Hypotension after Induction of Anesthesia as a Predictor of Hypotension after Opening the Dura Mater during Emergency Craniotomy
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawryluk, G.; Bell, R.; Jagoda, A.; Mangat, H.; Bobrow, B.; Ghajar, J. Guidelines for Prehospital Management of Traumatic Brain Injury 3rd Edition: Executive Summary. Neurosurgery 2023, 93, e159–e169. [Google Scholar] [CrossRef]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Sakamoto, T.; Ohnishi, H.; Karasawa, J.; Furuya, H. Preoperative predictors of reduction in arterial blood pressure following dural opening during surgical evacuation of acute subdural hematoma. J. Neurosurg. Anesthesiol. 1996, 8, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Kushi, H.; Sakurai, A.; Utagawa, A.; Saito, T.; Moriya, T.; Hayashi, N. Risk factors for intraoperative hypotension in traumatic intracranial hematoma. Resuscitation 2004, 60, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.; Mack, C.D.; Sammer, M.; Rozet, I.; Lee, L.A.; Muangman, S.; Wang, M.; Hollingworth, W.; Lam, A.M.; Vavilala, M.S. The incidence and risk factors for hypotension during emergent decompressive craniotomy in children with traumatic brain injury. Anesth. Analg. 2006, 103, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Brown, M.J.; Curry, P.; Noda, S.; Chesnut, R.M.; Vavilala, M.S. Prevalence and risk factors for intraoperative hypotension during craniotomy for traumatic brain injury. J. Neurosurg. Anesthesiol. 2012, 24, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Node, Y.; Yamamoto, Y.; Teramoto, A. Cardiopulmonary hemodynamic changes during acute subdural hematoma evacuation. Neurol. Med. Chir. 2006, 46, 219–225. [Google Scholar] [CrossRef]
- Wan, W.H.; Ang, B.T.; Wang, E. The Cushing Response: A case for a review of its role as a physiological reflex. J. Clin. Neurosci. 2008, 15, 223–228. [Google Scholar] [CrossRef]
- Chesnut, R.M.; Gautille, T.; Blunt, B.; Klauber, M.R.; Marshall, L.F. Neurogenic hypotension in patients with severe head injuries. J. Trauma Acute Care Surg. 1998, 44, 958–964. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef]
- Eastridge, B.J.; Salinas, J.; McManus, J.G.; Blackburn, L.; Bugler, E.M.; Cooke, W.H.; Convertino, V.A.; Wade, C.E.; Holcomb, J.B. Hypotension begins at 110 mm Hg: Redefining “hypotension” with data. J. Trauma 2007, 63, 291–297; discussion 297–299. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; He, Z.; Li, Z.; Gong, R.; Hui, J.; Weng, W.; Wu, X.; Yang, C.; Jiang, J.; Xie, L.; et al. Traumatic brain injury in elderly population: A global systematic review and meta-analysis of in-hospital mortality and risk factors among 2.22 million individuals. Ageing Res. Rev. 2024, 99, 102376. [Google Scholar] [CrossRef] [PubMed]
- Spaite, D.W.; Hu, C.; Bobrow, B.J.; Chikani, V.; Sherrill, D.; Barnhart, B.; Gaither, J.B.; Denninghoff, K.R.; Viscusi, C.; Mullins, T.; et al. Mortality and Prehospital Blood Pressure in Patients with Major Traumatic Brain Injury: Implications for the Hypotension Threshold. JAMA Surg. 2017, 152, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.W.; Hemphill, J.C.; Morabito, D.; Manley, G. A novel method of evaluating the impact of secondary brain insults on functional outcomes in traumatic brain-injured patients. Acad. Emerg. Med. 2005, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.; Knudson, M.M.; Morabito, D.; Damron, S.; Erickson, V.; Pitts, L. Hypotension, hypoxia, and head injury: Frequency, duration, and consequences. Arch. Surg. 2001, 136, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, V.; Vavilala, M.S.; Mills, B.; Rowhani-Rahbar, A. Demographic and clinical risk factors associated with hospital mortality after isolated severe traumatic brain injury: A cohort study. J. Intensive Care 2015, 3, 46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, H.B.; Smith, M. Systemic complications after head injury: A clinical review. Anaesthesia 2007, 62, 474–482. [Google Scholar] [CrossRef]
- Shiozaki, T. Hypertension and head injury. Curr. Hypertens. Rep. 2005, 7, 450–453. [Google Scholar] [CrossRef]
- Latson, T.W.; Ashmore, T.H.; Reinhart, D.J.; Klein, K.; Giesecke, A.H. Autonomic reflex dysfunction in patients presenting for elective surgery is associated with hypotension after anesthesia induction. Anesthesiology 1994, 80, 326–337. [Google Scholar] [CrossRef]
- Abdelhamid, B.M.; Yassin, A.; Ahmed, A.; Amin, S.M.; Abougabal, A. Perfusion index-derived parameters as predictors of hypotension after induction of general anaesthesia: A prospective cohort study. Anaesthesiol. Intensive Ther. 2022, 54, 34–41. [Google Scholar] [CrossRef]
- Czajka, S.; Putowski, Z.; Krzych, Ł.J. Post-induction hypotension and intraoperative hypotension as potential separate risk factors for the adverse outcome: A cohort study. J. Anesth. 2023, 37, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.A.; Dogan, L.; Sarikaya, Z.T.; Ulugol, H.; Gucyetmez, B.; Toraman, F. Hypotension after Anesthesia Induction: Target-Controlled Infusion Versus Manual Anesthesia Induction of Propofol. J. Clin. Med. 2023, 12, 5280. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R.; Chesnut, R.M.; Clifton, G.; Ghajar, J.; Marion, D.W.; Narayan, R.K.; Newell, D.W.; Pitts, L.H.; Rosner, M.J.; Wilberger, J.W.; et al. Guidelines for the management of severe head injury. Eur. J. Emerg. Med. 1996, 3, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kushi, H.; Makino, K.; Hayashi, N. The risk factors for the occurrence of acute brain swelling in acute subdural hematoma. Acta Neurochir. Suppl. 2003, 86, 351–354. [Google Scholar] [CrossRef]
- Saengrung, S.; Kaewborisutsakul, A.; Tunthanathip, T.; Phuenpathom, N.; Taweesomboonyat, C. Risk Factors for Intraoperative Hypotension During Decompressive Craniectomy in traumatic Brain Injury Patients. World Neurosurg. 2022, 162, e652–e658. [Google Scholar] [CrossRef] [PubMed]
- Kamiutsuri, K.; Tominaga, N.; Kobayashi, S. Preoperative elevated FDP may predict severe intraoperative hypotension after dural opening during decompressive craniectomy of traumatic brain injury. JA Clin. Rep. 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mayer, S.A.; Kurtz, P.; Wyman, A.; Sung, G.Y.; Multz, A.S.; Varon, J.; Granger, C.B.; Kleinschmidt, K.; Lapointe, M.; Peacock, W.F.; et al. Clinical practices, complications, and mortality in neurological patients with acute severe hypertension: The Studying the Treatment of Acute hyperTension registry. Crit. Care Med. 2011, 39, 2330–2336. [Google Scholar] [CrossRef]
- Seo, W. Paroxysmal Sympathetic Hyperactivity After Acquired Brain Injury: An Integrative Literature Review. Crit. Care Nurse. 2023, 43, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Shutter, L.A.; Narayan, R.K. Blood pressure management in traumatic brain injury. Ann. Emerg. Med. 2008, 51 (Suppl. S3), S37–S38. [Google Scholar] [CrossRef]
- Simard, J.M.; Bellefleur, M. Systemic arterial hypertension in head trauma. Am. J. Cardiol. 1989, 63, 32C–35C. [Google Scholar] [CrossRef]
- Meng, L.; Sun, Y.; Zhao, X.; Rasmussen, M.; Al-Tarshan, Y.; Meng, D.M.; Liu, Z.; Adams, D.C.; McDonagh, D.L. Noradrenaline-induced changes in cerebral blood flow in health, traumatic brain injury and critical illness: A systematic review with meta-analysis. Anaesthesia 2024, 79, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Bijker, J.B.; Persoon, S.; Peelen, L.M.; Moons, K.G.; Kalkman, C.J.; Kappelle, L.J.; Van Klei, W.A. Intraoperative hypotension and perioperative ischemic stroke after general surgery: A nested case-control study. Anesthesiology 2012, 116, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.P.; Freymond, D.; Ravussin, P. Use of mannitol in neuroanesthesia and neurointensive care. Ann. Fr. Anesth. Reanim. 1995, 14, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Servadei, F.; Nasi, M.T.; Giuliani, G.; Cremonini, A.M.; Cenni, P.; Zappi, D.; Taylor, G.S. CT prognostic factors in acute subdural haematomas: The value of the ‘worst’ CT scan. Br. J. Neurosurg. 2000, 14, 110–116. [Google Scholar] [CrossRef] [PubMed]
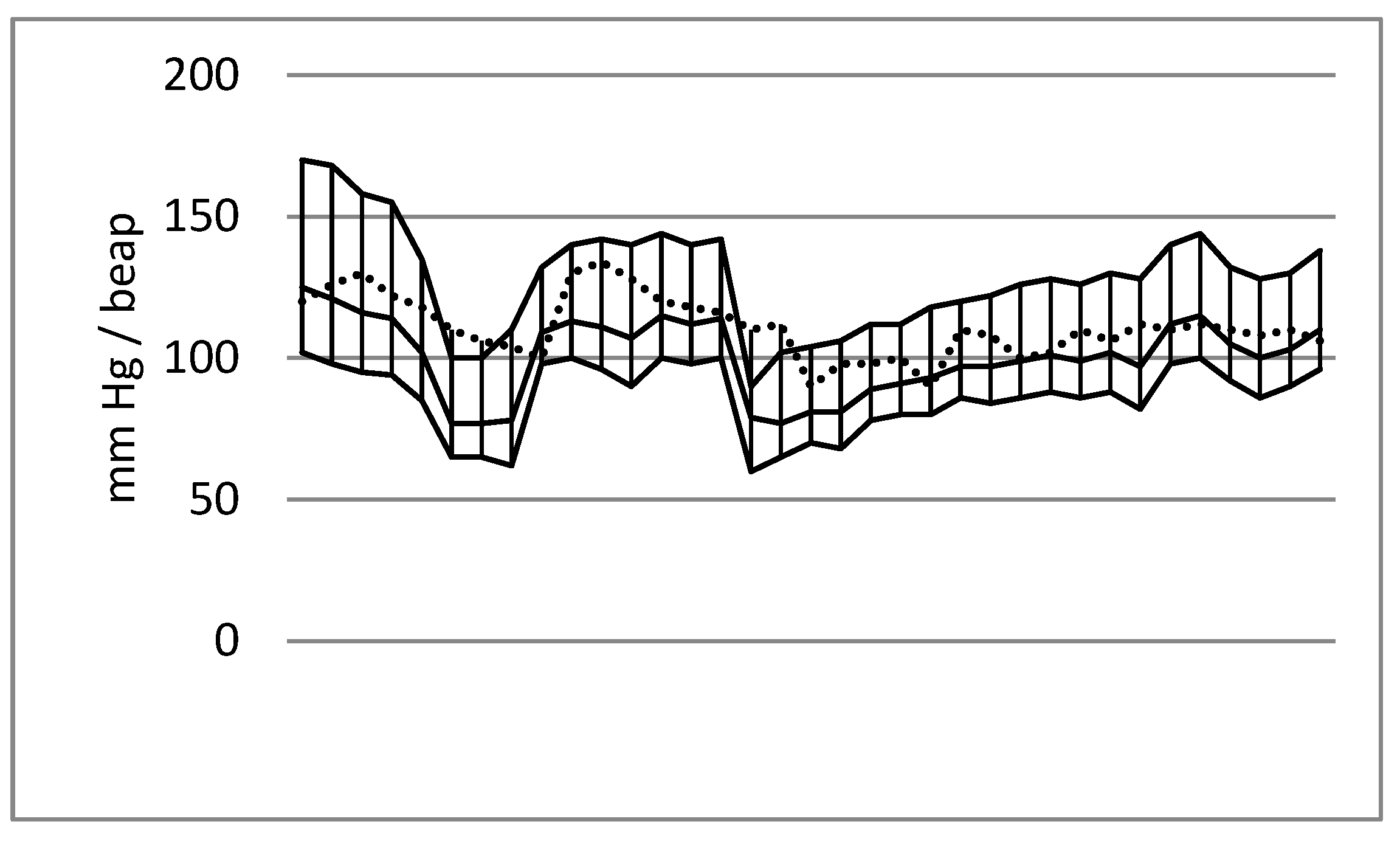

| HBP (n = 89) | NBP (n = 76) | p-Value | |
|---|---|---|---|
| Sex (M/F) | 75/14 | 56/20 | 0.0692 |
| Age (yr) | 57.8 ± 13.7 | 56.8 ± 14.3 | 0.6541 |
| GCS (range) | 7.4 ± 4.1 (3–15) | 8.7 ± 4.2 (3–15) | 0.0764 |
| Haematocrit (%) | 40.2 ± 5.1 | 37.9 ± 4.8 | 0.0075 * |
| Surgery time (min) | 108.5 ± 34.8 | 106.4 ± 35.1 | 0.6944 |
| HR on arrival to the OR (beat/min) | 100.7 ± 23.7 | 91.7 ± 22.8 | 0.0143 * |
| Total infusion (mL)—crystalloids | 1951.1 ± 902.6 | 2011.1 ± 810.6 | 0.6559 |
| Vasopressor use (n %)—norepinephrine | 12 (13.4%) | 11 (14.4%) | 0.5151 |
| Blood transfusion (n %) | 8 (8.9%) | 6 (7.8%) | 0.5139 |
| Total urine volume (ml) | 290.8 ± 366.5 | 494.4 ± 475.9 | 0.0022 * |
| Intraoperative brain oedema (n %) | 17 (19.1%) | 12 (15.7%) | 0.3638 |
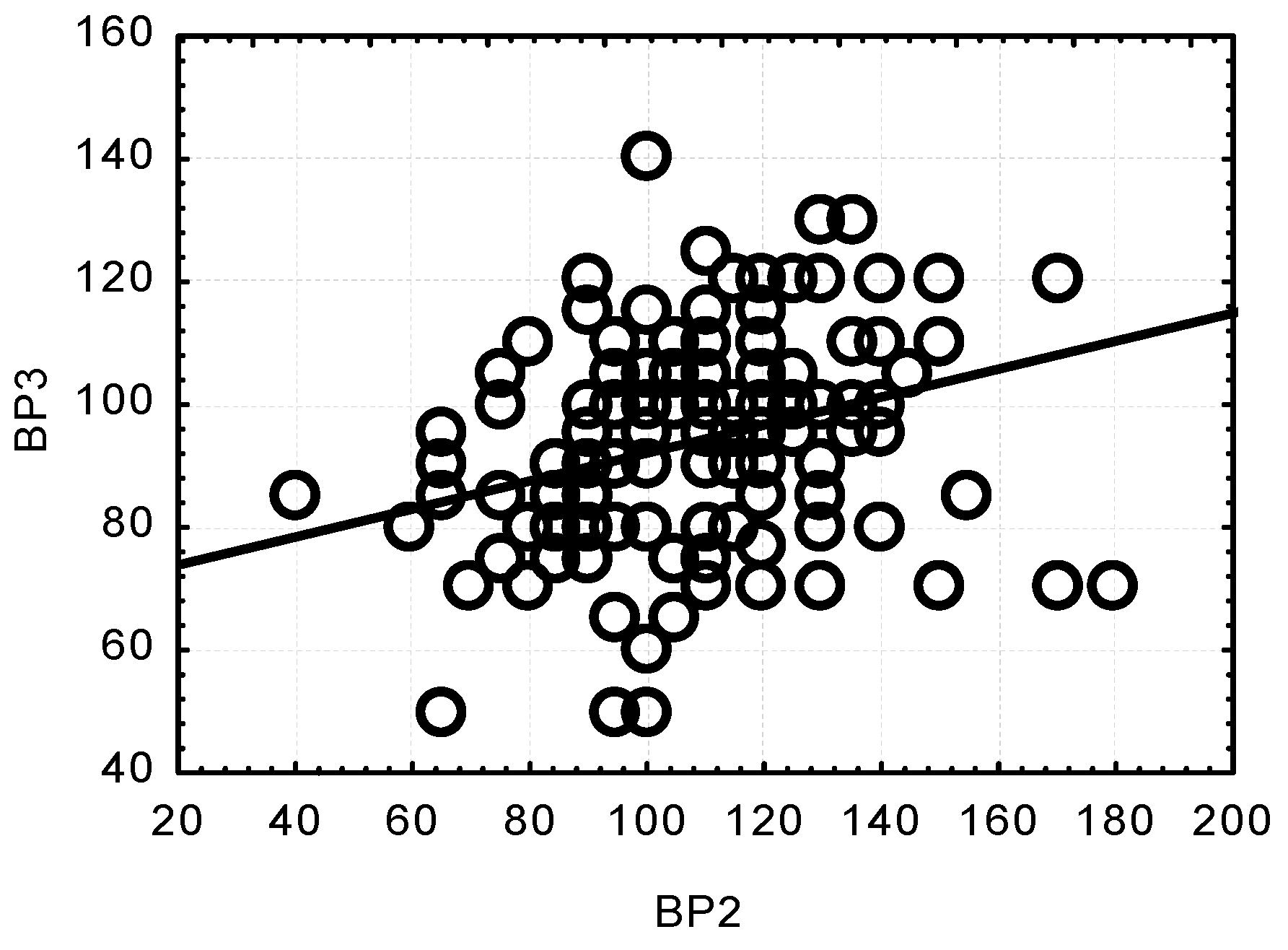
| BP 1 | BP 2 | BP 3 | BP 4 | |||||
|---|---|---|---|---|---|---|---|---|
| HBP | NBP | HBP | NBP | HBP | NBP | HBP | NBP | |
| Age (year) | r = 0.0266 | r = −0.1349 | r = −0.2670 | r = −0.1091 | r = −0.3830 | r = −0.1738 | r = 0.0160 | r = −0.1115 |
| p = 0.8170 | p = 0.2480 | p = 0.0180 * | p = 0.3510 | p = 0.0010 * | p = 0.1360 | p = 0.8900 | p = 0.3410 | |
| Glasgow Coma Scale | r = −0.2287 | r = 0.1997 | r = −0.1822 | r = −0.1106 | r = 0.2918 | r = 0.2369 | r = 0.2635 | r = 0.3193 |
| p = 0.0520 | p = 0.1080 | p = 0.1230 | p = 0.3770 | p = 0.0120 | p = 0.0550 | p = 0.0240 * | p = 0.0090 * | |
| HR (beap/min) | r = 0.2155 | r = −0.0981 | r = 0.1455 | r = 0.0967 | r = 0.0900 | r = −0.0778 | r = −0.0584 | r = −0.2915 |
| p = 0.0430 * | p = 0.3990 | p = 0.1740 | p = 0.4060 | p = 0.4010 | p = 0.5040 | p = 0.5870 | p = 0.0110 * | |
| Ht (%) | r = 0.2527 | r = 0.0445 | r = −0.1648 | r = 0.2006 | r = 0.0813 | r = 0.1824 | r = 0.1273 | r = −0.0336 |
| p = 0.0270 * | p = 0.7250 | p = 0.1520 | p = 0.1090 | p = 0.4820 | p = 0.1460 | p = 0.2700 | p = 0.7900 | |
| Intravenous fluids (mL) | r = 0.0916 | r = −0.3273 | r = −0.1296 | r = −0.2084 | r = 0.0012 | r = −0.0694 | r = 0.0674 | r = −0.0534 |
| p = 0.3930 | p = 0.0040 * | p = 0.2260 | p = 0.0710 | p = 0.9910 | p = 0.5510 | p = 0.5300 | p = 0.6470 | |
| Urine output (mL) | r = −0.0304 | r = 0.0035 | r = 0.0510 | r = 0.2558 | r = 0.0613 | r = −0.0132 | r = −0.0282 | r = −0.0735 |
| p = 0.7770 | p = 0.9760 | p = 0.6350 | p = 0.0260 * | p = 0.5680 | p = 0.9100 | p = 0.7930 | p = 0.5280 | |
| Glasgow Outcome Scale | r = −0.3222 | r = 0.2566 | r = −0.2493 | r = 0.0265 | r = 0.2770 | r = 0.5196 | r = 0.2091 | r = 0.3369 |
| p = 0.0050 * | p = 0.0440 * | p = 0.0330 * | p = 0.8380 | p = 0.0180 * | p< 0.0001 * | p = 0.0760 | p = 0.0070 * |
| HBP (n = 89) | NBP (n = 76) | p-Value | |
|---|---|---|---|
| Glasgow Outcome Scale | 2.26 ± 1.2 | 2.72 ± 1.2 | 0.0290 * |
| Deceased | 39 (43%) | 17 (22%) | 0.0276 * |
| Length of stay (range) | 12.2 ± 10.5 (1–60) days | 11.8 ± 8.8 (1–37) days | 0.8333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda, I.; Hofman, M.; Dymek, M.; Liberski, P.; Wojtacha, M.; Szczepańska, A. Hypotension after Induction of Anesthesia as a Predictor of Hypotension after Opening the Dura Mater during Emergency Craniotomy. J. Clin. Med. 2024, 13, 6021. https://doi.org/10.3390/jcm13196021
Duda I, Hofman M, Dymek M, Liberski P, Wojtacha M, Szczepańska A. Hypotension after Induction of Anesthesia as a Predictor of Hypotension after Opening the Dura Mater during Emergency Craniotomy. Journal of Clinical Medicine. 2024; 13(19):6021. https://doi.org/10.3390/jcm13196021
Chicago/Turabian StyleDuda, Izabela, Mariusz Hofman, Mikołaj Dymek, Piotr Liberski, Maciej Wojtacha, and Anna Szczepańska. 2024. "Hypotension after Induction of Anesthesia as a Predictor of Hypotension after Opening the Dura Mater during Emergency Craniotomy" Journal of Clinical Medicine 13, no. 19: 6021. https://doi.org/10.3390/jcm13196021
APA StyleDuda, I., Hofman, M., Dymek, M., Liberski, P., Wojtacha, M., & Szczepańska, A. (2024). Hypotension after Induction of Anesthesia as a Predictor of Hypotension after Opening the Dura Mater during Emergency Craniotomy. Journal of Clinical Medicine, 13(19), 6021. https://doi.org/10.3390/jcm13196021





