Enhancing Oral Cancer Detection: A Systematic Review of the Diagnostic Accuracy and Future Integration of Optical Coherence Tomography with Artificial Intelligence
Abstract
1. Introduction
1.1. Types and Advantages of OCT
1.2. Principles and Techniques of OCT
1.3. Applications of OCT in Oral Health
1.4. Study Aim
2. Materials and Methods
3. Results
4. Results of Individual Studies
5. Expanded Insights
6. Discussion
6.1. Advances in OCT Technology for Oral Pathology Assessment
6.2. Advances in Image Enhancement Technology for Oral Pathology Assessment
6.3. Integration with Other Imaging Modalities
6.4. Challenges, Limitations, and Potential Solutions
6.5. Future Directions
7. Conclusions
Funding
Conflicts of Interest
References
- Jerjes, W.; Upile, T.; Conn, B.; Hamdoon, Z.; Betz, C.S.; McKenzie, G.; Radhi, H.; Vourvachis, M.; El Maaytah, M.; Sandison, A.; et al. In vitro examination of suspicious oral lesions using optical coherence tomography. Br. J. Oral. Maxillofac. Surg. 2010, 48, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Przybek-Skrzypecka, J.; Skrzypecki, J.; Suh, L.; Szaflik, J.P. Corneal ring infiltrate-far more than Acanthamoeba keratitis: Review of pathophysiology, morphology, differential diagnosis and management. J. Ophthalmic Inflamm. Infect. 2023, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Janjua, O.S.; Jeelani, W.; Khan, M.I.; Qureshi, S.M.; Shaikh, M.S.; Zafar, M.S.; Khurshid, Z. Use of Optical Coherence Tomography in Dentistry. Int. J. Dent. 2023, 2023, 4179210. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Hamdoon, Z.; Jerjes, W.; McKenzie, G.; Jay, A.; Hopper, C. Optical coherence tomography in the assessment of oral squamous cell carcinoma resection margins. Photodiagn. Photodyn. Ther. 2016, 13, 211–217. [Google Scholar] [CrossRef]
- Gambino, A.; Martina, E.; Panzarella, V.; Ruggiero, T.; Haddad, G.E.; Broccoletti, R.; Arduino, P.G. Potential use of optical coherence tomography in oral potentially malignant disorders: In-vivo case series study. BMC Oral Health 2023, 23, 540. [Google Scholar]
- Yang, Z.; Pan, H.; Shang, J.; Zhang, J.; Liang, Y. Deep-Learning-Based Automated Identification and Visualization of Oral Cancer in Optical Coherence Tomography Images. Biomedicines 2023, 11, 802. [Google Scholar] [CrossRef]
- Mali, S.B. Role of in vivo imaging in Head and Neck cancer management. Oral. Oncol. 2023, 146, 106575. [Google Scholar] [CrossRef]
- Badhey, A.K.; Schwarz, J.S.; Laitman, B.M.; Veremis, B.M.; Westra, W.H.; Yao, M.; Teng, M.S.; Genden, E.M.; Miles, B.A. Intraoperative Use of Wide-Field Optical Coherence Tomography to Evaluate Tissue Microstructure in the Oral Cavity and Oropharynx. JAMA Otolaryngol. Head. Neck Surg. 2023, 149, 71–78. [Google Scholar] [CrossRef]
- Gambino, A.; Cafaro, A.; Broccoletti, R.; Turotti, L.; Karimi, D.; Haddad, G.E.; Hopper, C.; Porter, S.R.; Chiusa, L.; Arduino, P.G. In vivo evaluation of traumatic and malignant oral ulcers with optical coherence tomography: A comparison between histopathological and ultrastructural findings. Photodiagn. Photodyn. Ther. 2022, 39, 103019. [Google Scholar] [CrossRef]
- Yuan, W.; Yang, J.; Yin, B.; Fan, X.; Yang, J.; Sun, H.; Liu, Y.; Su, M.; Li, S.; Huang, X. Noninvasive diagnosis of oral squamous cell carcinoma by multi-level deep residual learning on optical coherence tomography images. Oral. Dis. 2023, 29, 3223–3231. [Google Scholar] [CrossRef]
- Le, N.; Lu, J.; Tang, P.; Chung, K.H.; Subhash, H.; Kilpatrick-Liverman, L.; Wang, R.K. Intraoral optical coherence tomography and angiography combined with autofluorescence for dental assessment. Biomed. Opt. Express 2022, 13, 3629–3646. [Google Scholar] [CrossRef] [PubMed]
- Saggu, A.; Maguluri, G.; Grimble, J.; Park, J.; Hasturk, H.; Iftimia, N.; Sima, C. Raman microspectroscopy/micro-optical coherence tomography approach for chairside diagnosis of periodontal diseases: A pilot study. J. Periodontol. 2022, 93, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Jena, S.; Jnaneswar, A.; Jha, K.; Suresan, V.; Singh, A. Advancements in diagnostic techniques for oral cancer detection. Minerva Dent. Oral. Sci. 2022, 71, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Noorlag, R.; de Bree, R.; Witjes, M.J.H. Image-guided surgery in oral cancer: Toward improved margin control. Curr. Opin. Oncol. 2022, 34, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Macey, R.; Ricketts, D.; Carrasco Labra, A.; Worthington, H.; Sutton, A.J.; Freeman, S.; Glenny, A.M.; Riley, P.; Clarkson, J.; et al. Enamel Caries Detection and Diagnosis: An Analysis of Systematic Reviews. J. Dent. Res. 2022, 101, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Classification of Salivary Gland Tumors Based on Quantitative Optical Coherence Tomography. Lasers Surg. Med. 2021, 53, 830–837. [Google Scholar] [CrossRef]
- Obade, A.Y.; Pandarathodiyil, A.K.; Oo, A.L.; Warnakulasuriya, S.; Ramanathan, A. Application of optical coherence tomography to study the structural features of oral mucosa in biopsy tissues of oral dysplasia and carcinomas. Clin. Oral. Investig. 2021, 25, 5411–5419. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Identification of oral precancerous and cancerous tissue by swept source optical coherence tomography. Lasers Surg. Med. 2022, 54, 320–328. [Google Scholar] [CrossRef]
- Ali, S.; Gilani, S.B.S.; Shabbir, J.; Almulhim, K.S.; Bugshan, A.; Farooq, I. Optical coherence tomography’s current clinical medical and dental applications: A review. F1000Res 2021, 10, 310. [Google Scholar] [CrossRef]
- Jerjes, W.; Hamdoon, Z.; Yousif, A.A.; Al-Rawi, N.H.; Hopper, C. Epithelial tissue thickness improves optical coherence tomography’s ability in detecting oral cancer. Photodiagn. Photodyn. Ther. 2019, 28, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Swinson, B.; Jerjes, W.; El-Maaytah, M.; Norris, P.; Hopper, C. Optical techniques in diagnosis of head and neck malignancy. Oral. Oncol. 2006, 42, 221–228. [Google Scholar] [CrossRef]
- Katkar, R.A.; Tadinada, S.A.; Amaechi, B.T.; Fried, D. Optical Coherence Tomography. Dent. Clin. N. Am. 2018, 62, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Schnabel, C.; Mueller, J.; Golde, J.; Koch, E.; Walther, J. In Vivo Endoscopic Optical Coherence Tomography of the Healthy Human Oral Mucosa: Qualitative and Quantitative Image Analysis. Diagnostics 2020, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Identification of oral cancer in OCT images based on an optical attenuation model. Lasers Med. Sci. 2020, 35, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.E.; Pham, T.T.; Ifegwu, I.; Burwell, R.; Armstrong, W.B.; Tjoson, T.; Whyte, S.; Giorgioni, C.; Wang, B.; Wong, B.J.F.; et al. The use of optical coherence tomography and convolutional neural networks to distinguish normal and abnormal oral mucosa. J. Biophotonics. 2020, 13, e201900221. [Google Scholar] [CrossRef] [PubMed]
- Stasio, D.D.; Lauritano, D.; Iquebal, H.; Romano, A.; Gentile, E.; Lucchese, A. Measurement of Oral Epithelial Thickness by Optical Coherence Tomography. Diagnostics 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Sunny, S.P.; Agarwal, S.; James, B.L.; Heidari, E.; Muralidharan, A.; Yadav, V.; Pillai, V.; Shetty, V.; Chen, Z.; Hedne, N.; et al. Intra-operative point-of-procedure delineation of oral cancer margins using optical coherence tomography. Oral. Oncol. 2019, 92, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, S.; Aoki, A.; Tsubokawa, M.; Lin, T.; Mizutani, K.; Koshy, G.; Sadr, A.; Oda, S.; Sumi, Y.; Izumi, Y. Observation and determination of periodontal tissue profile using optical coherence tomography. J. Periodontal Res. 2018, 53, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Kain, J.J.; Birkeland, A.C.; Udayakumar, N.; Morlandt, A.B.; Stevens, T.M.; Carroll, W.R.; Rosenthal, E.L.; Warram, J.M. Surgical margins in oral cavity squamous cell carcinoma: Current practices and future directions. Laryngoscope 2020, 130, 128–138. [Google Scholar] [CrossRef]
- Machoy, M.; Seeliger, J.; Szyszka-Sommerfeld, L.; Koprowski, R.; Gedrange, T.; Woźniak, K. The Use of Optical Coherence Tomography in Dental Diagnostics: A State-of-the-Art Review. J. Healthc. Eng. 2017, 2017, 7560645. [Google Scholar] [CrossRef] [PubMed]
- Gentile, E.; Maio, C.; Romano, A.; Laino, L.; Lucchese, A. The potential role of in vivo optical coherence tomography for evaluating oral soft tissue: A systematic review. J. Oral. Pathol. Med. 2017, 46, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Choi, W.J.; Wang, R.K. Microvascular imaging and monitoring of human oral cavity lesions in vivo by swept-source OCT-based angiography. Lasers Med. Sci. 2018, 33, 123–134. [Google Scholar] [CrossRef]
- Green, B.; Tsiroyannis, C.; Brennan, P.A. Optical diagnostic systems for assessing head and neck lesions. Oral. Dis. 2016, 22, 180–184. [Google Scholar] [CrossRef]
- Wang, T.A.; Trung, N.H.; Lee, H.C.; Lee, C.K.; Tsai, M.T.; Wang, Y.L. Quantitative Evaluation of Caries and Calculus with Ultrahigh-Resolution Optical Coherence Tomography. Bioengineering 2023, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Saberi, F.N.; Sukumaran, P.; Ung, N.M.; Liew, Y.M. Assessment of demineralized tooth lesions using optical coherence tomography and other state-of-the-art technologies: A review. Biomed. Eng. Online 2022, 21, 83. [Google Scholar] [CrossRef]
- Serban, C.; Lungeanu, D.; Bota, S.D.; Cotca, C.C.; Negrutiu, M.L.; Duma, V.F.; Sinescu, C.; Craciunescu, E.L. Emerging Technologies for Dentin Caries Detection-A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Roointan, S.; Tavakolian, P.; Sivagurunathan, K.S.; Mandelis, A.; Abrams, S.H. Detection and monitoring of early dental caries and erosion using three-dimensional enhanced truncated-correlation photothermal coherence tomography imaging. J. Biomed. Opt. 2021, 26, 046004. [Google Scholar] [CrossRef]
- Macey, R.; Walsh, T.; Riley, P.; Hogan, R.; Glenny, A.M.; Worthington, H.V.; Clarkson, J.E.; Ricketts, D. Transillumination and optical coherence tomography for the detection and diagnosis of enamel caries. Cochrane Database Syst. Rev. 2021, 1, CD013855. [Google Scholar]
- Fernandes, L.O.; Mota, C.C.B.O.; Oliveira, H.O.; Neves, J.K.; Santiago, L.M.; Gomes, A.S.L. Optical coherence tomography follow-up of patients treated from periodontal disease. J. Biophotonics. 2019, 12, e201800209. [Google Scholar] [CrossRef]
- Șurlin, P.; Camen, A.; Stratul, S.I.; Roman, A.; Gheorghe, D.N.; Herăscu, E.; Osiac, E.; Rogoveanu, I. Optical coherence tomography assessment of gingival epithelium inflammatory status in periodontal—Systemic affected patients. Ann. Anat. 2018, 219, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D. Identification of venular capillary remodelling: A possible link to the development of periodontitis? J. Periodontal Implant. Sci. 2022, 52, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Chiu, C.H.; Cai, Z.Q.; Lin, J.Y.; Yao, C.Y.; Lyu, D.Y.; Lee, S.Y.; Chen, K.W.; Chen, I.Y. OCT-Based Periodontal Inspection Framework. Sensors 2019, 19, 5496. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Chung, J.H.; Lee, J.S.; Kim, H.J.; Choi, S.H.; Jung, U.W. Comparisons of the diagnostic accuracies of optical coherence tomography, micro-computed tomography, and histology in periodontal disease: An ex vivo study. J. Periodontal Implant. Sci. 2017, 47, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Gruda, Y.; Albrecht, M.; Buckova, M.; Haim, D.; Lauer, G.; Koch, E.; Joehrens, K.; Schnabel, C.; Golde, J.; Li, J.; et al. Characteristics of Clinically Classified Oral Lichen Planus in Optical Coherence Tomography: A Descriptive Case-Series Study. Diagnostics 2023, 13, 2642. [Google Scholar] [CrossRef]
- Fiori, F.; Rullo, R.; Contaldo, M.; Inchingolo, F.; Romano, A. Noninvasive in-vivo imaging of oral mucosa: State-of-the-art. Minerva Dent. Oral. Sci. 2021, 70, 286–293. [Google Scholar] [CrossRef]
- Fomina, I.u.V.; Gladkova, N.D.; Leont’ev, V.K.; Urutina, M.N.; Gazhva, S.I.; Snopova, L.B.; Gelikonov, V.M.; Kamenskiĭ, V.A. Opticheskaia kogerentnaia tomografiia v otsenke sostoianiia slizistoĭ obolochki polosti rta. Soobshchenie II. Dobrokachestvennye i zlokachestvennye zabolevaniia [Optical coherence tomography in the evaluation of the oral cavity mucosa. Part II. Benign and malignant diseases]. Stomatologiia 2004, 83, 25–32. [Google Scholar]
- Tes, D.; Aber, A.; Zafar, M.; Horton, L.; Fotouhi, A.; Xu, Q.; Moiin, A.; Thompson, A.D.; Moraes Pinto Blumetti, T.C.; Daveluy, S.; et al. Granular Cell Tumor Imaging Using Optical Coherence Tomography. Biomed. Eng. Comput. Biol. 2018, 9, 1179597218790250. [Google Scholar] [CrossRef]
- Syomkin, V.A.; Rabinovich, O.F.; Agapitova, L.P.; Bezrukov, A.A.; Babichenko, I.I. Diagnosticheskaia tsennost’ metoda kogerentnoĭ tomografii u bol’nykh s leĭkoplakieĭ slizistoĭ obolochki rta [Diagnostic value of optical coherence tomography for oral leukoplakia assessment]. Stomatologiia 2018, 97, 37–39. [Google Scholar] [CrossRef]
- Chen, P.H.; Lee, H.Y.; Chen, Y.F.; Yeh, Y.C.; Chang, K.W.; Hou, M.C.; Kuo, W.C. Detection of Oral Dysplastic and Early Cancerous Lesions by Polarization-Sensitive Optical Coherence Tomography. Cancers 2020, 12, 2376. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Chen, Y.J.; Chen, Y.F.; Yeh, Y.C.; Chang, K.W.; Hou, M.C.; Kuo, W.C. Quantification of structural and microvascular changes for diagnosing early-stage oral cancer. Biomed. Opt. Express. 2020, 11, 1244–1256. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, L.; Yang, J.; Yin, B.; Fan, X.; Yang, J.; Li, S.; Zhong, J.; Huang, X. Noninvasive oral cancer screening based on local residual adaptation network using optical coherence tomography. Med. Biol. Eng. Comput. 2022, 60, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Ingato, D.; Wilder-Smith, P.; Chen, Z.; Kwon, Y.J. Stimuli-disassembling gold nanoclusters for diagnosis of early stage oral cancer by optical coherence tomography. Nano Converg. 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Classification of oral salivary gland tumors based on texture features in optical coherence tomography images. Lasers Med. Sci. 2022, 37, 1139–1146. [Google Scholar] [CrossRef]
- Keskinruzgar, A.; Ozcan-Kucuk, A.; Acibadem, E.; Yapici-Yavuz, G.; Karadag, A.S.; Simsek, A.; Koparal, M. Evaluation of neurodegenerative and inflammatory processes in temporomandibular joint disorders using optical coherence tomography. J. Stomatol. Oral. Maxillofac. Surg. 2021, 122, 151–155. [Google Scholar] [CrossRef]
- Albelasy, E.H.; Chen, R.; Fok, A.; Montasser, M.; Hamama, H.H.; Mahmoud, S.H.; Abdelrehim, T.; Chew, H.P. Inhibition of Caries around Restoration by Ion-Releasing Restorative Materials: An In Vitro Optical Coherence Tomography and Micro-Computed Tomography Evaluation. Materials 2023, 16, 5558. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, S.M.; Țuculină, M.J.; Osiac, E.; Camen, A.; Sălan, A.I.; Mărășescu, F.I.; Nicola, A.G.; Bechir, E.S.; Dascălu, I.T. Use of optical coherence tomography in orthodontics. Exp. Ther. Med. 2021, 22, 1424. [Google Scholar] [CrossRef] [PubMed]
- Ţogoe, M.M.; Crăciunescu, E.L.; Topală, F.I.; Sinescu, C.; Nica, L.M.; Ioniţă, C.; Duma, V.F.; Romînu, M.; Podoleanu, A.G.; Negruţiu, M.L. Endodontic fillings evaluated using en face OCT, microCT and SEM. Rom. J. Morphol. Embryol. 2021, 62, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.T.; Lee, H.C.; Lee, C.K.; Yu, C.H.; Chen, H.M.; Chiang, C.P.; Chang, C.C.; Wang, Y.M.; Yang, C.C. Effective indicators for diagnosis of oral cancer using optical coherence tomography. Opt. Express. 2008, 16, 15847–15862. [Google Scholar]
- Wilder-Smith, P.; Lee, K.; Guo, S.; Zhang, J.; Osann, K.; Chen, Z.; Messadi, D. In vivo diagnosis of oral dysplasia and malignancy using optical coherence tomography: Preliminary studies in 50 patients. Lasers Surg. Med. 2009, 41, 353–357. [Google Scholar] [CrossRef]
- Lee, C.K.; Chi, T.T.; Wu, C.T.; Tsai, M.T.; Chaing, C.P.; Yang, C.C. Diagnosis of oral precancer with optical coherence tomography. Biomed. Opt. Express. 2012, 3, 1632–1646. [Google Scholar] [CrossRef] [PubMed]
- James, B.L.; Sunny, S.P.; Heidari, A.E.; Ramanjinappa, R.D.; Lam, T.; Tran, A.V.; Kankanala, S.; Sil, S.; Tiwari, V.; Patrick, S.; et al. Validation of a Point-of-Care Optical Coherence Tomography Device with Machine Learning Algorithm for Detection of Oral Potentially Malignant and Malignant Lesions. Cancers 2021, 13, 3583. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Efficacy of optical coherence tomography in the diagnosing of oral cancerous lesion: Systematic review and meta-analysis. J. Sci. Spec. Head Neck 2022, 45, 473–481. [Google Scholar] [CrossRef]
- Ramezani, K.; Tofangchiha, M. Oral Cancer Screening by Artificial Intelligence-Oriented Interpretation of Optical Coherence Tomography Images. Radiol. Res. Pract. 2022, 2022, 1614838. [Google Scholar] [PubMed]



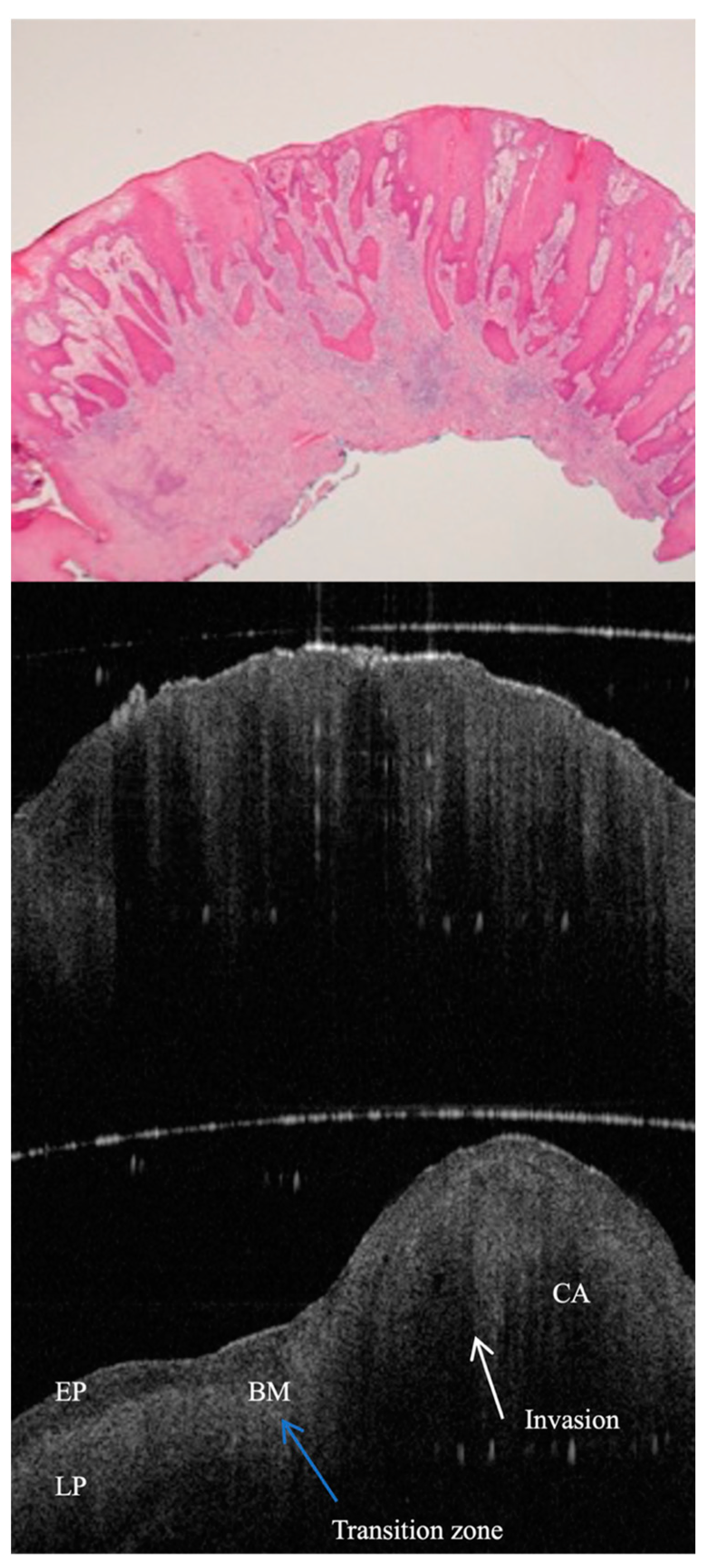

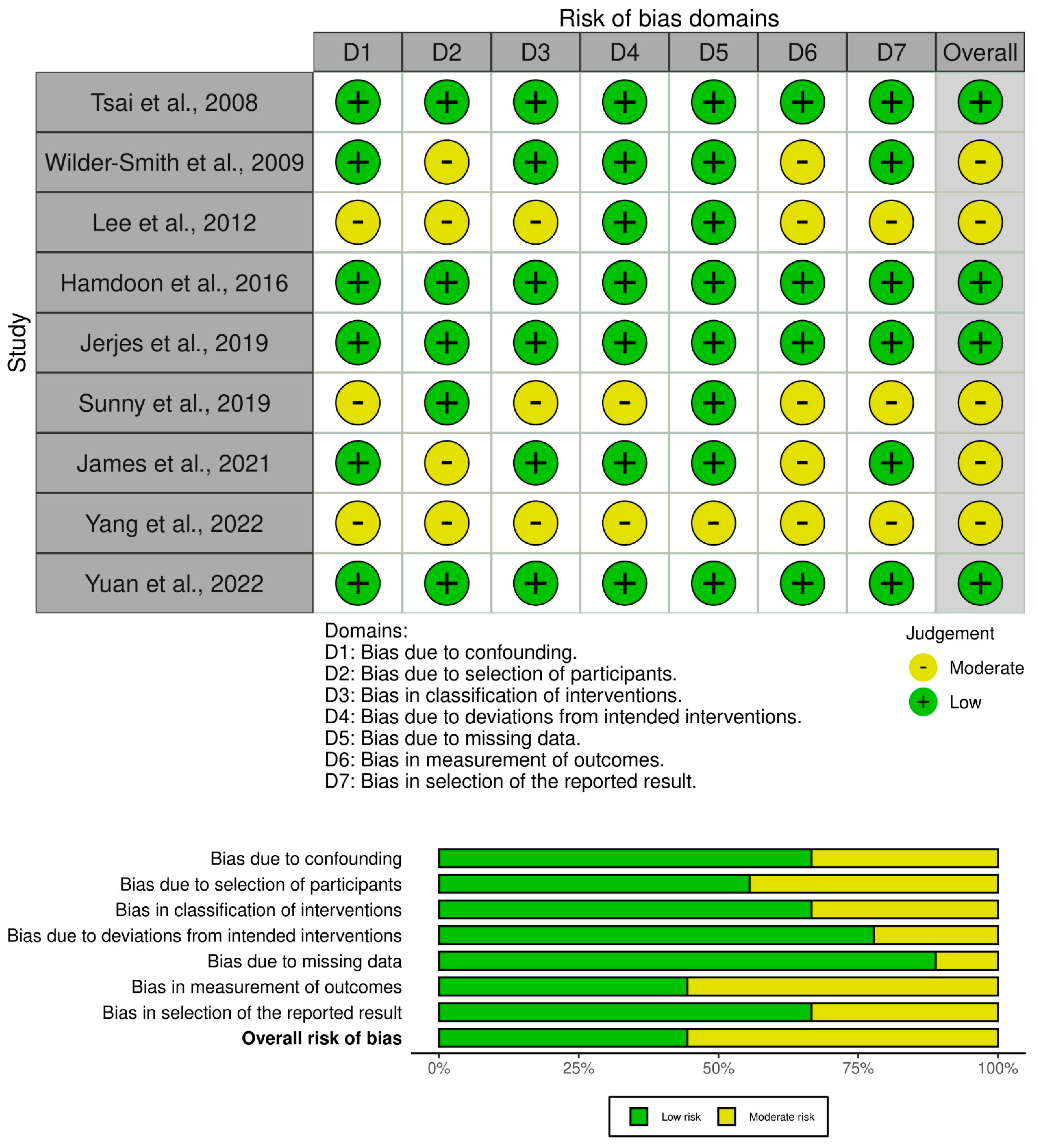
| Study Reference | OCT System and Approach | Key Findings | Clinical Importance |
|---|---|---|---|
| Tsai et al. [59], 2008 | Swept-source OCT | Identified effective diagnostic indicators for oral cancer. | Enhances early detection and diagnosis of oral cancer. |
| Wilder-Smith [60] et al., 2009 | In vivo OCT | Demonstrated high sensitivity and specificity in diagnosing oral dysplasia and malignancy. | Supports non-invasive diagnosis and monitoring of oral pathologies. |
| Lee et al. [61], 2012 | OCT image analysis | Distinguished between normal and precancerous oral mucosae with high diagnostic statistics. | Aids in the differentiation of oral lesions with potential for precancerous development. |
| Hamdoon et al. [5], 2016 | OCT for OSCC resection margins | Showed OCT’s value in assessing OSCC surgical margins. | Improves surgical outcomes by guiding resection margins. |
| Jerjes et al. [21], 2019 | OCT for epithelial thickness | Found epithelial thickness measurement improves OCT’s cancer detection ability. | Enhances accuracy in identifying cancerous changes in oral tissues. |
| Sunny et al. [28], 2019 | Intra-operative OCT | Demonstrated OCT’s high sensitivity and specificity in delineating oral cancer margins. | Facilitates precise surgical margin assessment during oral cancer surgery. |
| James et al. [62], 2021 | Portable OCT with machine learning | Validated a point-of-care OCT device for detecting oral potentially malignant and malignant lesions. | Offers a non-invasive tool for oral cancer screening and surveillance. |
| Yang et al. [19], 2022 | Swept-source OCT with texture analysis | Achieved high accuracy in identifying oral precancerous and cancerous tissues. | Enhances the diagnostic process through detailed tissue characterisation. |
| Yuan et al. [52], 2022 | OCT with deep learning for screening | Proposed a novel deep learning method for non-invasive oral cancer screening. | Streamlines oral cancer diagnosis with high accuracy and non-invasiveness. |
| Study Reference | OCT | Control | Sensitivity | Specificity | Image Interpretation | ||
|---|---|---|---|---|---|---|---|
| Event | Total | Event | Total | ||||
| Tsai et al. [59], 2008 | 21 | 36 | 7 | 43 | 75% | 71% | Clinician |
| Wilder-Smith et al. [60], 2009 | 32 | 33 | 3 | 17 | 91% | 93% | Clinician |
| Lee et al. [61], 2012 | 36 | 45 | 8 | 92 | 82% | 90% | Clinician |
| Hamdoon et al. [5], 2016 | 72 | 84 | 6 | 41 | 92% | 74% | Clinician |
| Jerjes et al. [21], 2019 | 46 | 65 | 5 | 174 | 90% | 90% | Clinician |
| Sunny et al. [28], 2019 | 24 | 24 | 0 | 23 | 100% | 100% | Algorithm-based |
| James et al. [62], 2021 | 91 | 96 | 5 | 21 | 95% | 76% | Algorithm-based |
| Yang et al. [19], 2022 | 416 | 421 | 8 | 525 | 98% | 99% | AI |
| Yuan et al. [52], 2022 | 132 | 141 | 12 | 123 | 92% | 92% | AI |
| Study | Limitations | Future Directions |
|---|---|---|
| Tsai et al. [59], 2008 | Limited by the sample size and the specificity in distinguishing between different types of dysplasia. | Expand sample size and refine diagnostic criteria for varying dysplasia grades. |
| Wilder-Smith et al. [60], 2009 | Study was preliminary and involved a small cohort of patients. | Conduct larger-scale studies to validate findings and improve diagnostic algorithms. |
| Lee et al. [61], 2012 | Focus was primarily on moderate dysplasia, with less emphasis on severe cases. | Include a wider range of dysplasia severity to enhance diagnostic applicability. |
| Hamdoon et al. [5], 2016 | Relied on ex vivo analysis of resection margins, which may not fully replicate in vivo conditions. | Develop techniques for in vivo margin assessment to guide real-time surgical decisions. |
| Jerjes W al. [21], 2019 | The effect of tissue processing on OCT measurements was noted, potentially affecting accuracy. | Investigate methods to account for tissue-processing effects and improve measurement consistency. |
| Sunny et al. [28], 2019 | Focused on a relatively small number of patients and surgical sites. | Increase the number of participants and sites for a more comprehensive evaluation. |
| James et al. [62], 2021 | Utilised machine learning algorithms that require extensive validation across diverse populations. | Test and refine algorithms in varied demographic and clinical settings to ensure generalisability. |
| Yang et al. [19], 2022 | Limited by the study’s reliance on ex vivo tissue samples. | Advance towards real-time, in vivo diagnostic applications to enhance clinical utility. |
| Yuan et al. [52], 2022 | Depended on a novel deep learning method that may require further optimisation. | Continue to refine and test the deep learning framework on larger datasets to validate efficacy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerjes, W.; Stevenson, H.; Ramsay, D.; Hamdoon, Z. Enhancing Oral Cancer Detection: A Systematic Review of the Diagnostic Accuracy and Future Integration of Optical Coherence Tomography with Artificial Intelligence. J. Clin. Med. 2024, 13, 5822. https://doi.org/10.3390/jcm13195822
Jerjes W, Stevenson H, Ramsay D, Hamdoon Z. Enhancing Oral Cancer Detection: A Systematic Review of the Diagnostic Accuracy and Future Integration of Optical Coherence Tomography with Artificial Intelligence. Journal of Clinical Medicine. 2024; 13(19):5822. https://doi.org/10.3390/jcm13195822
Chicago/Turabian StyleJerjes, Waseem, Harvey Stevenson, Daniele Ramsay, and Zaid Hamdoon. 2024. "Enhancing Oral Cancer Detection: A Systematic Review of the Diagnostic Accuracy and Future Integration of Optical Coherence Tomography with Artificial Intelligence" Journal of Clinical Medicine 13, no. 19: 5822. https://doi.org/10.3390/jcm13195822
APA StyleJerjes, W., Stevenson, H., Ramsay, D., & Hamdoon, Z. (2024). Enhancing Oral Cancer Detection: A Systematic Review of the Diagnostic Accuracy and Future Integration of Optical Coherence Tomography with Artificial Intelligence. Journal of Clinical Medicine, 13(19), 5822. https://doi.org/10.3390/jcm13195822






