The Impact of Nusinersen Treatment on Respiratory Function in Patients with Spinal Muscular Atrophy: A Systematic Review
Abstract
1. Introduction
- SMA type 1 (Werdnig–Hoffman disease): This is the most common form, presenting before six months of age. It causes severe symptoms, such as restricted mobility, muscle contractures, skeletal abnormalities, and respiratory problems. Without intervention, most affected children do not survive beyond two years, with respiratory disease being the leading cause of death [6,7,8].
- SMA type 3 (Kugelberg–Welander disease): This form of SMA manifests after 18 months. Children can walk independently but face mobility challenges. Complications include spinal curvature, contractures, and respiratory infections. With appropriate treatment, people with SMA type III can achieve an average life expectancy [6,7,8].
2. Materials and Methods
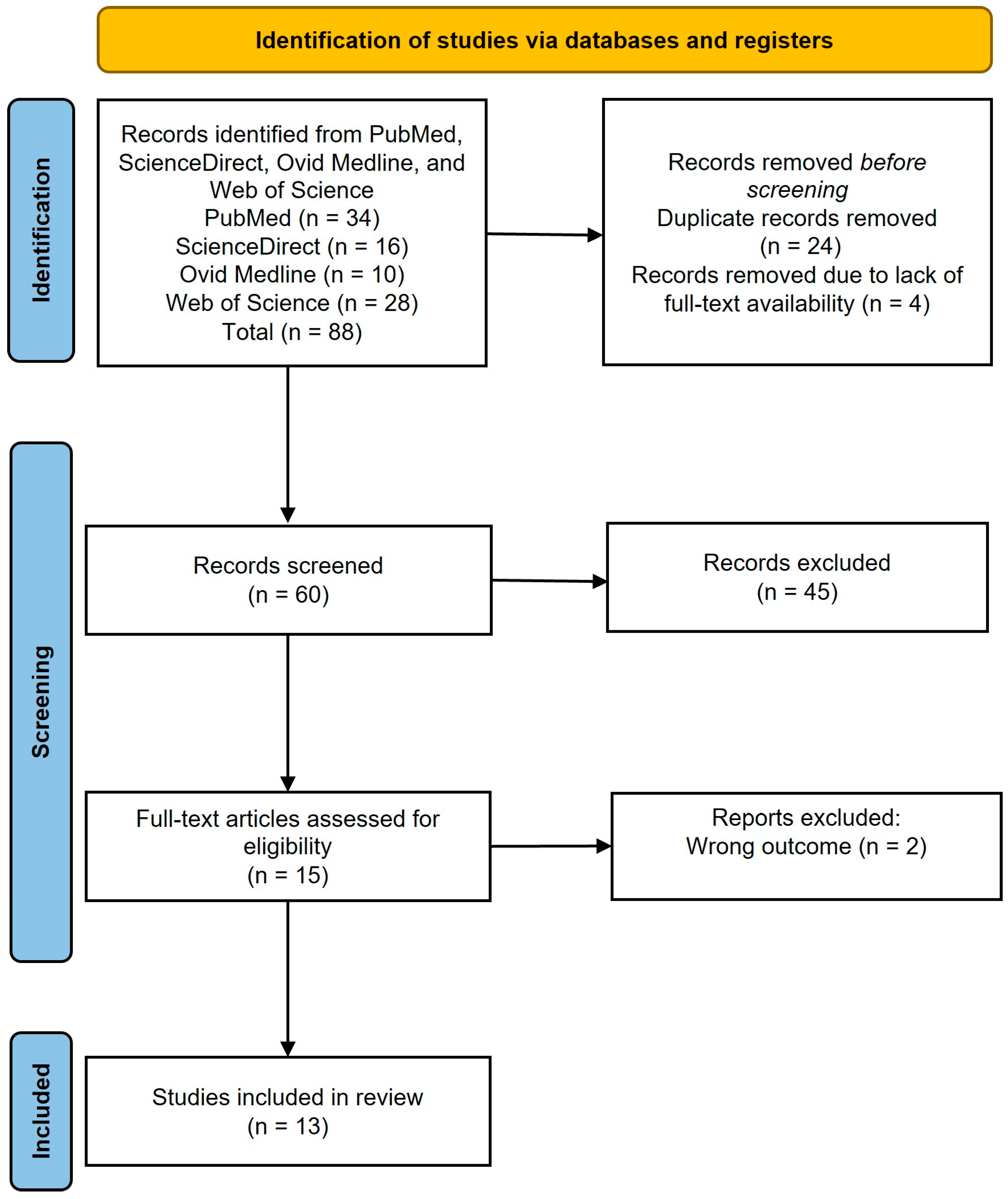
2.1. Literature Search and Eligibility Criteria
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis
3. Results
3.1. Characteristics of Included Studies
3.2. Quality Assessment
3.3. Changes in Pulmonary Function Tests
3.4. Improvement in Respiratory Muscle Strength
3.5. Changes in Ventilatory Support
3.6. Timing of Nusinersen Administration
3.7. Differences between Pediatric and Adult Patients
4. Discussion
4.1. Changes in FVC
4.2. Respiratory Complications
4.3. Implications of the Review
4.4. Limitations and Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmüller, H. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—A literature review. Orphanet. J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Mailman, M.D.; Heinz, J.W.; Papp, A.C.; Snyder, P.J.; Sedra, M.S.; Wirth, B.; Burghes, A.H.M.; Prior, T.W. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet. Med. 2002, 4, 20–26. [Google Scholar] [CrossRef]
- Feldkötter, M.; Schwarzer, V.; Wirth, R.; Wienker, T.F.; Wirth, B. Quantitative Analyses of SMN1 and SMN2 Based on Real-Time LightCycler PCR: Fast and Highly Reliable Carrier Testing and Prediction of Severity of Spinal Muscular Atrophy. Am. J. Hum. Genet. 2002, 70, 358–368. [Google Scholar] [CrossRef]
- Sari, D.M.; Wijaya, L.C.G.; Sitorus, W.D.R.; Dewi, M.M. Psychological burden in spinal muscular atrophy patients and their families: A systematic review. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 140. [Google Scholar] [CrossRef]
- Veldhoen, E.S.; Wijngaarde, C.A.; Hulzebos, E.H.J.; Wösten-van Asperen, R.M.; Wadman, R.I.; van Eijk, R.P.A.; Asselman, F.L.; Stam, M.; Otto, L.A.M.; Cuppen, I.; et al. Natural history of respiratory muscle strength in spinal muscular atrophy: A prospective national cohort study. Orphanet. J. Rare Dis. 2022, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; Paslier, D.L.; et al. Identification and Characterization of a Spinal Muscular Atrophy-Determining Gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef]
- Farrar, M.A.; Vucic, S.; Johnston, H.M.; Du Sart, D.; Kiernan, M.C. Pathophysiological insights derived by natural history and motor function of spinal muscular atrophy. J. Pediatr. 2013, 162, 155–159. [Google Scholar] [CrossRef]
- Grychtol, R.; Abel, F.; Fitzgerald, D.A. The role of sleep diagnostics and non-invasive ventilation in children with spinal muscular atrophy. Paediatr. Respir. Rev. 2018, 28, 18–25. [Google Scholar] [CrossRef]
- Hoy, S.M. Nusinersen: First Global Approval. Drugs 2017, 77, 473–479. [Google Scholar] [CrossRef]
- Maharshi, V.; Hasan, S. Nusinersen: The First Option Beyond Supportive Care for Spinal Muscular Atrophy. Clin. Drug Investig. 2017, 37, 807–817. [Google Scholar] [CrossRef]
- Gidaro, T.; Servais, L. Nusinersen treatment of spinal muscular atrophy: Current knowledge and existing gaps. Dev. Med. Child Neurol. 2019, 61, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, B.; Severyn, S.; Zhao, S.; Kline, D.; Linsenmayer, M.; Kelly, K.; Tellez, M.; Bartlett, A.; Heintzman, S.; Reynolds, J.; et al. Safety, Tolerability, and Effect of Nusinersen in Non-ambulatory Adults With Spinal Muscular Atrophy. Front. Neurol. 2021, 12, 650532. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ 2021, 372. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024); Cochrane: London, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 15 August 2024).
- Aromataris, E.; Fernandez, R.; Godfrey, C.; Holly, C.; Kahlil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an Umbrella review approach. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef]
- Bjelica, B.; Wohnrade, C.; Osmanovic, A.; Schreiber-Katz, O.; Petri, S. An observational cohort study on pulmonary function in adult patients with 5q-spinal muscular atrophy under nusinersen therapy. J. Neurol. 2023, 270, 3616–3622. [Google Scholar] [CrossRef]
- Heitschmidt, L.; Pichlmaier, L.; Eckerland, M.; Steindor, M.; Olivier, M.; Fuge, I.; Kölbel, H.; Hirtz, R.; Stehling, F. Nusinersen does not improve lung function in a cohort of children with spinal muscular atrophy—A single-center retrospective study. Eur. J. Paediatr. Neurol. 2021, 31, 88–91. [Google Scholar] [CrossRef]
- Sansone, V.A.; Pirola, A.; Albamonte, E.; Pane, M.; Lizio, A.; D’Amico, A.; Catteruccia, M.; Cutrera, R.; Bruno, C.; Pedemonte, M.; et al. Respiratory Needs in Patients with Type 1 Spinal Muscular Atrophy Treated with Nusinersen. J. Pediatr. 2020, 219, 223–228.e4. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.C.; Wenninger, S.; Thiele, S.; Stauber, J.; Hiebeler, M.; Greckl, E.; Stahl, K.; Pechmann, A.; Lochmüller, H.; Kirschner, J.; et al. Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type 3—A prospective observational study. J. Neuromuscul. Dis. 2019, 6, 453–465. [Google Scholar] [CrossRef]
- Duong, T.; Wolford, C.; McDermott, M.P.; Macpherson, C.E.; Pasternak, A.; Glanzman, A.M.; Martens, W.B.; Kichula, E.; Darras, B.T.; De Vivo, D.C.; et al. Nusinersen Treatment in Adults with Spinal Muscular Atrophy. Neurol. Clin. Pract. 2021, 11, E317–E327. [Google Scholar] [CrossRef]
- Chacko, A.; Sly, P.D.; Ware, R.S.; Begum, N.; Deegan, S.; Thomas, N.; Gauld, L.M. Effect of nusinersen on respiratory function in paediatric spinal muscular atrophy types 1–3. Thorax 2022, 77, 40–46. [Google Scholar] [CrossRef]
- Gómez-García de la Banda, M.; Amaddeo, A.; Khirani, S.; Pruvost, S.; Barnerias, C.; Dabaj, I.; Bénézit, A.; Durigneux, J.; Carlier, R.Y.; Desguerre, I.; et al. Assessment of respiratory muscles and motor function in children with SMA treated by nusinersen. Pediatr. Pulmonol. 2021, 56, 299–306. [Google Scholar] [CrossRef]
- Scheijmans, F.E.V.; Cuppen, I.; Van Eijk, R.P.A.; Wijngaarde, C.A.; Schoenmakers, M.A.G.C.; Van Der Woude, D.R.; Bartels, B.; Veldhoen, E.S.; Oude Lansink, I.L.B.; Groen, E.J.N.; et al. Population-based assessment of nusinersen efficacy in children with spinal muscular atrophy: A 3-year follow-up study. Brain Commun. 2022, 4, fcac269. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, A.; Behrens, M.; Dörnbrack, K.; Tassoni, A.; Stein, S.; Vogt, S.; Zöller, D.; Bernert, G.; Hagenacker, T.; Schara-Schmidt, U.; et al. Effect of nusinersen on motor, respiratory and bulbar function in early-onset spinal muscular atrophy. Brain 2023, 146, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Gonski, K.; Chuang, S.; Teng, A.; Thambipillay, G.; Farrar, M.A.; Menezes, M.P.; Fitzgerald, D.A. Respiratory and sleep outcomes in children with SMA treated with nusinersen-real world experience. Neuromuscul. Disord. 2023, 33, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Hepkaya, E.; Kılınç Sakallı, A.A.; Ülkersoy, İ.; Başkan, A.K.; Arslan, H.; Meral, Ö.; Dilek, T.D.; Güler, S.; Saltık, S.; Çokuğraş, H. The effects of nusinersen treatment on respiratory status of children with spinal muscular atrophy. Pediatr. Int. 2022, 64, e15310. [Google Scholar] [CrossRef]
- Fainmesser, Y.; Drory, V.E.; Ben-Shushan, S.; Lavon, A.; Spector, L.; Abramovich, B.; Abraham, A. Longer-term follow-up of nusinersen efficacy and safety in adult patients with spinal muscular atrophy types 2 and 3. Neuromuscul. Disord. 2022, 32, 451–459. [Google Scholar] [CrossRef]
- Edel, L.; Grime, C.; Robinson, V.; Manzur, A.; Abel, F.; Munot, P.; Ridout, D.; Scoto, M.; Muntoni, F.; Chan, E. A new respiratory scoring system for evaluation of respiratory outcomes in children with spinal muscular atrophy type1 (SMA1) on SMN enhancing drugs. Neuromuscul. Disord. 2021, 31, 300–309. [Google Scholar] [CrossRef]
- Salort-Campana, E.; Solé, G.; Magot, A.; Tard, C.; Noury, J.B.; Behin, A.; De La Cruz, E.; Boyer, F.; Lefeuvre, C.; Masingue, M.; et al. Multidisciplinary team meetings in treatment of spinal muscular atrophy adult patients: A real-life observatory for innovative treatments. Orphanet. J. Rare Dis. 2024, 19, 24. [Google Scholar] [CrossRef]
- Sumner, C.J. Molecular mechanisms of spinal muscular atrophy. J. Child Neurol. 2007, 22, 979–989. [Google Scholar] [CrossRef]
- Prior, T.W. Spinal muscular atrophy diagnostics. J. Child Neurol. 2007, 22, 952–956. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- De Wel, B.; De Schaepdryver, M.; Poesen, K.; Claeys, K.G. Biochemical and clinical biomarkers in adult SMA 3–4 patients treated with nusinersen for 22 months. Ann. Clin. Transl. Neurol. 2022, 9, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Darras, B.T.; Chiriboga, C.A.; Iannaccone, S.T.; Swoboda, K.J.; Montes, J.; Mignon, L.; Xia, S.; Bennett, C.F.; Bishop, K.M.; Shefner, J.M.; et al. Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies. Neurology 2019, 92, e2492–e2506. [Google Scholar] [CrossRef] [PubMed]
- Hagenacker, T.; Wurster, C.D.; Günther, R.; Schreiber-Katz, O.; Osmanovic, A.; Petri, S.; Weiler, M.; Ziegler, A.; Kuttler, J.; Koch, J.C.; et al. Nusinersen in adults with 5q spinal muscular atrophy: A non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020, 19, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Giess, D.; Erdos, J.; Wild, C. An updated systematic review on spinal muscular atrophy patients treated with nusinersen, onasemnogene abeparvovec (at least 24 months), risdiplam (at least 12 months) or combination therapies. Eur. J. Paediatr. Neurol. 2024, 51, 84–92. [Google Scholar] [CrossRef]
- Erdos, J.; Wild, C. Mid- and long-term (at least 12 months) follow-up of patients with spinal muscular atrophy (SMA) treated with nusinersen, onasemnogene abeparvovec, risdiplam or combination therapies: A systematic review of real-world study data. Eur. J. Paediatr. Neurol. 2022, 39, 1–10. [Google Scholar] [CrossRef]
| Database | Search Terms | Search Date |
|---|---|---|
| PubMed | (“nusinersen” [Title/Abstract] OR “Spinraza” [Title/Abstract]) AND (“spinal muscular atrophy” [Title/Abstract] OR “SMA” [Title/Abstract]) AND (“respiratory function” [Title/Abstract] OR “forced vital capacity” [Title/Abstract] OR “peak cough flow” [Title/Abstract]) | Up to January 2024 |
| Ovid Medline | (“nusinersen” OR “Spinraza”) AND (“spinal muscular atrophy” OR “SMA”) AND (“respiratory function” OR “forced vital capacity” OR “peak cough flow”) | Up to January 2024 |
| ScienceDirect | Title, abstract, keywords: (“nusinersen” OR “Spinraza”) AND (“spinal muscular atrophy” OR “SMA”) AND (“respiratory function” OR “forced vital capacity” OR “peak cough flow”) | Up to January 2024 |
| Web of Science | (AB = (“nusinersen” OR “Spinraza”)) AND AB = (“spinal muscular atrophy” OR “SMA”) AND AB = (“respiratory function” OR “forced vital capacity” OR “peak cough flow”) | Up to January 2024 |
| Study ID | Study Design | Sample Size | SMA Type | Age Mean (or Median) | Duration of the Intervention (Nusinersen) | Dosage | Main Results |
|---|---|---|---|---|---|---|---|
| Bjelica et al., 2023 [16] | Observational cohort study | 38 | SMA types 2 and 3 | 38.4 years | 30 months | NR | Mean FVC, FEV1, and PEF remained stable; for ambulatory patients, mean PEF showed a significant improvement at month 30 (+0.8 ± 0.5 L/min) compared to non-ambulatory patients (−0.0 ± 0.5 L/min), with a p-value < 0.05; PEF changes were significantly related to motor function, QoL, and fatigue. |
| Walter et al., 2019 [19] | Prospective cohort study | 17 | SMA type 3 | Mean age at start of therapy: 35.11 years | 300 days | Loading dose of 12 mg at baseline, then maintenance dose every four months | Peak cough flow: significant improvement at visit 5 compared to baseline. No significant changes: apart from peak cough flow, there were no other relevant significant changes in respiratory outcomes at visits 4, 5, or 6 compared to baseline. |
| Duong et al., 2021 [20] | Prospective cohort study | 42 | SMA types 2 and 3 | 34 years | Mean: 12.5 months | Loading dose of 12 mg, maintenance doses every 4 months | Positive changes in motor and respiratory functions; participants stated that they subjectively felt stronger, less fatigued, and had less breathlessness. |
| Chacko et al., 2021 [21] | Prospective observational study | 28 | SMA types 1, 2, and 3 | Median age: 8.71 years | 18 months | Multiple doses were administered over the study period | A reduced FVC rate by −0.25 compared to pre-treatment (−0.58), with a significant difference in decline rates (0.33, 95% CI: 0.02 to 0.66, p = 0.04); a better AHI, with significant reduction from a median of 5.5 events/hour to 2.7 events/hour, with a p-value of 0.02. |
| Gomez-García et al., 2021 [22] | Clinical trials using age-matched historical controls | 16 participants and 14 historical participants (controls) | SMA types 1c and 2 | Mean age: 9.4 ± 2.3 years for participants; mean age of controls: 9.3 ± 1.9 years | 14 months | Six injections of nusinersen | Respiratory muscle performance significantly improved in SMA type 2 patients compared to age-matched historical controls. This was assessed through maximal static inspiratory pressure, forced vital capacity (FVC), and esophageal pressure during a maximal sniff, with a p-value < 0.05. |
| Sansone et al., 2020 [18] | Observational, longitudinal cohort study | 118 | SMA type 1 | Median age: 42.8 months | 10 months | Intrathecal injections of nusinersen were administered on days 1, 15, 30, and 60 (loading doses) and then every four months (maintenance doses) | More than 80% of children treated before 2 years of age survived without requiring tracheostomy or non-invasive ventilation (NIV) for ≥16 h per day; a somewhat reduced total of NIV hours for children below 2 years of age. |
| Scheijmans et al., 2022 [23] | Single-center prospective cohort study | 71 | SMA types 1, 2, and 3 | Median age: 54 months | 38 months | Treatment started with a loading dose on days 0, 14, 28, and 63, followed by intrathecal injections every four months | Positive change in motor function, with stabilization in 18 percent of patients; no significant changes in respiratory function; 82 adverse effects, with none leading to treatment discontinuation. |
| Elsheikh et al., 2021 [12] | Prospective observational study | 19 | SMA types 2 and 3 | 39.7 ± 13.9 years | Assessed up to 14 months following nusinersen initiation | Participants received intrathecal nusinersen treatment on days 1, 15, 29, and 60, followed by maintenance doses administered every four months | FVC was stable, and functional measures were similar to baseline; CMAP and single motor unit potential amplitudes increased; motor unit counts stabilized. |
| Pechmann et al., 2023 [24] | Observational, longitudinal cohort study | 143 | SMA type 1 (with genetic confirmation of 5q SMA) | 8.4 ± 6.0 months; cohort 1b: mean age of 89.8 ± 58.4 months | Up to 38 months | NR | These included marked motor function gains, stabilization of respiratory and bulbar involvement, and higher dependency on ventilators and nasal feeds. |
| Gonski et al., 2023 [25] | Retrospective observational cohort study | 48 | SMA types 1, 2, and 3 | Mean age at first dose: 6.98 years (SD 5.25); SMA 1: mean age at first dose: 0.54 years (SD 0.33); SMA 2: mean age at first dose: 8.90 years (SD 4.96); SMA 3: mean age at first dose: 8.33 years (SD 4.04) | Data collected two years before date of first dose of nusinersen and then for two years after starting nusinersen | NR | Stabilization of respiratory outcomes; no significant changes in lung function and the majority of PSG measurements; significant improvement in oxygen nadir during sleep (mean increased from 87.9% to 92.3%, 95% CI: 1.24 to 7.63, p = 0.01); NIV use: 6 out of 21 patients discontinued nocturnal NIV post-treatment. |
| Hepkaya et al., 2022 [26] | Clinical trial | 43 | SMA types 1 (n = 18), 2 (n = 12), and 3 (n = 13) | SMA type 1: mean age at diagnosis: 4.39 ± 2.54 months; SMA type 2: mean age at diagnosis: 23.25 ± 21.49 months; SMA type 3: mean age at diagnosis: 64.54 ± 51.23 months | 13 months | Intrathecal injections with 12 mg of nusinersen: SMA type 1 (days 1, 15, 29, and 64), SMA types 2 and 3 (days 1, 29, 85, and 274) | Overall, hospitalization was reduced with early intervention, with a p-value of 0.026; enteral feeding was better in a ventilator-dependent subgroup. |
| Fainmesser et al., 2022 [27] | Cohort study | 37 | SMA types 2 (n = 15) and 3 (n = 22) | 38 years | 30 months | Intrathecal loading doses of 12 mg nusinersen on days 0, 14, 28, and 63, followed by maintenance doses every 4 months | Modest improvement in motor function up to 6 months, with stabilization thereafter; no significant change in respiratory function assessed by FEV1; only noted side effect was post-lumbar puncture headache. |
| Heitschmidt L et al., 2021 [17] | Retrospective cohort study | 12 | SMA types 2 and 3 | 8.6 years | 7 months | NR | FVC did not change significantly by end of study; respiratory function remained essentially unchanged. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldukain, M.; Aldukain, A.; Hobani, A.; Barakat, A.; Alsayyed, L.; Alomar, M.; Zain Al-Abeden, M.S.; Alzoum, N.; Asseri, A.A. The Impact of Nusinersen Treatment on Respiratory Function in Patients with Spinal Muscular Atrophy: A Systematic Review. J. Clin. Med. 2024, 13, 6306. https://doi.org/10.3390/jcm13216306
Aldukain M, Aldukain A, Hobani A, Barakat A, Alsayyed L, Alomar M, Zain Al-Abeden MS, Alzoum N, Asseri AA. The Impact of Nusinersen Treatment on Respiratory Function in Patients with Spinal Muscular Atrophy: A Systematic Review. Journal of Clinical Medicine. 2024; 13(21):6306. https://doi.org/10.3390/jcm13216306
Chicago/Turabian StyleAldukain, Mona, Ali Aldukain, Assal Hobani, Abdulmalik Barakat, Lujain Alsayyed, Maher Alomar, Maha Saad Zain Al-Abeden, Nora Alzoum, and Ali Alsuheel Asseri. 2024. "The Impact of Nusinersen Treatment on Respiratory Function in Patients with Spinal Muscular Atrophy: A Systematic Review" Journal of Clinical Medicine 13, no. 21: 6306. https://doi.org/10.3390/jcm13216306
APA StyleAldukain, M., Aldukain, A., Hobani, A., Barakat, A., Alsayyed, L., Alomar, M., Zain Al-Abeden, M. S., Alzoum, N., & Asseri, A. A. (2024). The Impact of Nusinersen Treatment on Respiratory Function in Patients with Spinal Muscular Atrophy: A Systematic Review. Journal of Clinical Medicine, 13(21), 6306. https://doi.org/10.3390/jcm13216306






