The Liver Transection Area Is a Novel Predictor for Surgical Difficulty in Laparoscopic Liver Resection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Data
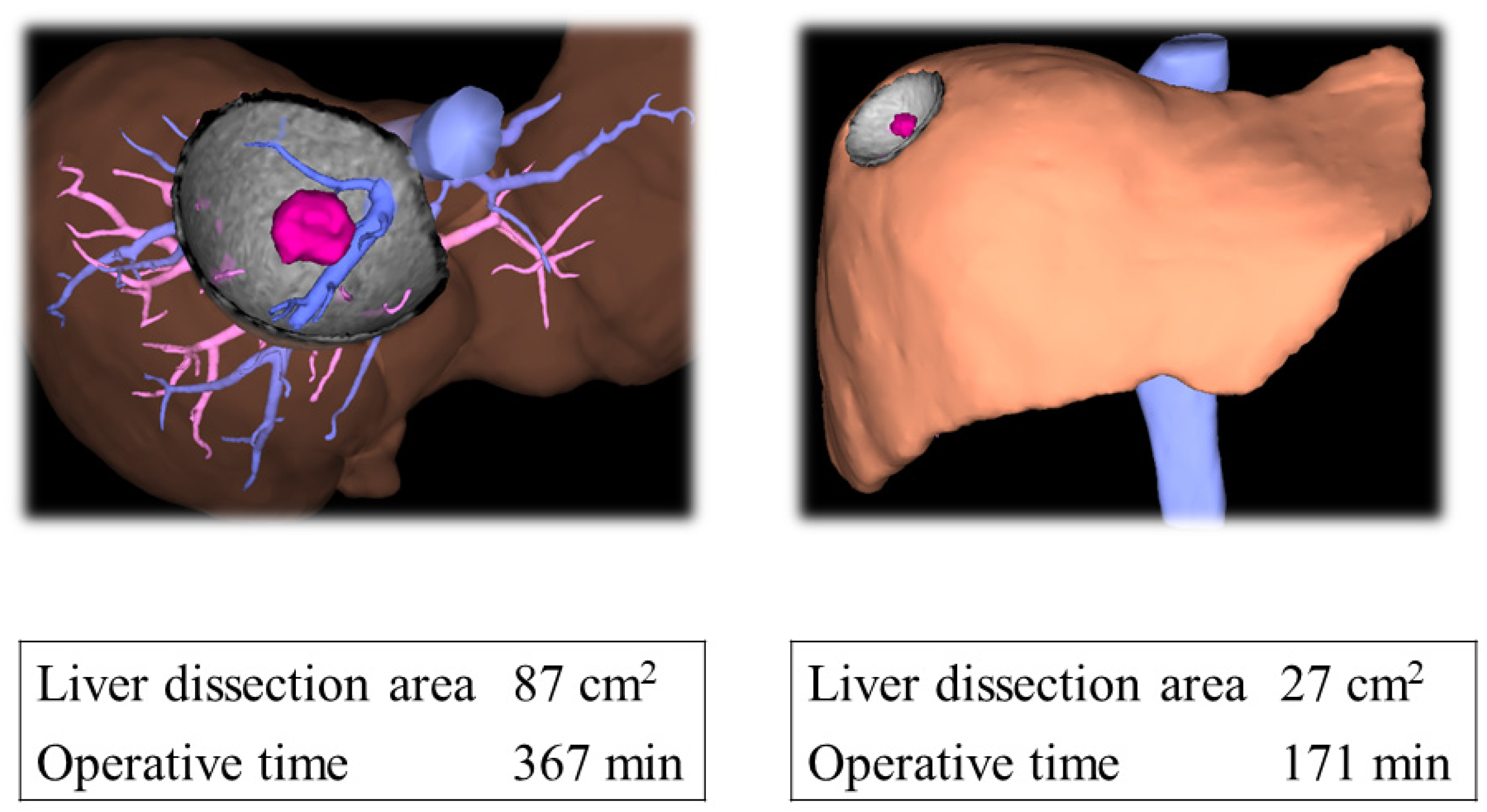
2.3. Liver Transection Area Measurement
2.4. Surgical Technique
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
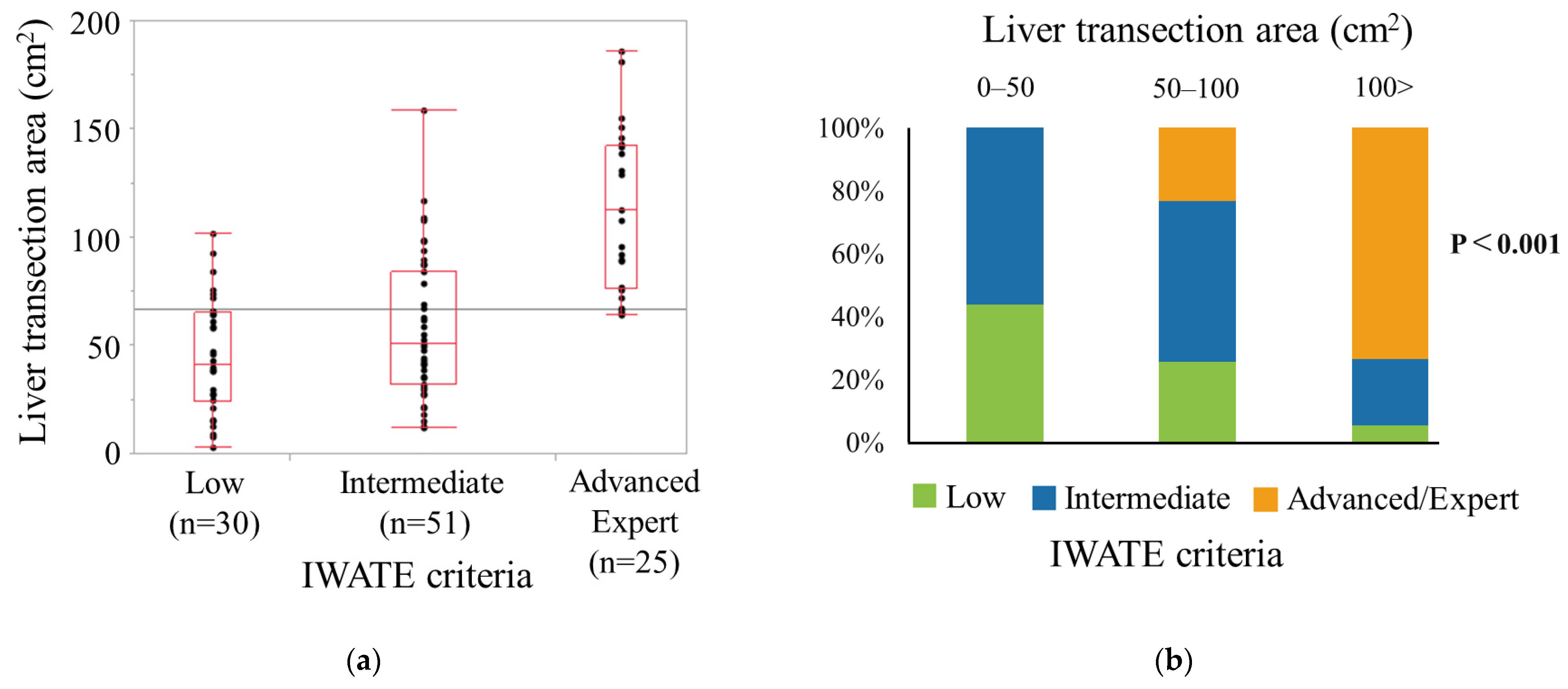
3.2. Association between Liver Transection Area and Difficulty Level
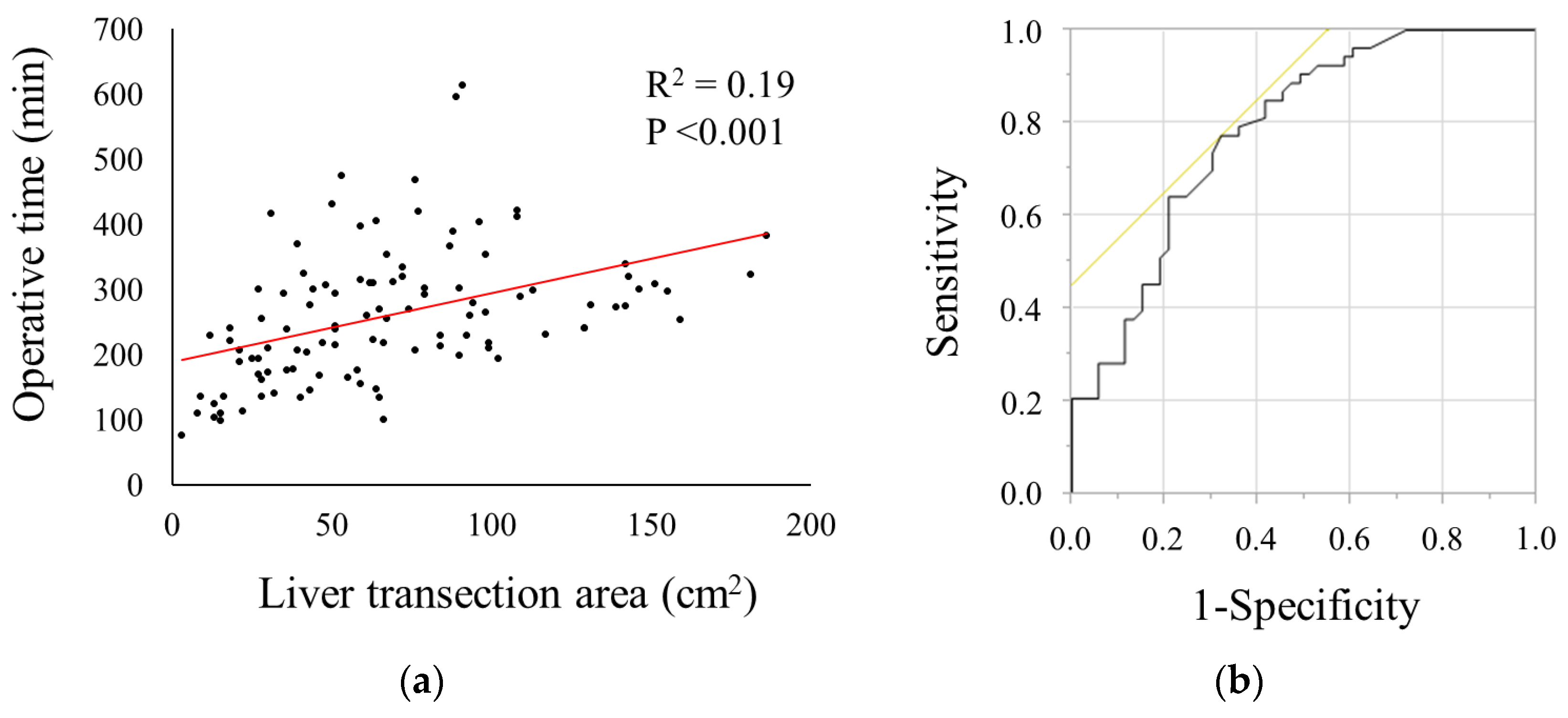
3.3. Association between Liver Transection Area and Operative Time
3.4. Predictive Factor Associated with Prolonged Operative Time
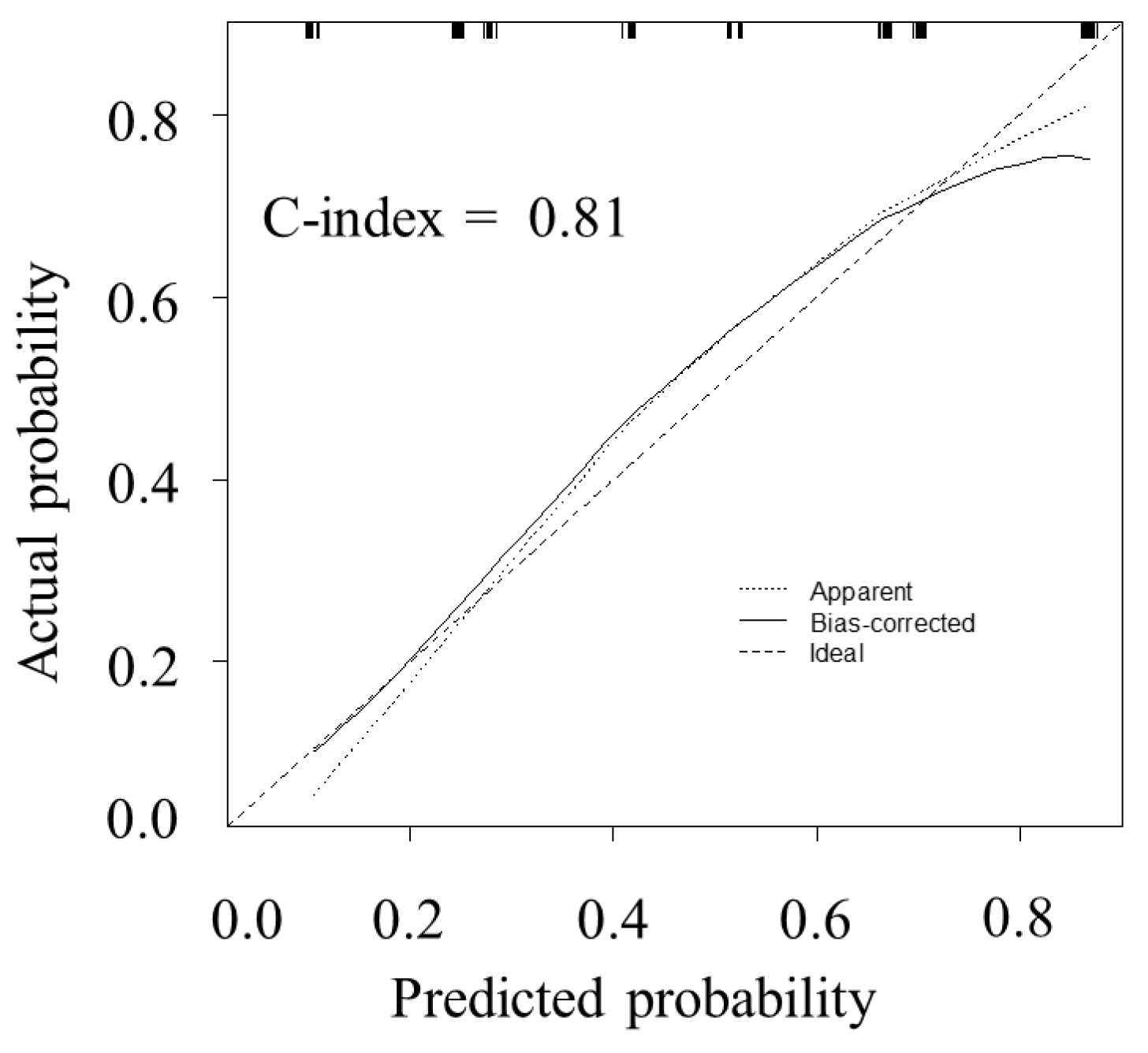
3.5. The Model Performance and Calibration of the Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozair, A.; Collings, A.; Adams, A.M.; Dirks, R.; Kushner, B.S.; Sucandy, I.; Morrell, D.; Abou-Setta, A.M.; Vreeland, T.; Whiteside, J.; et al. Minimally invasive versus open hepatectomy for the resection of colorectal liver metastases: A systematic review and meta-analysis. Surg. Endosc. 2022, 36, 7915–7937. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Gujjuri, R.R.; Hilal, M.A.; Manas, D.M.; White, S.A. Does minimally invasive liver resection improve long-term survival compared to open resection for hepatocellular carcinoma? A systematic review and meta-analysis. Scand. J. Surg. 2022, 111, 14574969211042455. [Google Scholar] [CrossRef] [PubMed]
- Gavriilidis, P.; Roberts, K.J.; Aldrighetti, L.; Sutcliffe, R.P. A comparison between robotic, laparoscopic and open hepatectomy: A systematic review and network meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Kimenai, H.J.A.N.; Terkivatan, T.; Tran, K.T.C.; Ijzermans, J.N.M.; Minnee, R.C. A novel difficulty grading system for laparoscopic living donor nephrectomy. Surg. Endosc. 2021, 35, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Ban, D.; Tanabe, M.; Ito, H.; Otsuka, Y.; Nitta, H.; Abe, Y.; Hasegawa, Y.; Katagiri, T.; Takagi, C.; Itano, O.; et al. A novel difficulty scoring system for laparoscopic liver resection. J. Hepato-Bil. Pancreat. Sci. 2014, 21, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kawaguchi, Y.; Kubo, S.; Kanazawa, A.; Takeda, Y.; Hirokawa, F.; Nitta, H.; Nakajima, T.; Kaizu, T.; Kaibori, M.; et al. Validation of index-based Iwate criteria as an improved difficulty scoring system for laparoscopic liver resection. Surgery 2019, 165, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Linn, Y.L.; Wu, A.G.; Han, H.S.; Liu, R.; Chen, K.H.; Fuks, D.; Soubrane, O.; Cherqui, D.; Geller, D.; Cheung, T.T.; et al. Systematic review and meta-analysis of difficulty scoring systems for laparoscopic and robotic liver resections. J. Hepato-Bil. Pancreat. Sci. 2023, 30, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Onda, S.; Furukawa, K.; Haruki, K.; Yasuda, J.; Shirai, Y.; Hamura, R.; Shiozaki, H.; Gocho, T.; Shiba, H.; Ikegami, T. Proposal for a revised system for classifying difficulty of laparoscopic partial liver resection. Langenbecks Arch. Surg. 2021, 406, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Yagi, T.; Yoshida, R.; Shinoura, S.; Umeda, Y.; Nobuoka, D.; Kuise, T.; Watanabe, N.; Fujiwara, T. Sarcopenia and American society of anesthesiologists physical status in the assessment of outcomes of hepatocellular carcinoma patients undergoing hepatectomy. Acta Med. Okayama 2016, 70, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Umeda, Y.; Kuise, T.; Yoshida, R.; Yoshida, K.; Yasui, K.; Tani, Y.; Yagi, T.; Fujiwara, T. A novel modified hanging maneuver in laparoscopic left hemihepatectomy. Int. J. Surg. Case Rep. 2020, 76, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Efron, B. Bootstrap methods: Another look at the jackknife. Ann. Statist. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Fuks, D.; Kokudo, N.; Gayet, B. Difficulty of laparoscopic liver resection: Proposal for a new classification. Ann. Surg. 2018, 267, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Halls, M.C.; Berardi, G.; Cipriani, F.; Barkhatov, L.; Lainas, P.; Harris, S.; D’Hondt, M.; Rotellar, F.; Dagher, I.; Aldrighetti, L.; et al. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br. J. Surg. 2018, 105, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
| Variable | n = 106 |
|---|---|
| Age, median, years | 68 (59–73) |
| Sex (men/women), n (%) | 63 (59.4)/43 (40.6) |
| BMI, median, kg/m2 | 23.4 (20.7–25.9) |
| ASA (1–2/3), n (%) | 98 (92.5)/8 (7.5) |
| Comorbidity, n (%) | |
| Diabetes | 20 (18.9) |
| Hypertension | 35 (33.0) |
| HBV positive | 32 (30.2) |
| HCV positive | 12 (11.3) |
| Previous abdominal surgery, n (%) | 46 (43.4) |
| Liver function | |
| Albumin, median, g/dL | 4.1 (3.8–4.3) |
| Platelet, median, ×104/μL | 21 (15.8–25.9) |
| Prothrombin time, median, % | 101 (94–110) |
| ICG-R15, median, % | 9.4 (5.9–14.1) |
| Child–Pugh score (A/B), n (%) | 106 (100)/0 (0) |
| Primary disease, n (%) | |
| Hepatocellular carcinoma | 45 (42.5) |
| Intrahepatic cholangiocarcinoma | 5 (4.7) |
| Metastatic tumor | 47 (44.3) |
| Benign tumor | 9 (8.5) |
| Tumor factor | |
| Tumor size, median, mm | 20 (12–40) |
| Tumor number (solitary/multiple), n (%) | 83 (78.3)/23 (21.7) |
| Iwate Difficulty score, n (%) | |
| Low (1–3) | 30 (28.3) |
| Intermediate (4–6) | 51 (48.1) |
| Advanced (7–9) | 18 (17.0) |
| Expert (10–12) | 7 (6.6) |
| VINCENT simulation | |
| Liver transection area, median, cm2 | 62.5 (36.0–91.8) |
| Operative factor | |
| Type of hepatectomy, n (%) | |
| Partial resection | 54 (50.9) |
| Left lateral sectionectomy | 19 (17.9) |
| Segmentectomy | 3 (2.8) |
| Sectionectomy (except lateral sectionectomy) | 18 (17.0) |
| Hemihepatectomy | 12 (11.3) |
| Operative time, median, min | 250 (195–310) |
| Blood loss, median, mL | 65 (10–170) |
| Postoperative factor | |
| Mortality, n (%) | 0 (0) |
| Major complications (CDc ≥ 3), n (%) | 4 (3.8) |
| Hospital stay, median, day | 8 (7–10) |
| Variables | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age (years) | ||||||
| ≥70 (vs. <70) | 0.73 | 0.33–1.59 | 0.43 | |||
| Gender | ||||||
| Male (vs. Female) | 1.48 | 0.68–3.26 | 0.32 | |||
| BMI (kg/m2) | ||||||
| ≥25 (vs. <25) | 1.47 | 0.64–3.45 | 0.36 | |||
| ASA | ||||||
| 3 (vs. 1–2) | 0.58 | 0.11–2.48 | 0.46 | |||
| Previous abdominal surgery | ||||||
| Presence (vs. absence) | 1 | 0.46–2.16 | 1 | |||
| Primary disease | ||||||
| Hepatocellular carcinoma (vs. others) | 3.37 | 1.51–7.78 | 0.003 | 3.05 | 1.17–8.36 | 0.02 |
| Tumor size (cm) | ||||||
| ≥3 (vs. <3) | 2.26 | 1.02–5.14 | 0.04 | 0.95 | 0.30–2.83 | 0.92 |
| Iwate location score | ||||||
| ≥4 (vs. <4) | 2.42 | 1.06–5.77 | 0.04 | 2.25 | 0.80–6.71 | 0.13 |
| Liver transection area (cm2) | ||||||
| ≥59 (vs. <59) | 7.24 | 3.13–17.8 | <0.001 | 6.07 | 2.38–16.6 | <0.001 |
| Type of hepatectomy | ||||||
| ≥Sectionectomy (vs. <Sectionectomy) | 3.82 | 1.73–8.74 | <0.001 | 3.79 | 1.35–11.4 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, M.; Takagi, K.; Fuji, T.; Yasui, K.; Kimura, J.; Nishiyama, T.; Nagai, Y.; Kanehira, N.; Fujiwara, T. The Liver Transection Area Is a Novel Predictor for Surgical Difficulty in Laparoscopic Liver Resection. J. Clin. Med. 2024, 13, 5686. https://doi.org/10.3390/jcm13195686
Yamada M, Takagi K, Fuji T, Yasui K, Kimura J, Nishiyama T, Nagai Y, Kanehira N, Fujiwara T. The Liver Transection Area Is a Novel Predictor for Surgical Difficulty in Laparoscopic Liver Resection. Journal of Clinical Medicine. 2024; 13(19):5686. https://doi.org/10.3390/jcm13195686
Chicago/Turabian StyleYamada, Motohiko, Kosei Takagi, Tomokazu Fuji, Kazuya Yasui, Jiro Kimura, Takeyoshi Nishiyama, Yasuo Nagai, Noriyuki Kanehira, and Toshiyoshi Fujiwara. 2024. "The Liver Transection Area Is a Novel Predictor for Surgical Difficulty in Laparoscopic Liver Resection" Journal of Clinical Medicine 13, no. 19: 5686. https://doi.org/10.3390/jcm13195686
APA StyleYamada, M., Takagi, K., Fuji, T., Yasui, K., Kimura, J., Nishiyama, T., Nagai, Y., Kanehira, N., & Fujiwara, T. (2024). The Liver Transection Area Is a Novel Predictor for Surgical Difficulty in Laparoscopic Liver Resection. Journal of Clinical Medicine, 13(19), 5686. https://doi.org/10.3390/jcm13195686







