Outcomes of Valve-in-Valve (VIV) Transcatheter Aortic Valve Replacement (TAVR) after Surgical Aortic Valve Replacement with Sutureless Surgical Aortic Valve Prostheses Perceval™: A Systematic Review of Published Cases
Abstract
1. Background
2. Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
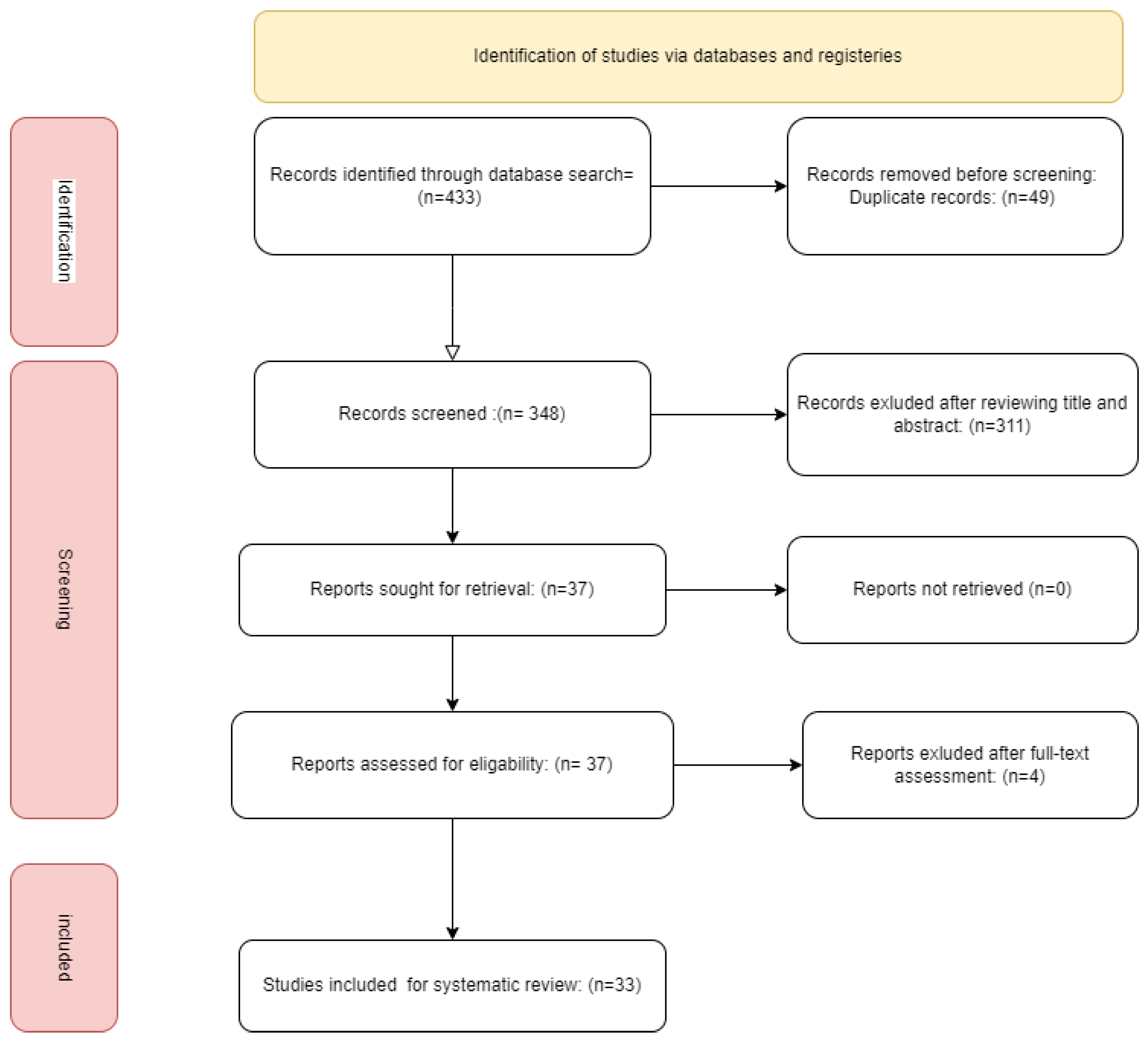
3.1. Database Searching and Screening
3.2. Baseline Characteristics
3.3. Clinical Outcomes
4. Discussion
5. Limitations
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AR | Aortic Regurgitation |
| AV | Atrioventricular |
| AVR | Aortic Valve Replacement |
| CPB | Cardiopulmonary Bypass |
| ESC | European Society of Cardiology |
| PPM | Permanent Pacemaker |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SAVR | Sutureless Aortic Valve Replacement |
| STS | Society of Thoracic Surgeons |
| TAVR | Transcatheter Aortic Valve Replacement |
| THV | Transcatheter Heart Valve |
| VARC-3 | Valve Academic Research Consortium 3 |
| VIV | Valve-in-Valve |
References
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2438–2488. [Google Scholar] [CrossRef]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; Evangelista, A.; et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardio-Thorac. Surg. 2012, 42, S1–S44. [Google Scholar]
- Goodney, P.P.; O’Connor, G.T.; Wennberg, D.E.; Birkmeyer, J.D. Do hospitals with low mortality rates in coronary artery bypass also perform well in valve replacement? Ann. Thorac. Surg. 2003, 76, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; O’Brien, S.M.; Wu, C.; Sikora, J.A.H.; Griffith, B.P.; Gammie, J.S. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2009, 137, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Fischlein, T.; Meuris, B.; Hakim-Meibodi, K.; Misfeld, M.; Carrel, T.; Zembala, M.; Gaggianesi, S.; Madonna, F.; Laborde, F.; Asch, F.; et al. The sutureless aortic valve at 1 year: A large multicenter cohort study. J. Thorac. Cardiovasc. Surg. 2016, 151, 1617–1626.e4. [Google Scholar] [CrossRef] [PubMed]
- Fischlein, T.; Pfeiffer, S.; Pollari, F.; Sirch, J.; Vogt, F.; Santarpino, G. Sutureless Valve Implantation via Mini J-Sternotomy: A Single Center Experience with 2 Years Mean Follow-up. Thorac. Cardiovasc. Surg. 2015, 63, 467–471. [Google Scholar] [CrossRef]
- Shrestha, M.; Fischlein, T.; Meuris, B.; Flameng, W.; Carrel, T.; Madonna, F.; Misfeld, M.; Folliguet, T.; Haverich, A.; Laborde, F. European multicentre experience with the sutureless Perceval valve: Clinical and haemodynamic outcomes up to 5 years in over 700 patients. Eur. J. Cardio-Thorac. Surg. 2016, 49, 234–241. [Google Scholar] [CrossRef]
- Concistré, G.; Baghai, M.; Santarpino, G.; Royse, A.; Scherner, M.; Troise, G.; Glauber, M.; Solinas, M. Clinical and hemodynamic outcomes of the Perceval sutureless aortic valve from a real-world registry. Interdiscip. CardioVasc. Thorac. Surg. 2023, 36, ivad103. [Google Scholar] [CrossRef]
- Santarpino, G.; Pfeiffer, S.; Concistré, G.; Grossmann, I.; Hinzmann, M.; Fischlein, T. The Perceval S aortic valve has the potential of shortening surgical time: Does it also result in improved outcome? Ann. Thorac. Surg. 2013, 96, 77–82. [Google Scholar] [CrossRef]
- Mahmoud, A.N.; Gad, M.M.; Elgendy, I.Y.; Mahmoud, A.A.; Taha, Y.; Elgendy, A.Y.; Ahuja, K.R.; Saad, A.M.; Simonato, M.; McCabe, J.M.; et al. Systematic review and meta-analysis of valve-in-valve transcatheter aortic valve replacement in patients with failed bioprosthetic aortic valves. EuroIntervention 2020, 16, 539–548. [Google Scholar] [CrossRef]
- Kay, R.T.; Linjawi, H.; Butler, C.; Mathew, A.; Muhll, I.V.; Khandekar, S.; Tyrrell, B.D.; Nagendran, J.; Taylor, D.; Welsh, R.C. Transcatheter Aortic Valve Implantation in a Failed Perceval Sutureless Valve, Complicated by Aortic Annular Rupture. CJC Open 2022, 4, 577–580. [Google Scholar] [CrossRef]
- Suleiman, T.; Tanseco, K.; Arunothayaraj, S.; Michail, M.; Cockburn, J.; Hadjivassilev, S.; Hildick-Smith, D. Valve-in-Valve Transcatheter Aortic Valve Implantation for the Failing Surgical Perceval Bioprosthesis. Cardiovasc. Revasculariz. Med. 2022, 40, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Belluschi, I.; Buzzatti, N.; Blasio, A.; Romano, V.; De Bonis, M.; Castiglioni, A.; Montorfano, M.; Alfieri, O. Self-expanding valve-in-valve treatment for failing sutureless aortic bioprosthesis. J. Card. Surg. 2020, 35, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Rayyan. Available online: https://rayyan.ai/ (accessed on 30 June 2024).
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Song, Z.; Soffer, D.; Pirris, J.P. Transcatheter aortic valve-in-valve implantation for early failure of sutureless aortic bioprosthesis. J. Card. Surg. 2018, 33, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, C.; Romano, M.; Camurri, N.; Niglio, T.; Serino, F.; Cionini, F.; Baccaglioni, N.; Buffoli, F.; Rosiello, R.; Rambaldini, M. Impianto transcatetere di valvola aortica in paziente con bioprotesi aortica sutureless degenerata: Descrizione di un caso e revisione della letteratura [Transcatheter valve-in-valve implantation in a patient with a degenerative sutureless aortic bioprosthesis: Case report and literature review]. G. Ital. Cardiol. 2017, 18 (Suppl. S1), 18S–21S. [Google Scholar]
- Mangner, N.; Holzhey, D.; Misfeld, M.; Linke, A. Treatment of a degenerated sutureless Sorin Perceval® valve using an Edwards SAPIEN 3. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 364–366. [Google Scholar] [CrossRef]
- Durand, E.; Tron, C.; Eltchaninoff, H. Emergency Transcatheter Aortic Valve Implantation for Acute and Early Failure of Sutureless Perceval Aortic Valve. Can. J. Cardiol. 2015, 31, 1204.e13–1204.e15. [Google Scholar] [CrossRef]
- Di Eusanio, M.; Saia, F.; Pellicciari, G.; Phan, K.; Ferlito, M.; Dall, G.; Di Bartolomeo, R.; Marzocchi, A. In the era of the valve-in-valve: Is transcatheter aortic valve implantation (TAVI) in sutureless valves feasible? Ann. Cardiothorac. Surg. 2015, 4, 214–217. [Google Scholar]
- Fujita, B.; Kütting, M.; Scholtz, S.; Utzenrath, M.; Hakim-Meibodi, K.; Paluszkiewicz, L.; Schmitz, C.; Börgermann, J.; Gummert, J.; Steinseifer, U.; et al. Development of an algorithm to plan and simulate a new interventional procedure. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 87–95. [Google Scholar] [CrossRef][Green Version]
- Amabile, N.; Zannis, K.; Veugeois, A.; Caussin, C. Early outcome of degenerated self-expanding sutureless aortic prostheses treated with transcatheter valve implantation: A pilot series. J. Thorac. Cardiovasc. Surg. 2016, 152, 1635–1637. [Google Scholar] [CrossRef][Green Version]
- Vondran, M.; Abt, B.; Nef, H.; Rastan, A.J. Allegra Transcatheter Heart Valve inside a Degenerated Sutureless Aortic Bioprosthesis. Thorac. Cardiovasc. Surg. Rep. 2021, 10, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Misfeld, M.; Abdel-Wahab, M.; Thiele, H.; Borger, M.A.; Holzhey, D. A series of four transcatheter aortic valve replacement in failed Perceval valves. Ann. Cardiothorac. Surg. 2020, 9, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Ellouze, M.; Mazine, A.; Carrier, M.; Bouchard, D. Sutureless and Transcatheter Aortic Valve Replacement: When Rivals Become Allies. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 427–430. [Google Scholar] [CrossRef]
- Vilalta, V.; Carrillo, X.; Fernández-Nofrerías, E.; González-Lopera, M.; Mauri, J.; Bayés-Genís, A. Valve-in-valve transcatheter aortic valve implantation for bioprosthetic aortic sutureless valve failure: A case series. Rev. Española Cardiol. (Engl. Ed.) 2021, 74, 269–272. [Google Scholar] [CrossRef]
- Raschpichler, M.C.; Chakravarty, T.; Lange, D.; Makkar, R.R. Balloon-expanding valve-in-valve for a deformed surgical bioprosthesis. Eur. Heart J. 2020, 41, 932. [Google Scholar] [CrossRef]
- Morales-Portano, J.D.; Zaldívar-Fujigaki, J.L.; García-García, J.F.; Madrid-Dour, E.A.; Muratalla-González, R.; Merino-Rajme, J.A. Acute dislocation of a Perceval valve treated with TAVI valve-in-valve: A literature review and case report. CIU Card. Image Updat. 2019, 1, 56–60. [Google Scholar]
- Laricchia, A.; Mangieri, A.; Colombo, A.; Giannini, F. Perceval sutureless valve migration treated by valve-in-valve with a CoreValve Evolut Pro. Catheter. Cardiovasc. Interv. 2020, 96, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, I.; Iakovou, I.; Leontiadis, E.; Sbarouni, E.; Georgiadou, P.; Bousoula, E.; Aravanis, N.; Stratinaki, M.; Voudris, V.; Mpalanika, M. The first transcatheter valve-in-valve implantation of a self-expanding valve for the treatment of a degenerated sutureless aortic bioprosthesis. Hell. J. Cardiol. 2020, 61, 49–50. [Google Scholar] [CrossRef]
- Koni, E.; Trianni, G.; Ravani, M.; Gasbarri, T.; Al Jabri, A.; Chiappino, D.; Berti, S. Bailout Balloon Predilatation and Buddy Wire Technique for Crossing a Degenerated Sutureless Perceval Bioprosthesis with SAPIEN 3 Ultra Device in a Transcatheter Valve-in-Valve Intervention. Cardiovasc. Revascularization Med. 2019, 20, 75–78. [Google Scholar] [CrossRef]
- Balghith, M.A. Degenerated Suturless Perceval with (Paravalvular Leak and AS) Treated by Valve in Valve using S3 Edward Valve. Heart Views 2019, 20, 166–169. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, J.; García-Puente, J.; Mateo-Martínez, A.; Pinar-Bermúdez, E.; Gutiérrez-García, F.; Valdés-Chávarri, M. Percutaneous Treatment of Early Denegeration of a Sutureless Bioprosthesis. Rev. Española Cardiol. (Engl. Ed.) 2018, 71, 398–399. [Google Scholar] [CrossRef]
- Oezpeker, U.C.; Feuchtner, G.; Bonaros, N. Cusp thrombosis of a self-expanding sutureless aortic valve treated by valve-in-valve transcatheter aortic valve implantation procedure: Case report. Eur. Heart J. Case Rep. 2018, 2, yty117. [Google Scholar] [PubMed]
- Tomai, F.; Weltert, L.; de Persio, G.; Salatino, T.; de Paulis, R. The matryoshka procedure. J. Card. Surg. 2021, 36, 3381–3383. [Google Scholar] [CrossRef] [PubMed]
- López-Tejero, S.; Núñez-García, J.C.; Antúnez-Muiños, P.; González-Calle, D.; Martín-Moreiras, J.; Diego-Nieto, A.; Rodríguez-Collado, J.; Herrero-Garibi, J.; Sánchez-Fernández, P.L.; Cruz-González, I. TAVR to Solve Perceval Sutureless Valve Migration. JACC Cardiovasc. Interv. 2022, 15, e65–e67. [Google Scholar] [CrossRef]
- Patterson, T.; Rajani, R.; Esposito, G.; Allen, C.; Adams, H.; Prendergast, B.; Young, C.; Redwood, S. Transcatheter Aortic Valve-in-Valve Implantation Complicated by Aorto-Right Ventricular Fistula. JACC Case Rep. 2020, 2, 309–311. [Google Scholar] [CrossRef]
- Erdoğan, M.; Kasapkara, A.; Öztürk, S.; Çöteli, C.; Baştuğ, S.; Bayram, N.A.; Akçay, M.; Durmaz, T.; Turkey, A. First experience in Turkey with Meril’s MyValTM transcatheter aortic valve-in valve replacement for degenerated PERCEVALTM bioprothesis valve. Anatol. J. Cardiol. 2022, 26, 143–146. [Google Scholar] [CrossRef]
- Arslan, Ş.; Bayar, N.; Erkal, Z.; Köklü, E.; Çağırcı, G. Transcatheter valve-in-valve implantation for sutureless bioprosthetic aortic paravalvular leak in the era of COVID-19. Anatol. J. Cardiol. 2021, 25, 209–211. [Google Scholar] [CrossRef]
- Kalra, A.; Reyes, M.; Yang, E.; Little, S.H.; Nabi, F.; Barker, C.M.; Ramchandani, M.; Reul, R.M.; Reardon, M.J.; Kleiman, N.S. Transcatheter Aortic Valve Replacement for Perceval Sutureless Aortic Valve Failure. J. Invasive Cardiol. 2017, 29, E65–E66. [Google Scholar] [PubMed]
- Landes, U.; Sagie, A.; Kornowski, R. Transcatheter aortic valve implantation in degenerative sutureless perceval aortic bioprosthesis. Catheter. Cardiovasc. Interv. 2018, 91, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Loforte, A.; Comentale, G.; Coppola, G.; Amodio, C.; Botta, L.; Saia, F.; Taglieri, N.; Marrozzini, C.; Savini, C.; Pacini, D. A rescue transcatheter solution for early sutureless basal ring infolding. J. Card. Surg. 2022, 37, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Medda, M.; Casilli, F.; Tespili, M.; Bande, M. The “Chaperone Technique”: Valve-in-Valve TAVR Procedure with a Self-Expandable Valve Inside a Degenerated Sutureless Prosthesis. JACC Cardiovasc. Interv. 2020, 13, e15–e18. [Google Scholar] [CrossRef] [PubMed]
- Dubios, C.; Minten, L.; Lamberigts, M.; Lesizza, P.; Jacobs, S.; Adriaenssens, T.; Verbrugghe, P.; Meuris, B. Valve-in-valve transcatheter aortic valve replacement for the degenerated rapid deployment Perceval prosthesis: Technical considerations. J. Vis. Surg. 2023, 9, 46. [Google Scholar] [CrossRef]
- Pibarot, P.; Herrmann, H.C.; Wu, C.; Hahn, R.T.; Otto, C.M.; Abbas, A.E.; Chambers, J.; Dweck, M.R.; Leipsic, J.A.; Simonato, M.; et al. Standardized Definitions for Bioprosthetic Valve Dysfunction Following Aortic or Mitral Valve Replacement. J. Am. Coll. Cardiol. 2022, 80, 545–561. [Google Scholar] [CrossRef]
- 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 14, 561–632.
- Meuris, B.; Flameng, W.J.; Laborde, F.; Folliguet, T.A.; Haverich, A.; Shrestha, M. Five-year results of the pilot trial of a sutureless valve. J. Thorac. Cardiovasc. Surg. 2015, 150, 84–88. [Google Scholar] [CrossRef]
- Concistré, G.; Gasbarri, T.; Ravani, M.; Al Jabri, A.; Trianni, G.; Bianchi, G.; Margaryan, R.; Chiaramonti, F.; Murzi, M.; Kallushi, E.; et al. Transcatheter Aortic Valve Replacement in Degenerated Perceval Bioprosthesis: Clinical and Technical Aspects in 32 Cases. J. Clin. Med. 2023, 12, 6265. [Google Scholar] [CrossRef]
- Ochi, A.; Cheng, K.; Zhao, B.; Hardikar, A.A.; Negishi, K. Patient Risk Factors for Bioprosthetic Aortic Valve Degeneration: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2020, 29, 668–678. [Google Scholar] [CrossRef]
- Cerillo, A.G.; Amoretti, F.; Mariani, M.; Cigala, E.; Murzi, M.; Gasbarri, T.; Solinas, M.; Chiappino, D. Increased Gradients After Aortic Valve Replacement with the Perceval Valve: The Role of Oversizing. Ann. Thorac. Surg. 2018, 106, 121–128. [Google Scholar] [CrossRef]
- Dvir, D.; Leipsic, J.; Blanke, P.; Ribeiro, H.B.; Kornowski, R.; Pichard, A.; Rodés-Cabau, J.; Wood, D.A.; Stub, D.; Ben-Dor, I.; et al. Coronary Obstruction in Transcatheter Aortic Valve-in-Valve Implantation. Circ. Cardiovasc. Interv. 2015, 8, e002079. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.; Zenses, A.-S.; Bernard, J.; Tastet, L.; Côté, N.; Guimarães, L.d.F.C.; Paradis, J.-M.; Beaudoin, J.; O’connor, K.; Bernier, M.; et al. Hemodynamic and Clinical Outcomes in Redo-Surgical Aortic Valve Replacement vs. Transcatheter Valve-in-Valve. Struct. Heart 2022, 6, 100106. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Mean | Standard Deviation (SD) | |
|---|---|---|---|
| Age | 76 | 4.4 | |
| STS score % | 6.1 | 4.3 | |
| Months after Perceval implant | 9.8 | 8.9 | |
| TAVI size (mm) | 24.7 | 1.9 | |
| Number | % | ||
| Gender | Males | 18 | 31.5% |
| Female | 39 | 68.4% | |
| Perceval size | Small | 18 | 31.5% |
| Medium | 23 | 40.3% | |
| Large | 13 | 22.8% | |
| X-large | 3 | 5.2% | |
| Reason for ViV | Stenosis | 18 | 31.57% |
| Regurgitation | 13 | 22.8% | |
| Steno-insufficiency | 26 | 45.6% | |
| Cause | Degeneration | 36 | 63.1% |
| Stent folding | 13 | 22.8% | |
| Migration | 3 | 5.2% | |
| Cusp thrombosis | 2 | 3.5% | |
| Endocarditis | 1 | 1.7% | |
| TAVI implanted | Balloon-expanding | 35 | 61.4% |
| Self-expanding | 22 | 38.6% | |
| TAVI model | Sapien | 33 | 57.8% |
| Evolut/Core | 19 | 33.3% | |
| Myval | 2 | 3.5% | |
| Others (Accurate, Portico, Allegra) | 3 | 5.2% | |
| Characteristic | Mean | Standard Deviation | |
|---|---|---|---|
| Follow-up (months) | 9.8 | 8.9 | |
| Mean gradients at discharge (mmHg) | 15 | 5.9 | |
| Mean gradients at follow-up (mmHg) | 13 | 4.2 | |
| Number | Percentage | ||
| Status at follow-up, n = 16 | Asymptomatic or improved symptoms | 16 | 100% |
| With symptoms | 0 | 0% | |
| Complications | Yes | 9 | 15.7% |
| No | 38 | 84.3% | |
| Coronary obstruction, n = 45 | Yes | 0 | 0% |
| No | 57 | 100% | |
| N | Author | Year | Age (yrs) | G | STS % | P Size | Days after P Implant | Reason for ViV | Cause | TAVI Model | TAVI Size (mm) | mPG | FU (m) | mPG FU (mmHg) | Comp. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sun | 2018 | 71 | M | 16.4 | S | 21 | steno-insufficiency | Stent folding | Edwards SAPIEN 3 | 26 | 9.7 | 6 | 9.1 | no |
| 2 | Lettieri | 2017 | 84 | F | M | 1825 | steno-insufficiency | Degeneration and stent folding | Corevalve Evolute R | 26 | no | ||||
| 3 | Mangner | 2018 | 75 | F | 8.1 | S | 1825 | steno-insufficiency | Degeneration, regurgitation, and stenosis | Edwards SAPIEN 3 | 23 | 21 | no | ||
| 5 | Amabile | 2015 | 78 | F | S | 1095 | regurgitation | Failure | Edwards SAPIEN 3 | 23 | 1 | no | |||
| 6 | Di Eusanio | 2015 | 80 | F | M | 5 | regurgitation | Stent folding | Edwards SAPIEN XT | 23 | no | ||||
| 7 | Karla | 2018 | 75 | F | M | 21 | steno-insufficiency | Stent folding | Edwards SAPIEN 3 | 20 | no | ||||
| 9 | Landes | 2018 | 80 | F | S | 730 | steno-insufficiency | Stent folding | Edwards SAPIEN XT | 23 | 10 | 6 | no | ||
| 10 | Fujita | 2015 | 77 | F | 4.2 | S | 1095 | steno-insufficiency | Degeneration | Edwards SAPIEN XT | 23 | 8 | 2 | no | |
| 11 | Amabile | 2016 | 83.2 | F | 9.6 | M | 1144.45 | steno-insufficiency | Degeneration | Evolut R | 26 | 23 | 3 | 10 | no |
| 11 | Amabile | 2016 | 82.9 | F | 7.2 | M | 2425.87 | stenosis | Degeneration | Evolut R | 26 | 33 | 3 | 20 | no |
| 11 | Amabile | 2016 | 83.8 | F | 6 | M | 2212.81 | steno-insufficiency | Degeneration | Evolut R | 26 | 16 | 3 | 8 | no |
| 11 | Amabile | 2016 | 80.9 | M | 4.7 | L | 1999.74 | stenosis | Degeneration | Evolut R | 26 | 16 | 3 | 19 | no |
| 11 | Amabile | 2016 | 72.4 | F | 16.3 | M | 1905.39 | steno-insufficiency | Degeneration | Corevalve | 23 | 32 | 3 | 15 | no |
| 12 | Belluschi | 2021 | 67 | M | L | 2920 | regurgitation | Failure | Myval | 26 | 10 | 1 | 10 | no | |
| 13 | Vondran | 2021 | 85 | F | 11.9 | M | 1095 | steno-insufficiency | Degeneration | Allegra | 23 | 9 | no | ||
| 14 | Misfeld | 2020 | 77 | F | S | 2920 | stenosis | Degeneration | Sapien 3 | 20 | 24 | 12 | 13 | no | |
| 14 | Misfeld | 2020 | 82 | F | L | 2190 | stenosis | Degeneration | Evolut R | 29 | 11 | 3 | 6 | no | |
| 14 | Misfeld | 2020 | 76 | F | S | 2190 | steno-insufficiency | Degeneration | Sapien 3 | 23 | 23 | 3 | 17 | bleeding into the right groin | |
| 14 | Misfeld | 2020 | 85 | F | S | 1460 | stenosis | Degeneration | Sapien 3 | 20 | 3 | 17 | no | ||
| 15 | Ellouze | 2020 | 79 | F | M | 1095 | steno-insufficiency | Degeneration | CoreValve | 26 | 9 | 8 | no | ||
| 15 | Ellouze | 2020 | 84 | F | S | 1460 | steno-insufficiency | Degeneration | Sapien | 23 | 7 | 6 | no | ||
| 15 | Ellouze | 2020 | 86 | M | L | 1461 | stenosis | Degeneration | Sapien | 26 | 10 | 1 | AV block with PPI, 8 days post-TAVR | ||
| 17 | Vilalta | 2020 | S | 350 | stenosis | Stent folding | Evolut PRO | 23 | 27 | 6 | 15 | AV block III | |||
| 17 | Vilalta | 2020 | S | 1139 | steno-insufficiency | Stent folding | Acurate Neo | 23 | 19 | 6 | 17 | AV block III | |||
| 17 | Vilalta | 2020 | S | 1249 | steno-insufficiency | Degeneration | Sapien 3 | 23 | 16 | 6 | 10 | no | |||
| 17 | Vilalta | 2020 | M | 1811 | steno-insufficiency | Degeneration | Sapien 3 | 23 | 10 | 6 | 7 | no | |||
| 17 | Vilalta | 2020 | XL | 978 | regurgitation | Failure | Sapien 3 | 26 | 9 | 6 | 7 | no | |||
| 18 | Raschpichler | 2019 | 81 | F | M | 213 | regurgitation | Stent folding | Sapien 3 | 23 | 10 | 6 | no | ||
| 19 | Morales-Portano | 2019 | 76 | M | 4 | M | 1 | regurgitation | Dislocation | Corevalve | 29 | 10 | no | ||
| 20 | Laricchia | 2019 | 73 | M | M | 0.25 | regurgitation | Partial migration | Evolut PRO | 29 | 1 | no | |||
| 21 | Kosmas | 2019 | 71 | F | S | 2191 | steno-insufficiency | Degeneration | Evolut PRO | 23 | 35.8 | no | |||
| 22 | Koni | 2019 | 88 | F | L | 2555 | steno-insufficiency | Degeneration | Sapien 3 Ultra | 26 | 16 | no | |||
| 23 | Belluschi | 2019 | 74 | M | 2 | XL | 365 | steno-insufficiency | Stent deformation | Evolut R | 29 | 12 | no | ||
| 24 | Balghith | 2019 | 70 | F | M | 1825 | steno-insufficiency | Degeneration | Sapien 3 | 26 | 15 | no | |||
| 25 | Garcia-Lara | 2018 | 79 | F | 4.3 | S | 1826 | stenosis | Degeneration | Sapien 3 | 23 | 10 | 2 | AV block III | |
| 26 | Oezpeker | 2018 | 77 | F | 10.01 | M | 304 | cusps thrombosis | Cusp thrombosis | Sapien 3 | 23 | 10 | 3 | 10 | no |
| 27 | Suleiman | 2021 | 78 | F | M | 1917 | regurgitation | Degeneration | Sapien 3 | 23 | 13,2 | Vasc. access pouch stented | |||
| 27 | Suleiman | 2021 | 72 | F | S | 1339 | regurgitation | Degeneration | CoreValve | 23 | 9 | 10 | 9 | no | |
| 27 | Suleiman | 2021 | 73 | F | M | 1369 | stenosis | Degeneration | CoreValve | 23 | 30 | 3 | no | ||
| 27 | Suleiman | 2021 | 66 | F | L | 2130 | stenosis | Degeneration | CoreValve | 26 | 7 | 4 | 7 | no | |
| 28 | Tomai | 2021 | 73 | M | M | 240 | steno-insufficiency | Endocarditis leading to degeneration | Sapien 3 | 23 mm | 16 | no | |||
| 29 | Medda | 2020 | 84 | F | 17,4 | L | 2190 | steno-insufficiency | Malfunction | Abbott Portico | 25 | no | |||
| 30 | Loforte | 2022 | 83 | F | M | 3 | steno-insufficiency | Stent folding | Sapien | 23 | 48 | no | |||
| 31 | López-Tejero | 2022 | 67 | F | 1,02 | S | 0.25 | regurgitation and occlusion ofthe left main coronary artery | Migration | Evolut Pro | 26 | death | |||
| 32 | Kay Robert T. | 2022 | 75 | F | M | 1095 | stenosis | Stent folding | SAPIEN 3 | 23 | annular rupture and death | ||||
| 33 | Patterson T. | 2020 | 81 | F | S | 730 | regurgitation | Stent folding | SAPIEN 3 | 23 | NA | annular rupture | |||
| 34 | Erdogan | 2022 | 70 | F | 4,5 | L | 1826 | stenosis | Degeneration | Myval | 26 | 7 | no | ||
| 35 | Arslan | 2021 | 70 | M | M | 93 | regurgitation | Stent folding and paravalvular failure jet | SAPIEN 3 | 23 | 11 | no | |||
| 36 | Dubois | 2023 | 74 | F | L | 1825 | regurgitation | Degeneration | sapien 3 | 26 | 13 | no | |||
| 36 | Dubois | 2023 | 83 | F | L | 2217 | stenosis | Degeneration | Sapien 3 | 23 | 18 | no | |||
| 36 | Dubois | 2023 | 75 | f | L | 1715 | stenosis | Degeneration | Sapien 3 | 23 | 17 | no | |||
| 36 | Dubois | 2023 | 82 | M | M | 1314 | stenosis | Degeneration | Sapien 3 | 23 | 12 | no | |||
| 36 | Dubois | 2023 | 76 | M | L | 2774 | stenosis | Degeneration | Sapien | 26 | 14 | no | |||
| 36 | Dubois | 2023 | 80 | F | L | 2372 | stenoso-insufficency | Degeneration | Evolut R | 26 | 11 | no | |||
| 36 | Dubois | 2023 | 78 | M | M | 1788 | steno-insufficiency | Degeneration | Sapien 3 | 23 | 28 | no | |||
| 36 | Dubois | 2023 | 79 | F | S | 1643 | steno-insufficiency | Degeneration | sapien 3 | 23 | 8 | no | |||
| 36 | Dubois | 2023 | 82 | M | XL | 2226 | stenosis | Degeneration | Evolut R | 29 | 17 | no |
| Study ID | Outcomes |
|---|---|
| Kay et al., 2022 [11]. | TTE on the same day showed a well-seated SAPIEN valve, with normal gradients and no regurgitation. However, a significant compression of the right ventricular outflow tract from an extrinsic source was seen, which was not evident on previous imaging. An emergent gated CT angiogram of the chest revealed an aortic annular rupture inferior to the origin of the left coronary artery with extravasation of contrast and a large hematoma compressing the right ventricular outflow tract. Upon review of previous imaging, the rupture site appeared to correspond to the location of the previous infolded portion of the Perceval valve. The patient was stable through the day, with sudden deterioration in the early morning, when she passed away. |
| Suleiman et al., 2022 [12] | The patient was discharged successfully two days after the procedure and is clinically much improved, and no gradient/regurgitation. |
| Suleiman et al., 2022 [12] | The patient tolerated the procedure well with no gradient or regurgitant jet apparent across the Perceval CoreValve combination. Owing to the acuity and complexity of the patient’s condition, their total hospital stay was 25 days. However, she was discharged home 2 days following ViV-TAVI and was asymptomatic from a cardiac standpoint when seen in clinic 6 months post-procedure. An echocardiogram 10 months post-procedure showed a well-seated valve with no regurgitation apparent and a MG of 9 mmHg. |
| Suleiman et al., 2022 [12] | From an aortic valve standpoint, the Perceval-CoreValve apparatus remained well seated on echocardiogram 3 months post-procedure with no regurgitant jet. There was a higher than expected peak velocity of 3.7 m·s−1 without apparent valve leaflet dysfunction and this has been attributed to effective patient prosthesis mismatch, anemia, and associated hyperdynamic circulation. |
| Suleiman et al., 2022 [12] | Good hemodynamic effect was demonstrated with minimal gradient across the valve. The patient had an uneventful recovery and was discharged day 1 post-procedure. Echocardiography 4 months post-procedure showed an MG of 7 mmHg and no para-valvular leak. |
| Belluschi, et al., 2021 [13] | Excellent angiographic results, with mild AR. No complications. After uneventful in-hospital stay, the patient was discharged 4 days later, maintaining similar echo parameters at 30-day follow-up. |
| Sun et al., 2018 [18] | No complications, excellent angiographic results, proper valve-in-valve function with no significant gradients or regurgitation, and uncomplicated postoperative course. Patient remained asymptomatic at 6 months. |
| Lettieri et al., 2017 [19] | No complications. The pre-discharge multidetector CT showed the correct positioning of the distal margin of the Evolut R approximately 2 mm above the distal ring of the sutureless valve, with minimal compression of the transcatheter valve at that level, but with circular shape of the perimeter in correspondence to the Evolotu R leaflets. The patient was discharged and at 90 days, the postoperative course was uneventful. |
| Mangner et al., 2018 [20] | No complications. After ViV there was an immediate improvement of the regurgitation and of the stenosis. Echo predischarge showed no regurgitation, a mean gradient of 21 mmHg and an aortic valve area of 1.3 cm2, indicating a moderate patient prosthesis mismatch already existing directly after first operation. |
| Durand et al., 2015 [21] | No complications. A final supra-aortic angiogram showed no residual aortic regurgitation and hemodynamic status improved rapidly. The clinical status of the patient improved dramatically with rapid normalization of liver and renal function tests within 72 h. Clinical course was uneventful and the patient was discharged home 5 days later. At 30-day follow-up, the patient was asymptomatic with return to normal life. |
| Di Eusanio, et al., 2015 [22] | No complications. The TAVI was successfully deployed with excellent angiographic results. The post-procedural course was uneventful; renal function improved and TTE at discharge showed no significant leaks and gradients across the TAVI implanted. |
| Fujita et al., 2015 [23] | No complications occurred. |
| Amabile et al., 2016 [24] | The success rate was 100%. There was no device migration, neither death or periprocedural adverse events, and no need for a second device in any patient. A mild post-procedural aortic regurgitation was noted in 2 patients. The 30-day post-procedural clinical course was uneventful in all subjects. Mean transaortic gradient significantly decreased over time: median mean transprosthetic gradient was 45 ± 26 mmHg before procedure, 24 ± 16 mmHg immediately after, and 14 ± 9 Hg mm 30 days after TAVI (p < 0.05 vs. baseline for D0 and D30, p < 0.05 for D30 vs. D0, t Student paired test). Comparable results were observed at 90 days. |
| Vondran et al., 2021 [25] | No postdilatation was needed in view of the excellent hemodynamic results with an invasive transvalvular gradient of 5 mmHg. Based on the Valve Academic Research Consortium II criteria, no major adverse event occurred during the hospital stay. The patient was discharged 9 days after the procedure to a rehabilitation center with a maximum/mean transvalvular pressure gradient measured by transthoracic echocardiography of 19/9 mm Hg and no apparent paravalvular leakage. |
| Misfeld et al., 2020 [26] | The patient left the hybrid operation room in a hemodynamically stable condition. Postoperative transthoracic echocardiography (TTE) demonstrated normal LV function and a mean gradient across the valve of 24 mmHg. Effective orifice area was calculated at 1.0 cm2. There was only trivial paravalvular leak. The patient was discharged on postoperative day two. At 12-month follow-up, the patient showed improvement in clinical symptoms (NYHA I–II). Pressure gradients had further decreased (mean gradient 13 mmHg) and the effective orifice area was measured at 1.2 cm2. There was only trivial paravalvular leakage. |
| Misfeld et al., 2020 [26] | The postoperative course was uneventful. On TTE, LV function had slightly improved (LV ejection fraction 47%). Mean pressure gradient was 11 mmHg and effective orifice area was calculated at 1.8 cm2. There was mild residual aortic regurgitation. The patient was discharged on postoperative day three. At 3-month follow-up, clinical symptoms had improved (NYHA I–II°). TTE showed an LV ejection fraction of 48% and a low mean pressure gradient of max 6 mmHg with an effective orifice area of 1.9 cm2. There was still mild aortic regurgitation. |
| Misfeld et al., 2020 [26] | Analysis of the cerebral protection system revealed large debris in both filters. The early course was complicated by bleeding into the right groin, which was treated conservatively. On TTE, LV ejection fraction was 47%, mean gradient was slightly increased (23 mmHg), and the effective orifice area was 1.1 cm2. There was only trivial residual aortic regurgitation. The patient was discharged on POD five. At 3-month follow-up, this patient had also clinically improved (NYHA I–II). LV function also improved (LV ejection fraction 57%) and mean gradient was 17 mmHg with an effective orifice area of 1.5 cm2. There was only trivial residual aortic regurgitation. |
| Misfeld et al., 2020 [26] | Analysis of the cerebral protection system revealed debris in both filters. The early course was uncomplicated and the patient was discharged on postoperative day 3. At 3-month follow-up, this patient had also clinically improved, but still had dyspnea (NYHA II–III). LV function was normal and mean gradient across the aortic valve prosthesis was 17 mmHg with an effective orifice area of 1.2 cm2. There was no residual aortic regurgitation. During the last admission, an Amplatzer Vascular Plug III (St. Jude Medical Inc., MN, USA) was implanted to close the residual gap in the LAA. |
| Ellouze et al., 2020 [27] | (a) ViV transcatheter aortic valve implantation in sutureless valves was feasible and safe; (b) challenging cases such as small degenerated valves were successfully treated with self-expanding valves and acceptable gradients, (c) the rate of procedural complications was low and good in-hospital and mid-term outcomes were acheived with different types of transcatheter aortic valves. |
| Vilalta et al., 2020 [28] | NR |
| Raschpichler et al., 2019 [29] | At 6-month follow-up, no change respecting the prosthesis implanted was observed, with CT showing full expansion of the surgical valve frame, circular geometry of the ViV complex, and no evidence of frame recoil |
| Morales-Portano et al., 2019 [30] | Successful with no complications. |
| Laricchia et al., 2019 [31] | No complications. Trivial residual AR. The patient was discharged on the sixth postoperative day in good general conditions. After 1 month, he repeated a CT scan demonstrating good positioning and shaping of the ViV. |
| Kosmas et al., 2019 [32] | NR |
| Koni et al., 2019 [33] | Good hemodynamic results without leak and uneventful hospitalization. No complications |
| Balghith et al., 2019 [34] | Excellent final results, no PVL and the gradient was 15 mmHg. The patient was discharged from hospital after 2 days in a very good condition. No complications |
| Garcia-Lara et al., 2018 [35] | (After implantation): The hemodynamic outcome was optimal with a resulting maximum gradient of 20 mmHg and a mean gradient of 10 mmHg, with no significant regurgitation. Angiography showed that the prosthesis remained in a position slightly below the lower edge of the Perceval prosthesis. After implantation, the patient experienced complete atrioventricular block. A provisional pacemaker was implanted with implantation of a definitive VVIR device after 48 h. The patient progressed favorably and was discharged 5 days after implantation. Two months later, she remained asymptomatic. |
| Oezpeker et al., 2018 [36] | No complications. Post-operative echocardiography 5 days and 6 and 12 weeks after TAVI showed regular AV function with a constant minimal MPG (≤10 mmHg) and excluded valvular regurgitation. CT scan on Day 5 after TAVI confirmed perfect anatomical positioning of the AV with a completely circular shape of the annulus [diameter 20.4 × 20.1 mm]. The course of NT-proBNP levels was 2260 ng/L before initial aortic valve replacement, 2892 ng/L before the ViV procedure, and 1475 ng/L immediately thereafter. The calculated systolic PAP decreased from 40 mmHg before ViV-TAVI to 30 mmHg. The patient presented with NYHA I 1 month after ViV-TAVI. |
| Tomai et al., 2021 [37] | Angiography and echo-color-Doppler evaluation both indicated the absence of aortic regurgitation or paravalvular leaks. The patient was discharged 5 days later, in good clinical conditions. The so-called Matryoshka procedure, that is, a valve-in-valve-in-valve TAVI procedure with a balloon-expanding Sapien 3 valve following a surgical valve-in-valve procedure with a suture-less bioprosthesis implanted into a degenerated stented aortic valve, is safe and feasible (operative mean gradient). |
| López-Tejero et al., 2022 [38] | Neither regurgitation nor significant gradient was detected after implantation. After the initial recovery, the patient died 10 days later due to complications derived from the mechanical support implanted the day after the procedure. |
| Patterson et al., 2020 [39] | Post-procedural imaging demonstrated bioprosthetic valve frame protrusion and contained annular rupture, which required operative intervention (Level of Difficulty: Intermediate). |
| Erdogan et al., 2022 [40] | There was no significant gradient and/or paravalvular regurgitation on control aortic root angiography after THV implantation, and both the coronary arteries were patent. |
| Arslan et al., 2021 [41] | Symptoms of heart failure started during the follow-up, which suggests that the stent-infolding of the valve might occur in the late postoperative period. |
| Kalra, et al., 2018 [42] | There was complete resolution of transaoortic gradient and restoration of circular valve conformation, and no complications occurred. |
| Landes, et al., 2018 [43] | The TAVI was successfully deployed with excellent angiographic results. The rest of the hospitalization course was uneventful. At discharge, echocardiography showed proper ViV functioning with no significant leaks or gradients across the Sapien XT valve. The patient remained asymptomatic for the following 6 months. |
| Loforte et al., 2022 [44] | She presented type III AV block on the third day and progressive worsening of hemodynamics and respiratory function. The echocardiogram revealed the presence of moderate aortic regurgitation and high pressure gradients through the implanted valve (max/mean gradients: 52/31 mmHg; prosthetic valve area: 1.15 cm2) that appeared to be “heart shaped” as a result of a partial collapse of the basal ring at the level of the right coronary sinus; this structural distortion jailed the right coronary cusp and caused moderate-to-severe aortic regurgitation. Urgent transcatheter balloon valvuloplasty was successfully performed to reshape the valve and to stabilize the hemodynamics, but the patients developed the same imaging and clinical pattern 9 days after. |
| Medda et al., 2020 [45] | Good results were obtained without complications. |
| Dubois et al., 2023 [46] | Good results were obtained without complications. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owais, T.; Bisht, O.; El Din Moawad, M.H.; El-Garhy, M.; Stock, S.; Girdauskas, E.; Kuntze, T.; Amer, M.; Lauten, P. Outcomes of Valve-in-Valve (VIV) Transcatheter Aortic Valve Replacement (TAVR) after Surgical Aortic Valve Replacement with Sutureless Surgical Aortic Valve Prostheses Perceval™: A Systematic Review of Published Cases. J. Clin. Med. 2024, 13, 5164. https://doi.org/10.3390/jcm13175164
Owais T, Bisht O, El Din Moawad MH, El-Garhy M, Stock S, Girdauskas E, Kuntze T, Amer M, Lauten P. Outcomes of Valve-in-Valve (VIV) Transcatheter Aortic Valve Replacement (TAVR) after Surgical Aortic Valve Replacement with Sutureless Surgical Aortic Valve Prostheses Perceval™: A Systematic Review of Published Cases. Journal of Clinical Medicine. 2024; 13(17):5164. https://doi.org/10.3390/jcm13175164
Chicago/Turabian StyleOwais, Tamer, Osama Bisht, Mostafa Hossam El Din Moawad, Mohammad El-Garhy, Sina Stock, Evaldas Girdauskas, Thomas Kuntze, Mohamed Amer, and Philipp Lauten. 2024. "Outcomes of Valve-in-Valve (VIV) Transcatheter Aortic Valve Replacement (TAVR) after Surgical Aortic Valve Replacement with Sutureless Surgical Aortic Valve Prostheses Perceval™: A Systematic Review of Published Cases" Journal of Clinical Medicine 13, no. 17: 5164. https://doi.org/10.3390/jcm13175164
APA StyleOwais, T., Bisht, O., El Din Moawad, M. H., El-Garhy, M., Stock, S., Girdauskas, E., Kuntze, T., Amer, M., & Lauten, P. (2024). Outcomes of Valve-in-Valve (VIV) Transcatheter Aortic Valve Replacement (TAVR) after Surgical Aortic Valve Replacement with Sutureless Surgical Aortic Valve Prostheses Perceval™: A Systematic Review of Published Cases. Journal of Clinical Medicine, 13(17), 5164. https://doi.org/10.3390/jcm13175164






