex. Journal of Clinical Medicine, 13(21), 6372. https://doi.org/10.3390/jcm13216372






