Development and Validation of Artificial Intelligence Models for Prognosis Prediction of Juvenile Myoclonic Epilepsy with Clinical and Radiological Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Participants
2.2. Image Acquisition and Processing
2.3. Machine Learning Models
3. Results
3.1. Clinical Characteristics
3.2. Radiological Characteristics
3.3. Performances of Machine Learning Models
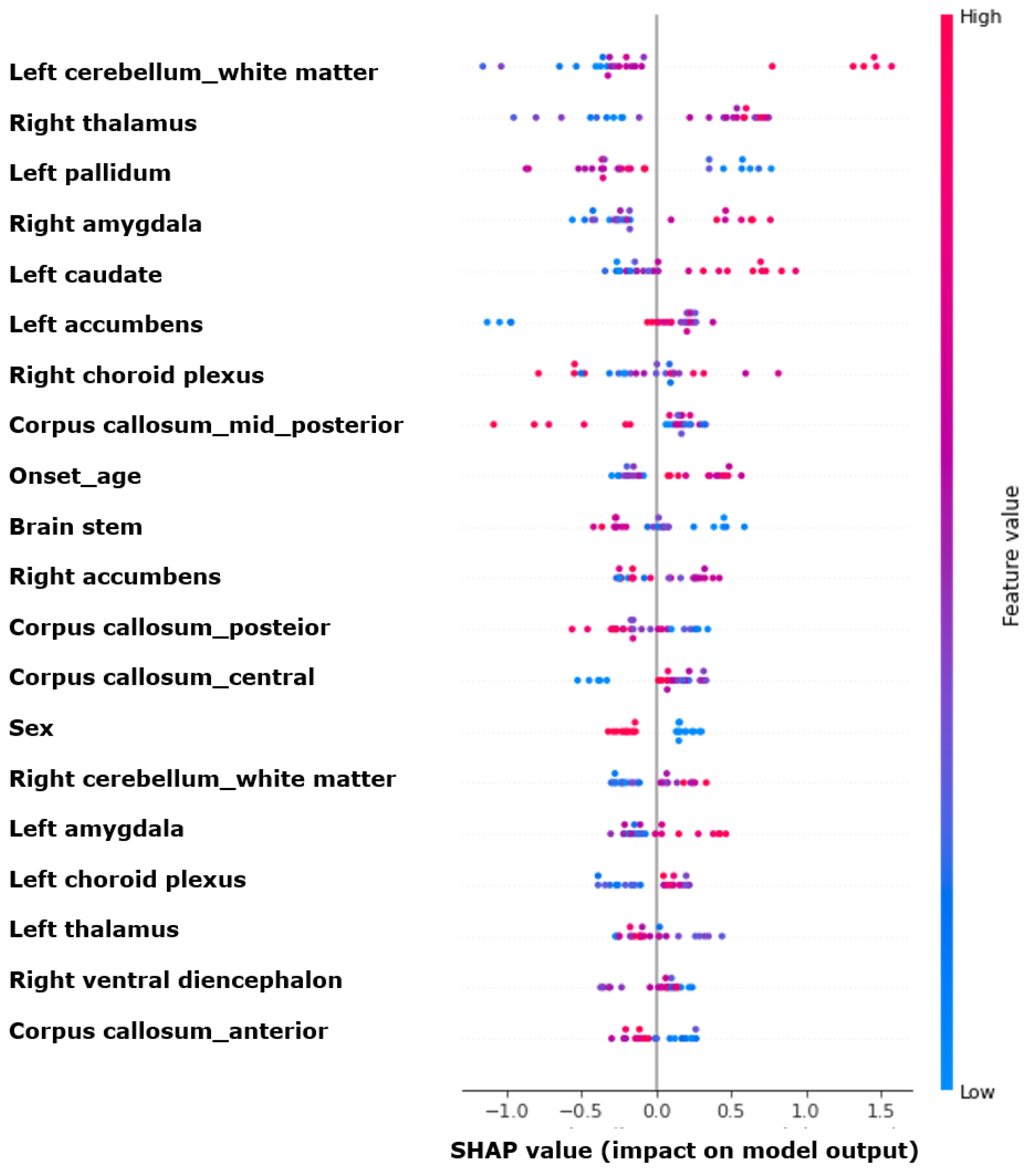
3.4. Feature Importances
4. Discussion
- The volumes of the left amygdala and right hippocampus, along with male gender, were associated with prognosis;The XGBoost model demonstrated a performance of approximately 0.700 in predicting prognosis, with higher performance observed when combining the clinical and radiological variables;
- The cerebellum, thalamus, and globus pallidus were crucial for the machine learning model’s prediction of prognosis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hauser, W.A. The prevalence and incidence of convulsive disorders in children. Epilepsia 1994, 35 (Suppl. 2), S1–S6. [Google Scholar] [CrossRef]
- Grünewald, R.A.; Panayiotopoulos, C.P. Juvenile myoclonic epilepsy: A review. Arch. Neurol. 1993, 50, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Proposal for classification of epilepsies and epileptic syndromes. Commission on classification and terminology of the international league against epilepsy. Epilepsia 1985, 26, 268–278. [Google Scholar]
- Baykan, B.; Wolf, P. Juvenile myoclonic epilepsy as a spectrum disorder: A focused review. Seizure 2017, 49, 36–41. [Google Scholar] [CrossRef]
- Baykan, B.; Martínez-Juárez, I.E.; Altindag, E.A.; Camfield, C.S.; Camfield, P.R. Lifetime prognosis of juvenile myoclonic epilepsy. Epilepsy Behav. 2013, 28 (Suppl. 1), S18–S24. [Google Scholar] [CrossRef]
- Giuliano, L.; Mainieri, G.; Aguglia, U.; Bilo, L.; Durante, V.; Ermio, C.; Galimberti, C.A.; La Neve, A.; Monti, G.; Ranzato, F.; et al. Long-term prognosis of juvenile myoclonic epilepsy: A systematic review searching for sex differences. Seizure 2021, 86, 41–48. [Google Scholar] [CrossRef]
- Fayad, C.; Saad, K.; Kahwagi, G.J.; Hallit, S.; Griffin, D.; Abou-Khalil, R.; El-Hayek, E. A systematic review and meta-analysis of factors related to first line drugs refractoriness in patients with juvenile myoclonic epilepsy (jme). PLoS ONE 2024, 19, e0300930. [Google Scholar] [CrossRef] [PubMed]
- Stevelink, R.; Koeleman, B.P.C.; Sander, J.W.; Jansen, F.E.; Braun, K.P.J. Refractory juvenile myoclonic epilepsy: A meta-analysis of prevalence and risk factors. Eur. J. Neurol. 2019, 26, 856–864. [Google Scholar] [CrossRef]
- Guaranha, M.S.; Filho, G.M.; Lin, K.; Guilhoto, L.M.; Caboclo, L.O.; Yacubian, E.M. Prognosis of juvenile myoclonic epilepsy is related to endophenotypes. Seizure 2011, 20, 42–48. [Google Scholar] [CrossRef]
- Rubboli, G.; Beier, C.P.; Selmer, K.K.; Syvertsen, M.; Shakeshaft, A.; Collingwood, A.; Hall, A.; Andrade, D.M.; Fong, C.Y.; Gesche, J.; et al. Variation in prognosis and treatment outcome in juvenile myoclonic epilepsy: A biology of juvenile myoclonic epilepsy consortium proposal for a practical definition and stratified medicine classifications. Brain Commun. 2023, 5, fcad182. [Google Scholar] [CrossRef] [PubMed]
- Domin, M.; Bartels, S.; Geithner, J.; Wang, Z.I.; Runge, U.; Grothe, M.; Langner, S.; von Podewils, F. Juvenile myoclonic epilepsy shows potential structural white matter abnormalities: A tbss study. Front. Neurol. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Suh, S.i.; Park, S.Y.; Seo, W.K.; Koh, I.; Koh, S.B.; Seol, H.Y. Microstructural white matter abnormality and frontal cognitive dysfunctions in juvenile myoclonic epilepsy. Epilepsia 2012, 53, 1371–1378. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, Z.; Yang, Y.; Zhang, J.; Wang, Y. Development and validation of an interpretable machine learning model for predicting post-stroke epilepsy. Epilepsy Res. 2024, 205, 107397. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Liu, C.; Friedman, D. Artificial intelligence/machine learning for epilepsy and seizure diagnosis. Epilepsy Behav. 2024, 155, 109736. [Google Scholar] [CrossRef]
- Kaushik, M.; Mahajan, S.; Machahary, N.; Thakran, S.; Chopra, S.; Tomar, R.V.; Kushwaha, S.S.; Agarwal, R.; Sharma, S.; Kukreti, R.; et al. Predicting efficacy of antiseizure medication treatment with machine learning algorithms in north indian population. Epilepsy Res. 2024, 205, 107404. [Google Scholar] [CrossRef]
- Hakeem, H.; Feng, W.; Chen, Z.; Choong, J.; Brodie, M.J.; Fong, S.L.; Lim, K.S.; Wu, J.; Wang, X.; Lawn, N.; et al. Development and validation of a deep learning model for predicting treatment response in patients with newly diagnosed epilepsy. JAMA Neurol. 2022, 79, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.S.; Cho, Y.-J.; Jang, S.H.; Lee, M.K.; Lee, B.I.; Heo, K. The localizing and lateralizing value of auras in lesional partial epilepsy patients. Yonsei Med. J. 2012, 53, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Ségonne, F.; Dale, A.M.; Busa, E.; Glessner, M.; Salat, D.; Hahn, H.K.; Fischl, B. A hybrid approach to the skull stripping problem in mri. Neuroimage 2004, 22, 1060–1075. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Ségonne, F.; Pacheco, J.; Fischl, B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging 2007, 26, 518–529. [Google Scholar] [CrossRef]
- Giuste, F.; Shi, W.; Zhu, Y.; Naren, T.; Isgut, M.; Sha, Y.; Tong, L.; Gupte, M.; Wang, M.D. Explainable artificial intelligence methods in combating pandemics: A systematic review. IEEE Rev. Biomed. Eng. 2023, 16, 5–21. [Google Scholar] [CrossRef]
- Cho, S.; Joo, B.; Park, M.; Ahn, S.J.; Suh, S.H.; Park, Y.W.; Ahn, S.S.; Lee, S.K. A radiomics-based model for potentially more accurate identification of subtypes of breast cancer brain metastases. Yonsei Med. J. 2023, 64, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Shakeshaft, A.; Panjwani, N.; McDowall, R.; Crudgington, H.; Peña Ceballos, J.; Andrade, D.M.; Beier, C.P.; Fong, C.Y.; Gesche, J.; Greenberg, D.A.; et al. Trait impulsivity in juvenile myoclonic epilepsy. Ann. Clin. Transl. Neurol. 2021, 8, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Jiang, D.; Zhao, X.; Yang, J.; Liang, D.; Wang, H.; Zhao, C.; Liao, J. Predicting drug treatment outcomes in childrens with tuberous sclerosis complex-related epilepsy: A clinical radiomics study. AJNR Am. J. Neuroradiol. 2023, 44, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Hwang, H.; Sohn, B.; Park, K.; Han, K.; Ahn, S.S.; Lee, W.; Chu, M.K.; Heo, K.; Lee, S.K. Development and validation of mri-based radiomics models for diagnosing juvenile myoclonic epilepsy. Korean J. Radiol. 2022, 23, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; An, H.; Shin, D.W.; Lee, H.W. Retention rates and successful treatment with antiseizure medications in newly-diagnosed epilepsy patients. Yonsei Med. J. 2024, 65, 89–97. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, D.A.; Park, K.M. Altered cerebellar volumes and intrinsic cerebellar network in juvenile myoclonic epilepsy. Acta Neurol. Scand. 2023, 2023, 7907887. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.B.; Suh, S.-i. Alteration of cerebello-thalamocortical spontaneous low-frequency oscillations in juvenile myoclonic epilepsy. Acta Neurol. Scand. 2019, 140, 252–258. [Google Scholar] [CrossRef]
- Mory, S.B.; Betting, L.E.; Fernandes, P.T.; Lopes-Cendes, I.; Guerreiro, M.M.; Guerreiro, C.A.; Cendes, F.; Li, L.M. Structural abnormalities of the thalamus in juvenile myoclonic epilepsy. Epilepsy Behav. 2011, 21, 407–411. [Google Scholar] [CrossRef]
- Betting, L.E.; Mory, S.B.; Lopes-Cendes, I.; Li, L.M.; Guerreiro, M.M.; Guerreiro, C.A.M.; Cendes, F. Mri reveals structural abnormalities in patients with idiopathic generalized epilepsy. Neurology 2006, 67, 848–852. [Google Scholar] [CrossRef]
- O’Muircheartaigh, J.; Vollmar, C.; Barker, G.J.; Kumari, V.; Symms, M.R.; Thompson, P.; Duncan, J.S.; Koepp, M.J.; Richardson, M.P. Abnormal thalamocortical structural and functional connectivity in juvenile myoclonic epilepsy. Brain 2012, 135, 3635–3644. [Google Scholar] [CrossRef] [PubMed]
- Gauffin, H.; Landtblom, A.-M.; Vigren, P.; Frick, A.; Engström, M.; McAllister, A.; Karlsson, T. Similar profile and magnitude of cognitive impairments in focal and generalized epilepsy: A pilot study. Front. Neurol. 2022, 12, 746381. [Google Scholar] [CrossRef] [PubMed]


| Favorable Prognosis (n = 85) | Poor Prognosis (n = 40) | p-Value | |
|---|---|---|---|
| Age (years) | 23.5 ± 8.7 | 23.2 ± 7.7 | 0.843 |
| Male sex, n (%) | 51 (60.0) | 16 (40.0) | 0.036 |
| Onset age (years) | 15.2 ± 4.5 | 15.2 ± 4.8 | 0.991 |
| Epilepsy duration (years) | 8.3 ± 9.4 | 8.0 ± 8.2 | 0.888 |
| Family history, n (%) | 16 (18.8) | 4 (10.0) | 0.209 |
| Febrile seizure history *, n (%) | 9 (10.6) | 5 (12.5) | 0.767 |
| Absence seizure, n (%) | 31 (36.5) | 14 (35.0) | 0.873 |
| Treated, n (%) | 44 (51.8) | 24 (60.0) | 0.388 |
| Number of ASMs | 2 (1–3) | 2 (1–3) | 0.577 |
| VPA, n (%) | 65 (76.5) | 30 (75.0) | 0.857 |
| LTG, n (%) | 39 (45.9) | 21 (52.5) | 0.490 |
| LEV, n (%) | 40 (47.1) | 24 (60.0) | 0.177 |
| TPM, n (%) | 17 (20.0) | 6 (15.0) | 0.501 |
| Follow-up duration (years) | 13.9 ± 6.7 | 11.1 ± 7.2 | 0.178 |
| Favorable Prognosis (n = 85) | Poor Prognosis (n = 40) | p-Value | |
|---|---|---|---|
| Left | |||
| Thalamus | 8054.0 ± 844.4 | 7748.2 ± 1393.7 | 0.131 |
| Caudate | 3569.1 ± 433.8 | 3458.2 ± 643.6 | 0.259 |
| Putamen | 5075.5 ± 619.2 | 4902.2 ± 895.1 | 0.211 |
| Pallidum | 2073.8 ± 244.5 | 2017.6 ± 353.9 | 0.303 |
| Hippocampus | 4180.6 ± 415.2 | 4027.1 ± 630.2 | 0.107 |
| Amygdala | 1739.9 ± 263.4 | 1601.7 ± 358.2 | 0.017 |
| Nucleus accumbens | 511.4 ± 98.5 | 286.6 ± 133.8 | 0.246 |
| Ventral diencephalon | 4175.7 ± 457.7 | 4003.7 ± 621.1 | 0.084 |
| Choroid plexus | 437.0 ± 163.7 | 427.5 ± 159.2 | 0.765 |
| Cerebellum–cortex | 56,595.1 ± 5734.4 | 54,113.8 ± 8452.0 | 0.057 |
| Cerebellum–white matter | 14,800.9 ± 1846.9 | 14,133.2 ± 2293.1 | 0.084 |
| Right | |||
| Thalamus | 7574.4 ± 775.1 | 7219.0 ± 1275.0 | 0.056 |
| Caudate | 3637.9 ± 444.1 | 3544.3 ± 567.1 | 0.318 |
| Putamen | 5130.4 ± 617.7 | 4985.8 ± 809.3 | 0.273 |
| Pallidum | 1977.6 ± 238.8 | 1942.0 ± 284.8 | 0.467 |
| Hippocampus | 4396.6 ± 417.7 | 4128.8 ± 825.7 | 0.017 |
| Amygdala | 1848.7 ± 279.3 | 1744.6 ± 361.0 | 0.080 |
| Nucleus accumbens | 577.2 ± 105.1 | 554.5 ± 117.2 | 0.280 |
| Ventral diencephalon | 4173.3 ± 444.1 | 4017.0 ± 562.6 | 0.095 |
| Choroid plexus | 430.6 ± 154.9 | 422.9 ± 188.5 | 0.809 |
| Cerebellum–cortex | 56,266.6 ± 5838.5 | 53,800.4 ± 8392.4 | 0.059 |
| Cerebellum–white matter | 14,242.4 ± 2001.1 | 13,540.7 ± 2200.4 | 0.079 |
| Midline | |||
| Brainstem | 21,182.7 ± 2266.9 | 20,565.8 ± 3631.8 | 0.248 |
| Optic-chiasm | 154.3 ± 58.7 | 140.1 ± 60.8 | 0.213 |
| Corpus callosum | |||
| Anterior | 862.7 ± 141.4 | 836.8 ± 158.5 | 0.360 |
| Mid-anterior | 669.4 ± 181.2 | 666.1 ± 171.8 | 0.922 |
| Central | 688.8 ± 172.1 | 660.2 ± 171.5 | 0.388 |
| Mid-posterior | 552.8 ± 103.9 | 560.6 ± 128.1 | 0.720 |
| Posterior | 990.4 ± 178.0 | 982.0 ± 205.2 | 0.815 |
| Total intracranial volume | 1,581,418.5 ± 175,060.8 | 1,502,151.6 ± 250,957.1 | 0.077 |
| Models | Accuracy | Precision | Recall | F1-Score | AUROC |
|---|---|---|---|---|---|
| Logistic Regression | 0.600 | 0.560 | 0.600 | 0.565 | 0.431 |
| Random Forest | 0.680 | 0.664 | 0.680 | 0.652 | 0.580 |
| XGBoost | 0.680 | 0.816 | 0.680 | 0.712 | 0.700 |
| Light GBM | 0.560 | 0.486 | 0.560 | 0.505 | 0.618 |
| SVM | 0.640 | 0.410 | 0.640 | 0.500 | 0.500 |
| ANN | 0.600 | 0.400 | 0.600 | 0.480 | 0.425 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.M.; Choi, B.K.; Ha, W.-S.; Cho, S.; Chu, M.K.; Heo, K.; Kim, W.-J. Development and Validation of Artificial Intelligence Models for Prognosis Prediction of Juvenile Myoclonic Epilepsy with Clinical and Radiological Features. J. Clin. Med. 2024, 13, 5080. https://doi.org/10.3390/jcm13175080
Kim KM, Choi BK, Ha W-S, Cho S, Chu MK, Heo K, Kim W-J. Development and Validation of Artificial Intelligence Models for Prognosis Prediction of Juvenile Myoclonic Epilepsy with Clinical and Radiological Features. Journal of Clinical Medicine. 2024; 13(17):5080. https://doi.org/10.3390/jcm13175080
Chicago/Turabian StyleKim, Kyung Min, Bo Kyu Choi, Woo-Seok Ha, Soomi Cho, Min Kyung Chu, Kyoung Heo, and Won-Joo Kim. 2024. "Development and Validation of Artificial Intelligence Models for Prognosis Prediction of Juvenile Myoclonic Epilepsy with Clinical and Radiological Features" Journal of Clinical Medicine 13, no. 17: 5080. https://doi.org/10.3390/jcm13175080
APA StyleKim, K. M., Choi, B. K., Ha, W.-S., Cho, S., Chu, M. K., Heo, K., & Kim, W.-J. (2024). Development and Validation of Artificial Intelligence Models for Prognosis Prediction of Juvenile Myoclonic Epilepsy with Clinical and Radiological Features. Journal of Clinical Medicine, 13(17), 5080. https://doi.org/10.3390/jcm13175080






