Performance of the Mammoth Balloon Catheter in Patients with Severe Aortic Valve Stenosis Undergoing Percutaneous Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Objective and Type of Study/Study Design
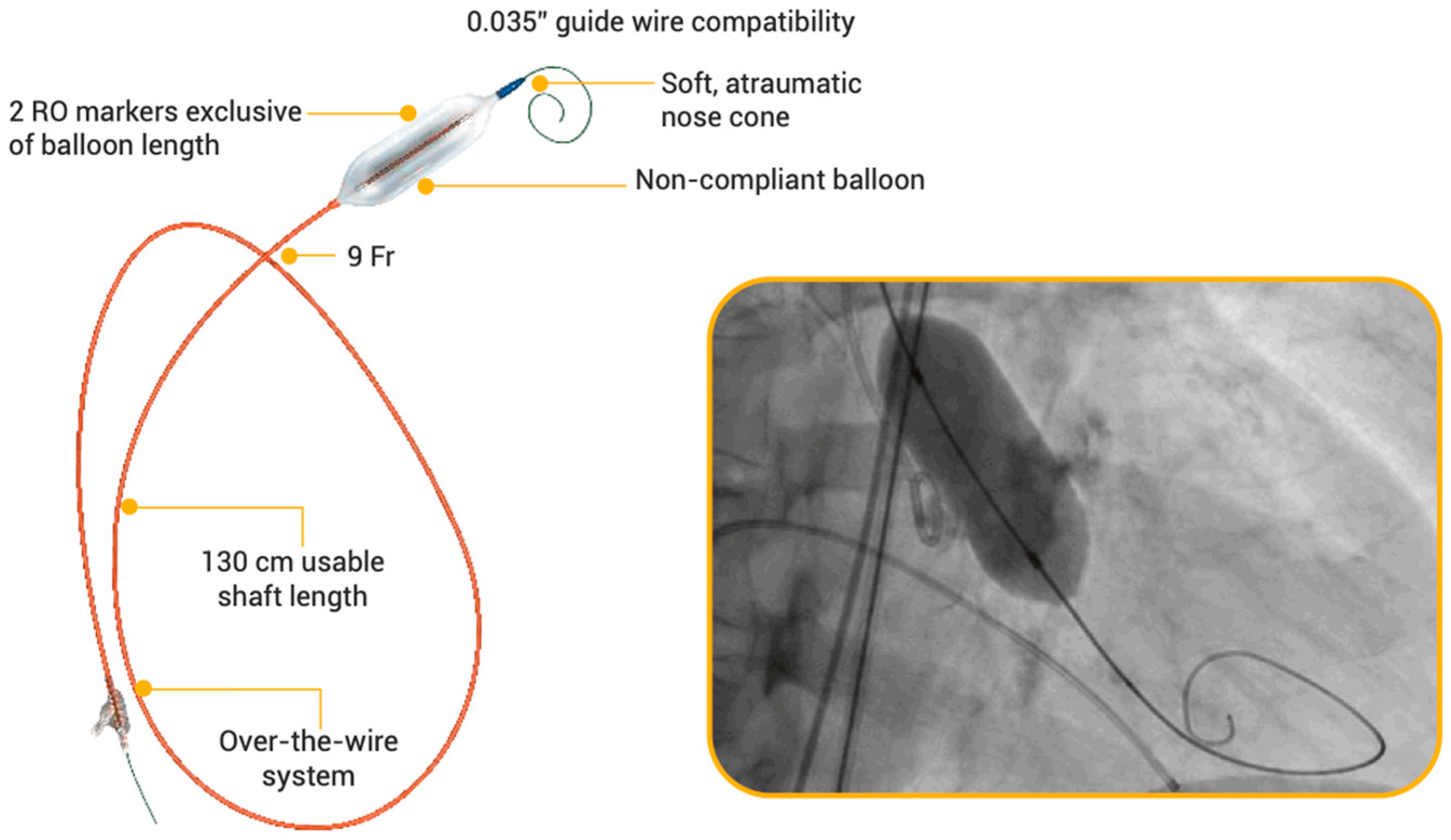
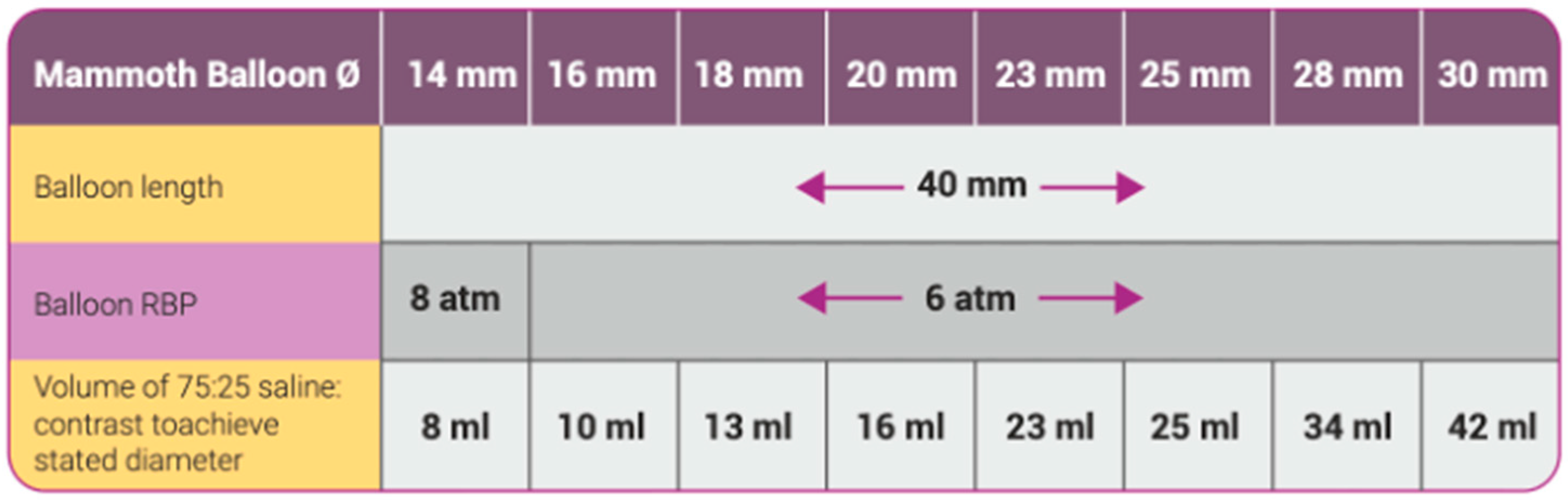
2.2. Study Device
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Pre-TAVR BAV vs. Direct TAVR: Available Data and Current Recommendations
4.2. BAV as Stand-Alone Procedure for Aortic Stenosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ternacle, J.; Al-Azizi, K.; Szerlip, M.; Potluri, S.; Hamandi, M.; Blanke, P.; Leipsic, J.; Dahou, A.; Salaun, E.; Vincent, F.; et al. Impact of Predilation During Transcatheter Aortic Valve Replacement: Insights from the PARTNER 3 Trial. Circ. Cardiovasc. Interv. 2021, 14, 7. [Google Scholar] [CrossRef]
- Rodés-Cabau, J. Transcatheter Aortic Valve Implantation: Current and Future Approaches. Nat. Rev. Cardiol. 2012, 9, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Husser, O.; Pellegrini, C.; Kessler, T.; Burgdorf, C.; Thaller, H.; Mayr, N.P.; Kasel, A.M.; Kastrati, A.; Schunkert, H.; Hengstenberg, C. Predictors of Permanent Pacemaker Implantations and New-Onset Conduction Abnormalities with the SAPIEN 3 Balloon-Expandable Transcatheter Heart Valve. JACC Cardiovasc. Interv. 2016, 9, 244–254. [Google Scholar] [CrossRef]
- Meredith, I.T.; Walters, D.L.; Dumonteil, N.; Worthley, S.G.; Tchétché, D.; Manoharan, G.; Blackman, D.J.; Rioufol, G.; Hildick-Smith, D.; Whitbourn, R.J.; et al. 1-Year Outcomes with the Fully Repositionable and Retrievable Lotus Transcatheter Aortic Replacement Valve in 120 High-Risk Surgical Patients with Severe Aortic Stenosis. JACC Cardiovasc. Interv. 2016, 9, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, H.; Zhang, L.; Li, H.; Mao, H.; Pei, Y.; Jing, Z.; Lu, Q. Transcatheter Aortic Valve Replacement with Balloon-Expandable Valve. Herz 2018, 43, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Mangi, A.A.; Popma, J.J.; Khabbaz, K.; Reardon, M.J.; Kleiman, N.S.; Yakubov, S.J.; Watson, D.; Kodali, S.; George, I.; et al. Early Outcomes with the Evolut PRO Repositionable Self-Expanding Transcatheter Aortic Valve with Pericardial Wrap. JACC Cardiovasc. Interv. 2018, 11, 160–168. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, I.; Rogers, T.; Satler, L.F.; Waksman, R. A Word of Caution Using Self-Expanding Transcatheter Aortic Valve-Frame Infolding. Catheter. Cardiovasc. Interv. 2019, 93, 555–558. [Google Scholar] [CrossRef]
- Bernardi, F.L.M.; Ribeiro, H.B.; Carvalho, L.A.; Sarmento-Leite, R.; Mangione, J.A.; Lemos, P.A.; Abizaid, A.; Grube, E.; Rodés-Cabau, J.; de Brito, F.S. Direct Transcatheter Heart Valve Implantation Versus Implantation with Balloon Predilatation. Circ. Cardiovasc. Interv. 2016, 9, 97–104. [Google Scholar] [CrossRef]
- Chan, P.H.; Mario, C.D.; Moat, N. Transcatheter Aortic Valve Implantation without Balloon Predilatation. Catheter. Cardiovasc. Interv. 2013, 82, 328–332. [Google Scholar] [CrossRef]
- Kleczynski, P.; Kulbat, A.; Brzychczy, P.; Dziewierz, A.; Trebacz, J.; Stapor, M.; Sorysz, D.; Rzeszutko, L.; Bartus, S.; Dudek, D.; et al. Balloon Aortic Valvuloplasty for Severe Aortic Stenosis as Rescue or Bridge Therapy. J. Clin. Med. 2021, 10, 4657. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Kamp, N.; Bansal, A.; Kumar, A.; Puri, R.; Krishnaswamy, A.; Kapadia, S.; Reed, G.W. Balloon Aortic Valvuloplasty in the Modern Era: A Review of Outcomes, Indications, and Technical Advances. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101002. [Google Scholar] [CrossRef] [PubMed]
- Montonati, C.; Pellegrini, D.; d’Atri, D.O.; Pellicano, M.; Briguglia, D.; Giannini, F.; De Blasio, G.; Guagliumi, G.; Tespili, M.; Ielasi, A. A Novel Balloon-Expandable Transcatheter Aortic Valve Bioprosthesis: Myval and Myval Octacor. Expert. Rev. Cardiovasc. Ther. 2024, 22, 325–337. [Google Scholar] [CrossRef]
- Barbanti, M.; Yang, T.-H.; Rodès Cabau, J.; Tamburino, C.; Wood, D.A.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; Latib, A.; Colombo, A.; et al. Anatomical and Procedural Features Associated with Aortic Root Rupture During Balloon-Expandable Transcatheter Aortic Valve Replacement. Circulation 2013, 128, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Tops, L.F.; Wood, D.A.; Delgado, V.; Schuijf, J.D.; Mayo, J.R.; Pasupati, S.; Lamers, F.P.L.; van der Wall, E.E.; Schalij, M.J.; Webb, J.G.; et al. Noninvasive Evaluation of the Aortic Root with Multislice Computed Tomography. JACC Cardiovasc. Imaging 2008, 1, 321–330. [Google Scholar] [CrossRef]
- Mach, M.; Szalkiewicz, P.; Poschner, T.; Hasan, W.; Andreas, M.; Winkler, B.; Hasimbegovic, E.; Steinkellner, T.; Strouhal, A.; Adlbrecht, C.; et al. The Use of Semi-compliant versus Non-compliant Balloon Systems for Predilatation during the Implantation of Self-expandable Transcatheter Aortic Valves. Eur. J. Clin. Investig. 2021, 51, e13570. [Google Scholar] [CrossRef]
- Arslan, S.; Bayar, N.; Uslu, S. Treatment of Main Coronary Obstruction with Renal Stent Implantation after Transcatheter Aortic Valve Implantation. Anatol. J. Cardiol. 2021, 25, 593–594. [Google Scholar] [CrossRef]
- Maluenda, G.; Baeza, C.; Giacaman, A.; Sepúlveda, E.; Cuevas, Ó.; Alfaro, M.; Moreno, Ó.; Baza, R. Resultados Clínicos Del Reemplazo Valvular Aórtico Percutáneo Con Dispositivo Balón Expandible MyVal En Estenosis Aórtica Severa de Alto Riesgo. Rev. Chil. Cardiol. 2020, 39, 237–246. [Google Scholar] [CrossRef]
- Testa, L.; Criscione, E.; Popolo Rubbio, A.; Squillace, M.; Ielasi, A.; Tespili, M.; Brambilla, N.; Bedogni, F. Safety and Performance Parameters of the Myval Transcatheter Aortic Valve Bioprosthesis: The SAPPHIRE Prospective Registry. Cardiovasc. Revascularization Med. 2023, 55, 22–27. [Google Scholar] [CrossRef]
- Magyari, B.; Kittka, B.; Goják, I.; Kasza, G.; Schönfeld, K.; Szapáry, L.B.; Simon, M.; Kiss, R.; Bertalan, A.; Várady, E.; et al. Single Center Experience with the Balloon-expandable Myval Transcatheter Aortic Valve System with the First 100 Patients: 30-day and 1-year Follow-up. Catheter. Cardiovasc. Interv. 2023, 102, 1317–1330. [Google Scholar] [CrossRef]
- Baumbach, A.; van Royen, N.; Amat-Santos, I.J.; Hudec, M.; Bunc, M.; Ijsselmuiden, A.; Laanmets, P.; Unic, D.; Merkely, B.; Hermanides, R.S.; et al. LANDMARK Comparison of Early Outcomes of Newer-Generation Myval Transcatheter Heart Valve Series with Contemporary Valves (Sapien and Evolut) in Real-World Individuals with Severe Symptomatic Native Aortic Stenosis: A Randomised Non-Inferiority Trial. Lancet 2024, 403, 2695–2708. [Google Scholar] [CrossRef]
- Moscarella, E.; Ielasi, A.; Montonati, C.; Pellegrini, D.; Pellicano, M.; Briguglia, D.; D’Alessandro, V.; Giannini, F.; Gamardella, M.; Medda, M.; et al. Comparing Two-Year Outcomes of Balloon-Expandable Myval and Self-Expanding Evolut R in Severe Aortic Valve Stenosis. Int. J. Cardiol. 2024, 400, 131701. [Google Scholar] [CrossRef]
- Santos-Martínez, S.; Amat-Santos, I.J.; Serrador, A.; Rodríguez-Gabella, T.; Gutiérrez, H.; San Román, A. Balloon-Expandable Myval Transcatheter Aortic Valve Implantation. First Experience in Spain. Rev. Española Cardiol. (Engl. Ed.) 2020, 73, 596–597. [Google Scholar] [CrossRef]
- Chopra, A.; Subban, V.; Rao, R.; Ajit, M.; Abraham, G. Successful Transcatheter Aortic Valve Replacement in a Kidney Allograft Patient on Rapamycin. Indian J. Transplant. 2019, 13, 303. [Google Scholar] [CrossRef]
- Gupta, H.; Kaur, N.; Sharma, Y.; Barwad, P. ROTAVI: Simultaneous Left Main Rotablation and Transcutaneous Aortic Valve Implantation in Calcified Coronaries and Severe Aortic Stenosis—A Case Report. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Ray, S. Trans-Catheter Aortic Valve Implantation in a Severe Calcific Aortic Stenosis. In Proceedings of the Cardiovascular Summit Transcatheter Cardiovascular Therapeutics Asia Pacific (TCTAP) & AP VALVES 2020 Virtual, Seoul, Republic of Korea, 6–9 August 2020. [Google Scholar]
- Barros de Toledo, J.F.; Teixeirense, P.T.; Guimaraes, W.; Motta, J.; Joaquim, M.R.; Odone, V.; Mantovani, J.; Alves, R.; Gubolino, L.A. In-Series Transcatheter Aortic Valve Replacement-in-Transcatheter Aortic Valve Replacement: ACURATE Neo Transcatheter Heart Valve Degeneration Successfully Managed with Myval, Avoiding Coronary Flow Obstruction—A Case Report. Struct. Heart 2023, 7, 100181. [Google Scholar] [CrossRef]
- Sharma, S.K.; Rao, R.S.; Chandra, P.; Goel, P.K.; Bharadwaj, P.; Joseph, G.; Jose, J.; Mahajan, A.U.; Mehrotra, S.; Sengottovelu, G.; et al. First-in-Human Evaluation of a Novel Balloon-Expandable Transcatheter Heart Valve in Patients with Severe Symptomatic Native Aortic Stenosis: The MyVal-1 Study. EuroIntervention 2020, 16, 421–429. [Google Scholar] [CrossRef]
- Pagnesi, M.; Baldetti, L.; Del Sole, P.; Mangieri, A.; Ancona, M.B.; Regazzoli, D.; Buzzatti, N.; Giannini, F.; Colombo, A.; Latib, A. Predilatation Prior to Transcatheter Aortic Valve Implantation: Is It Still a Prerequisite? Interv. Cardiol. Rev. 2017, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.P.; Sperrin, M.; Bagur, R.; de Belder, M.A.; Buchan, I.; Gunning, M.; Ludman, P.F.; Mamas, M.A. Pre-Implantation Balloon Aortic Valvuloplasty and Clinical Outcomes Following Transcatheter Aortic Valve Implantation: A Propensity Score Analysis of the UK Registry. J. Am. Heart Assoc. 2017, 6, e004695. [Google Scholar] [CrossRef]
- Dumonteil, N.; Terkelsen, C.; Frerker, C.; Collart, F.; Wöhrle, J.; Butter, C.; Hovorka, T.; Pinaud, F.; Baumgartner, H.; Tarantini, G.; et al. Outcomes of Transcatheter Aortic Valve Replacement without Predilation of the Aortic Valve: Insights from 1544 Patients Included in the SOURCE 3 Registry. Int. J. Cardiol. 2019, 296, 32–37. [Google Scholar] [CrossRef]
- Schymik, G.; Rudolph, T.; Jacobshagen, C.; Rothe, J.; Treede, H.; Kerber, S.; Frank, D.; Sykorova, L.; Okamoto, M.; Thoenes, M.; et al. Balloon-Expandable Transfemoral Transcatheter Aortic Valve Implantation with or without Predilation: Findings from the Prospective EASE-IT TF Multicentre Registry. Open Heart 2019, 6, e001082. [Google Scholar] [CrossRef] [PubMed]
- Pagnesi, M.; Kim, W.-K.; Conradi, L.; Barbanti, M.; Stefanini, G.G.; Schofer, J.; Hildick-Smith, D.; Pilgrim, T.; Abizaid, A.; Zweiker, D.; et al. Impact of Predilatation Prior to Transcatheter Aortic Valve Implantation with the Self-Expanding Acurate Neo Device (from the Multicenter NEOPRO Registry). Am. J. Cardiol. 2020, 125, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Toutouzas, K.; Latsios, G.; Stathogiannis, K.; Drakopoulou, M.; Synetos, A.; Sanidas, E.; Mastrokostopoulos, A.; Trantalis, G.; Kaitozis, O.; Lazaros, G.; et al. One-Year Outcomes after Direct Transcatheter Aortic Valve Implantation with a Self-Expanding Bioprosthesis. A Two-Center International Experience. Int. J. Cardiol. 2016, 202, 631–635. [Google Scholar] [CrossRef]
- Deharo, P.; Jaussaud, N.; Grisoli, D.; Camus, O.; Resseguier, N.; Le Breton, H.; Auffret, V.; Verhoye, J.P.; Koning, R.; Lefevre, T.; et al. Impact of Direct Transcatheter Aortic Valve Replacement Without Balloon Aortic Valvuloplasty on Procedural and Clinical Outcomes. JACC Cardiovasc. Interv. 2018, 11, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Spaziano, M.; Sawaya, F.; Chevalier, B.; Roy, A.; Neylon, A.; Garot, P.; Hovasse, T.; Benamer, H.; Romano, M.; Unterseeh, T.; et al. Comparison of Systematic Predilation, Selective Predilation, and Direct Transcatheter Aortic Valve Implantation with the SAPIEN S3 Valve. Can. J. Cardiol. 2017, 33, 260–268. [Google Scholar] [CrossRef]
- Auffret, V.; Regueiro, A.; Campelo-Parada, F.; del Trigo, M.; Chiche, O.; Chamandi, C.; Puri, R.; Rodés-Cabau, J. Feasibility, Safety, and Efficacy of Transcatheter Aortic Valve Replacement without Balloon Predilation: A Systematic Review and Meta-analysis. Catheter. Cardiovasc. Interv. 2017, 90, 839–850. [Google Scholar] [CrossRef]
- Islas, F.; Almería, C.; García-Fernández, E.; Jiménez, P.; Nombela-Franco, L.; Olmos, C.; Marcos-Alberca, P.; Cuadrado, A.; Fernández-Ortiz, A.; Macaya, C.; et al. Usefulness of Echocardiographic Criteria for Transcatheter Aortic Valve Implantation without Balloon Predilation: A Single-Center Experience. J. Am. Soc. Echocardiogr. 2015, 28, 423–429. [Google Scholar] [CrossRef]
- Otto, C.M.; Kumbhani, D.J.; Alexander, K.P.; Calhoon, J.H.; Desai, M.Y.; Kaul, S.; Lee, J.C.; Ruiz, C.E.; Vassileva, C.M. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults with Aortic Stenosis. J. Am. Coll. Cardiol. 2017, 69, 1313–1346. [Google Scholar] [CrossRef]
- McInerney, A.; Vera-Urquiza, R.; Tirado-Conte, G.; Marroquin, L.; Jimenez-Quevedo, P.; Nuñez-Gil, I.; Pozo, E.; Gonzalo, N.; de Agustín, J.A.; Escaned, J.; et al. Pre-Dilation and Post-Dilation in Transcatheter Aortic Valve Replacement: Indications, Benefits and Risks. Interv. Cardiol. Rev. Res. Resour. 2021, 16, e28. [Google Scholar] [CrossRef]
- Bruno, M.; Iannopollo, G.; Cardelli, L.S.; Capecchi, A.; Lanzilotti, V.; Verardi, R.; Pedone, C.; Nobile, G.; Casella, G. Efficacy and Safety of a Minimalistic Balloon Aortic Valvuloplasty Strategy in a Centre without Heart Surgery. AsiaIntervention 2024, 10, 40–50. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]

| Baseline Clinical Characteristics | n = 121 |
|---|---|
| Age (years), mean ± SD | 81.3 ± 8.9 |
| Male gender, n (%) | 80 (66) |
| CAD, n (%) | 72 (60) |
| Prior MI, n (%) | 17 (14) |
| Prior PCI, n (%) | 52 (43) |
| Prior CABG, n (%) | 6 (5) |
| History of atrial fibrillation, n (%) | 38 (31) |
| Prior PM/ICD, n (%) | 6 (5) |
| COPD, n (%) | 11 (9) |
| Peripheral artery disease, n (%) | 31 (25.6) |
| Severe chronic kidney disease (eGFR ≤ 30 mL/min) | 20 (16.5) |
| Severe chronic kidney disease (eGFR ≤ 30 mL/min) in BAV-only patients | 10/16 (62.5) |
| Cardiovascular risk factors | |
| Diabetes mellitus, n (%) | 25 (21) |
| Arterial hypertension, n (%) | 109 (90) |
| Hypercholesterolemia, n (%) | 73 (60) |
| Active or former smoker, n (%) | 38 (31) |
| Severe obesity (BMI > 40 kg/m2) n (%) | 2 (2) |
| NYHA class ≥ 3, n (%) | 60 (50.5) |
| EuroSCORE II (%), mean ± SD | 4.4 ± 1.9 |
| STS-PROM score (%), mean ± SD | 3.9 ± 2.1 |
| Baseline trans-thoracic echocardiography assessment | n = 121 |
| Trans-valvular gradient (mmHg), mean ± SD | 47.9 ± 17.5 |
| Aortic valve area (cm2), mean ± SD | 0.65 ± 0.20 |
| LVEF (%), mean ± SD | 52.7 ± 14.8 |
| Aortic regurgitation ≥ moderate, n (%) | 29 (24) |
| Baseline MSCT assessment | n = 111 |
| Aortic annulus diameter (mm), mean ± SD | 23.6 ± 2.4 |
| Aortic annulus area (mm2), mean ± SD | 466.1 ± 106.5 |
| Aortic annulus average perimeter (mm), mean ± SD | 74.1 ± 6.8 |
| Degree of moderate aortic leaflet calcification, n (%) | 50 /111(45.0) |
| Degree of severe aortic leaflet calcification, n (%) | 61/111 (54.9) |
| Degree of LVOT calcification ≥ moderate, n (%) | 5/111 (4.5) |
| Left common femoral artery diameter, (mm) mean ± SD | 6.4 ± 0.6 |
| Right common femoral artery diameter, (mm) mean ± SD | 6.8 ± 0.9 |
| Procedural Characteristics | n = 121 |
|---|---|
| Trans-femoral access, n (%) | 121 (100) |
| BAV procedure time (mins), mean ± SD | 19.4 ± 9.2 |
| BAV fluoroscopy time (mins), mean ± SD | 24.4 ± 12.8 |
| BAV total contrast volume (ml), mean ± SD | 24.3 ± 11.9 |
| Local anesthesia plus mild sedation, n (%) | 121 (100) |
| Safari S wire in the left ventricle, n (%) | 91 (75.2) |
| Safari XS wire in the left ventricle, n (%) | 23 (19) |
| Innowi wire in the left ventricle, n (%) | 2 (1.6) |
| Lunderquist wire in the left ventricle, n (%) | 5 (4.1) |
| Post-dilatation required after TAVR, n (%) | 3/111 (2.7) |
| Haemodynamics | Overall Population n= 121 |
|---|---|
| Baseline peak-to-peak transvalvular gradient (mmHg), mean ± SD | 59.8 ± 22.6 |
| Post-BAV peak-to-peak transvalvular gradient (mmHg), mean ± SD | 24.3 ± 11.4 |
| Haemodynamics | BAV alone n = 16 |
| Baseline transvalvular gradient (mmHg), mean ± SD | 42.9 ± 10.7 |
| Post-BAV transvalvular gradient (mmHg), mean ± SD | 21.0 ± 6.9 |
| Trans-thoracic Echocardiogram | BAV alone n = 16 |
| Baseline transvalvular gradient (mmHg), mean ± SD | 44.1 ± 9.8 |
| Post-procedural transvalvular gradient (mmHg), mean ± SD | 21.0 ± 2.0 |
| Baseline effective orifice area (mm2), mean ± SD | 0.82 ± 0.20 |
| Post-procedural effective orifice area (mm2) | 1.12 ± 0.22 |
| Balloon Type | Balloon Name | Manufacturer | Balloon Length (cm) n= | Shaft Length (cm) n= | Rated Burst Pressure (atm) | Total Sizes n= | Material |
|---|---|---|---|---|---|---|---|
| Non- compliant | VACS III | Osypka Medical | 2–6 | 100 | 4–15 | 18 | NA |
| Mammoth | Meril | 4 | 130 | 24 | 6 | Vestamid Care ML21 | |
| Tyshak II | B. Braun Interventional Systems, Inc. (Bethlehem, PA, USA) | 2–8 | 70–100 | 1.5–6 | 74 | Polymeric, DEHP-free | |
| True Dilatation | Bard | 4.5 | 110 | 6 | 9 | Fiber | |
| Atlas Gold | Bard | 2–4–6 | 80–120 | 16 | 40 | NA | |
| Z-MED™ | B. Braun Interventional Systems, Inc. | 2–6 | 100 | 1.5–3 | 52 | NA | |
| Z-MED™II | 3–4 | 77 | |||||
| SIM-valve force | Simeks Medical | 2–6 | 100 | 4, 6, 8, 12 | 65 | Nylon 12 | |
| NuCLEUS NuCLEUS X | B. Braun Interventional Systems, Inc. | 3–6 4–6 | 110 | 2–9 2–4 | 54 | NA | |
| Semi- compliant | VACS II | Osypka Medical | 2–6 | 100 | 1.5–6 | 17 | NA |
| Cristal | BALT extrusion | 3–6 | 110 | 6 | 9 | NA | |
| Valver | Balton | 2.5–6 | 110 | 3–5 | 15 | NA |
| Author and Year of Publication | Patient Details | Procedure | Outcomes |
|---|---|---|---|
| Santos-Martınez et al., 2018 [23] | 83-year-old male. NYHA class III due to severe AS. Mean gradient 78 mmHg and AVA 0.6 cm2 with preserved LVEF. Euroscore II 2.9%. | Pre-dilatation with an 18 mm Mammoth balloon catheter (BC), then a 24.5 mm Myval implanted | THV correctly positioned, with mild PVL. Mean gradient 9 mmHg. Discharged at dat 4 without complications |
| Chopra et al., 2020 [24] | 59-year-old male, post-renal transplant (on immunosuppressant therapy). NYHA Class III. Severe degenerative aortic valve disease, bicuspid leaflet with heavy calcification. Aortic valve mean gradient 70 mmHg and AVA 0.7 cm2 with normal LVEF. STS mortality score 4.8% with combined mortality and morbidity score 21.7% | Pre-dilatation with a 20 mm Mammoth BC, then a 23 mm Myval was deployed | Residual mean gradient of 4 mm Hg with no PVL |
| Maluenda et al., 2020 [18] | 14 patients with severe AS at high surgical risk. Mean age 82.5 ± 7.8 years. Mean STS score mortality 11.6 ± 5.1% at 30-day. Mean aortic valve gradient 47 ± 9 mm Hg. Mean AVA 0.6 ± 0.2 cm2. Mean aortic annulus area 435 ± 88 mm2. Mean aortic annular perimeter 71 ± 15 mm | Routine pre-dilatation with a Mammoth BC was recommended. Post-dilatation was recommended in cases with more than mild to moderate PVL | Device and procedural success 86%. Substantial drop in mean aortic gradient, persistent at 6-month follow-up without more than mild aortic regurgitation. Device failure in 2 patients, one due to delivery failure and the other due to ventricular embolization. One early death due to dissection/rupture of the aorta and 2 major hemorrhages. |
| Gupta et al., 2020 [25] | 75-year-old frail female with insulin-dependent diabetes mellitus and CKD. Severe AS with calcified left main distal and ostial left anterior descending artery lesions. NYHA Class. Maximum and mean gradient of 110 and 65 mmHg and AVA of 0.6 cm2. STS risk score of 16 and EUROSCORE risk of in-hospital mortality of 8.25% | Pre-dilatation with a 16 mm Mammoth BC followed by deployment of a 21 mm Myval THV | THV placed and expanded successfully without PVL. At 6-month follow-up, patient in NYHA Class I. |
| Ray, 2020 [26] | 71-year-old male presented in emergency with chest pain and severe shortness of breath. Severe calcific AS with peak gradient of 56 mm/Hg and mean gradient of 43 mm/Hg; severe systolic dysfunction | Pre-dilatation with a 16 mm Mammoth BC at 7 atm, then Myval 20 mm implantation | THV placed and expanded successfully. Mild PVL. Uneventful recovery. |
| Arslan et al., 2021 [17] | 83-year-old male, NYHA class III. Severe AS (mean gradient, 59 mm Hg; AVA: 0.8 cm2). Aortic annulus area and perimeter 508 mm2 and 81 mm, respectively. STS risk score 4.2. Left coronary ostium height, left leaflet length, sinus curvature length, and sinus of Valsalva diameter: 14.4 mm, 15.4 mm, 15.1 mm, and 34 mm, respectively. Bulky calcifications, more pronounced in the left aortic leaflet. | Pre-dilatation with 23 mm Mammoth BC. | No PVL, but partial eft main coronary artery (LMCA) obstruction. Despite repeated balloon inflations, LMCA recoil occurred. A 6.0×18 mm renal stent was successfully implanted in the LMCA. Uneventful recovery. |
| De Toledo et al., 2022 [27] | 73-year-old female. History of TAVR with a 25 mm Acurate Neo THV to treat severe symptomatic AS. Structural valve degeneration with severe aortic regurgitation (AR), intra and para-prosthetic. LVEF 74%. | Neo THV was crossed followed by a partially inflation of a 20 mm Mammoth BC to confirm central crossing. Myval 26 mm THV was implanted below the nadir of the leaflets of the degenerated THV with an oversizing to the annulus of 12.3% | Mean gradient of 3 mmHg, angiogram confirmed patency of coronary arteries, and no residual AR. At 1-month follow-up, the patient remained asymptomatic with mean gradient: 9 mmHg, AVA: 2.3 cm2, and no leaks. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscardelli, S.; Caminiti, R.; Montonati, C.; Ceresa, F.; De Blasio, G.; Vizzari, G.; Pellegrini, D.; Pellicano, M.; Guagliumi, G.; Patanè, F.; et al. Performance of the Mammoth Balloon Catheter in Patients with Severe Aortic Valve Stenosis Undergoing Percutaneous Treatment. J. Clin. Med. 2024, 13, 5986. https://doi.org/10.3390/jcm13195986
Moscardelli S, Caminiti R, Montonati C, Ceresa F, De Blasio G, Vizzari G, Pellegrini D, Pellicano M, Guagliumi G, Patanè F, et al. Performance of the Mammoth Balloon Catheter in Patients with Severe Aortic Valve Stenosis Undergoing Percutaneous Treatment. Journal of Clinical Medicine. 2024; 13(19):5986. https://doi.org/10.3390/jcm13195986
Chicago/Turabian StyleMoscardelli, Silvia, Rodolfo Caminiti, Carolina Montonati, Fabrizio Ceresa, Giuseppe De Blasio, Giampiero Vizzari, Dario Pellegrini, Mariano Pellicano, Giulio Guagliumi, Francesco Patanè, and et al. 2024. "Performance of the Mammoth Balloon Catheter in Patients with Severe Aortic Valve Stenosis Undergoing Percutaneous Treatment" Journal of Clinical Medicine 13, no. 19: 5986. https://doi.org/10.3390/jcm13195986
APA StyleMoscardelli, S., Caminiti, R., Montonati, C., Ceresa, F., De Blasio, G., Vizzari, G., Pellegrini, D., Pellicano, M., Guagliumi, G., Patanè, F., Tespili, M., Micari, A., & Ielasi, A. (2024). Performance of the Mammoth Balloon Catheter in Patients with Severe Aortic Valve Stenosis Undergoing Percutaneous Treatment. Journal of Clinical Medicine, 13(19), 5986. https://doi.org/10.3390/jcm13195986






