Antiphospholipid Antibody Testing in a Maximum Care Hospital: Method-Dependent Differences
Abstract
1. Introduction
2. Materials and Methods
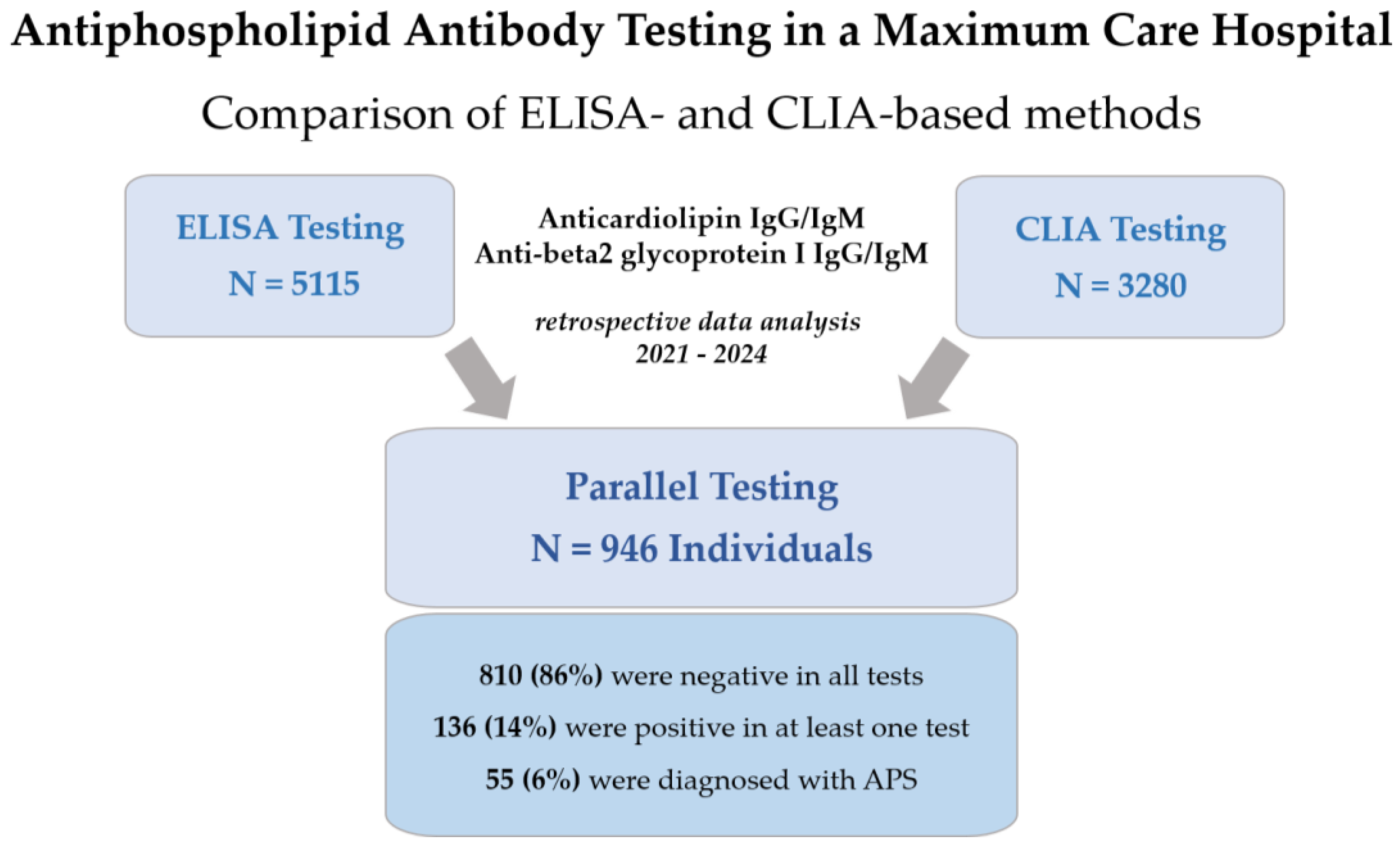
2.1. Data Collection
2.2. Laboratory Assays
2.3. Statistical Analysis
3. Results
3.1. Concordances of Antiphospholipid Antibody Assays
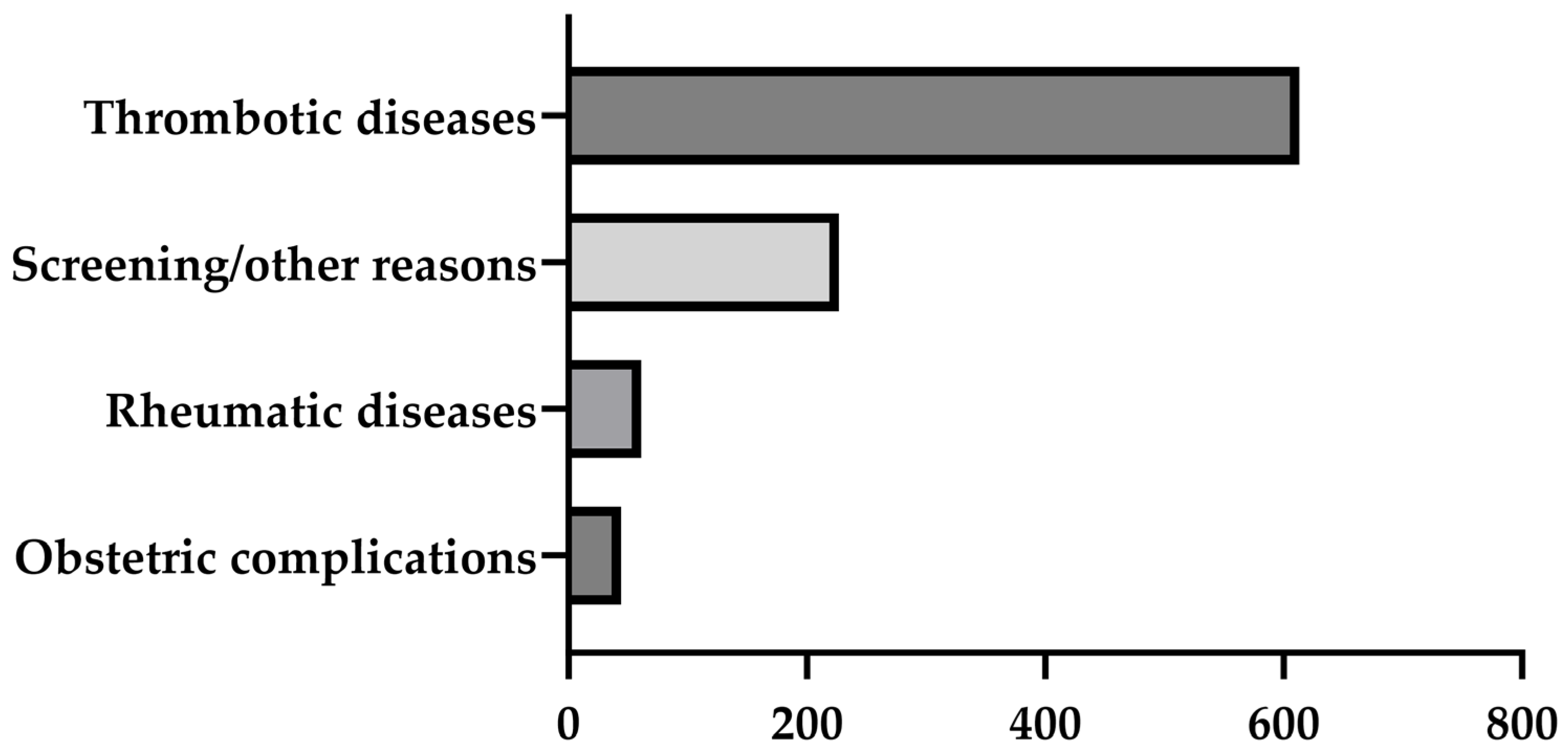
3.1.1. Results of APL Measurements in the Entire Cohort
3.1.2. Results of APL Measurements in Patients with APS
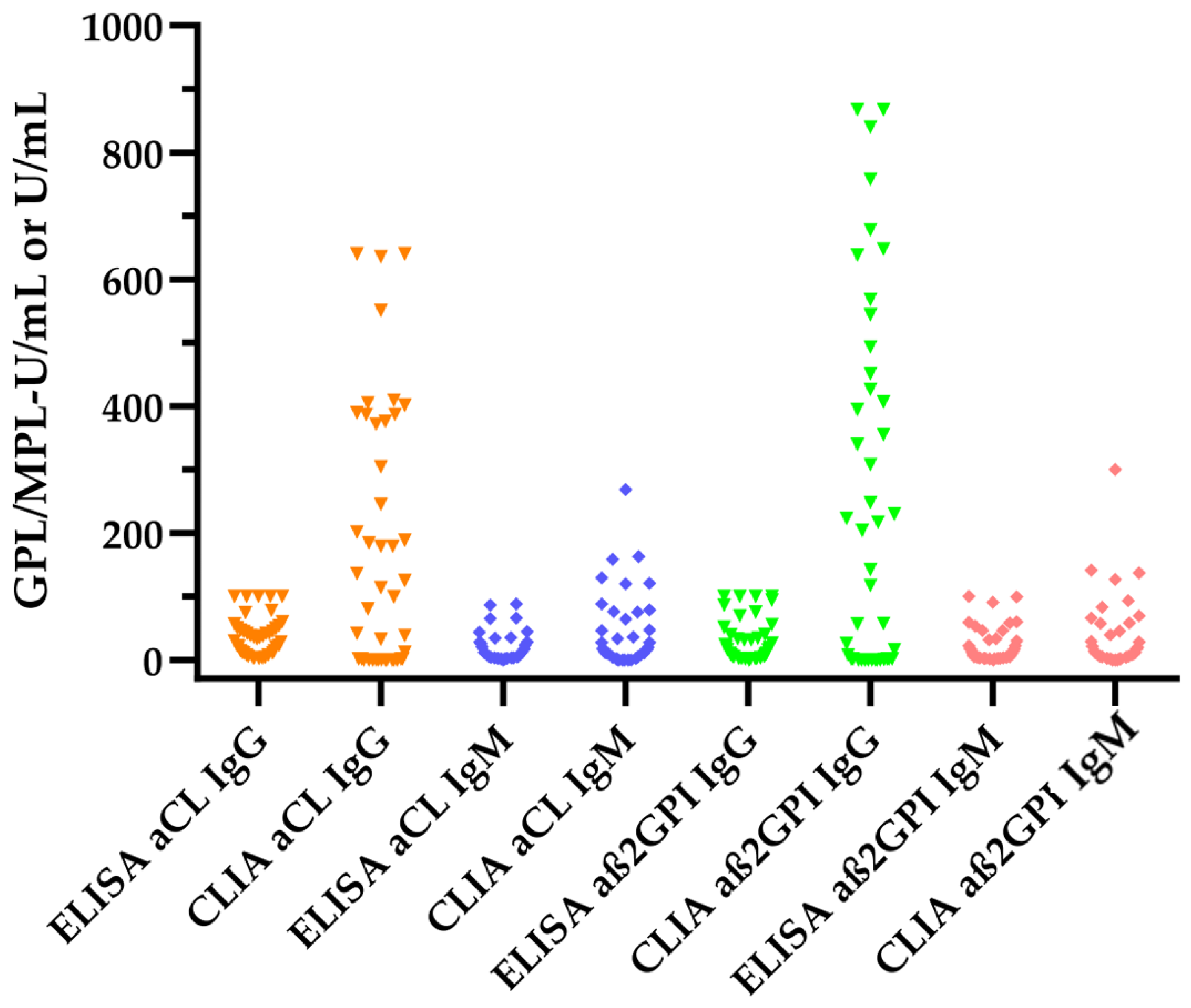
3.2. Comparison of Antiphospholipid Antibody Levels between ELISA and CLIA Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arreola-Diaz, R.; Majluf-Cruz, A.; Sanchez-Torres, L.E.; Hernandez-Juarez, J. The Pathophysiology of The Antiphospholipid Syndrome: A Perspective From The Blood Coagulation System. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221088576. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, M.L.; Gomez, S.; Pareja, J.F.; Theodoridou, A.; Sanna, G.; Hughes, G.R.; Khamashta, M.A. Antiphospholipid antibody tests: Spreading the net. Ann. Rheum. Dis. 2005, 64, 1639–1643. [Google Scholar] [CrossRef]
- Fischer, M.J.; Rauch, J.; Levine, J.S. The antiphospholipid syndrome. Semin. Nephrol. 2007, 27, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Branch, D.W.; Ortel, T.L. Antiphospholipid syndrome: Advances in diagnosis, pathogenesis, and management. BMJ 2023, 380, e069717. [Google Scholar] [CrossRef]
- Meroni, P.L.; Borghi, M.O.; Raschi, E.; Tedesco, F. Pathogenesis of antiphospholipid syndrome: Understanding the antibodies. Nat. Rev. Rheumatol. 2011, 7, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Rodriguez-Pinto, I.; Espinosa, G. The diagnosis and clinical management of the catastrophic antiphospholipid syndrome: A comprehensive review. J. Autoimmun. 2018, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Shi, H.; Li, C.; Knight, J.S. Antiphospholipid syndrome: A clinical perspective. Chin. Med. J. 2020, 133, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; de Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.A.; Gharavi, A.E.; Koike, T.; Lockshin, M.D.; Branch, D.W.; Piette, J.C.; Brey, R.; Derksen, R.; Harris, E.N.; Hughes, G.R.; et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: Report of an international workshop. Arthritis. Rheum. 1999, 42, 1309–1311. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. The 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis. Rheumatol. 2023, 75, 1687–1702. [Google Scholar] [CrossRef]
- Devreese, K.M.; Poncet, A.; Lindhoff-Last, E.; Musial, J.; de Moerloose, P.; Fontana, P. A multicenter study to assess the reproducibility of antiphospholipid antibody results produced by an automated system. J. Thromb. Haemost. 2017, 15, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.J. Testing for antiphospholipid antibodies: Advances and best practices. Int. J. Lab. Hematol. 2020, 42 (Suppl. S1), 49–58. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.J.; de Groot, P.G.; de Laat, B.; Erkan, D.; Favaloro, E.J.; Mackie, I.; Martinuzzo, M.; Ortel, T.L.; Pengo, V.; Rand, J.H.; et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: Update of the guidelines for lupus anticoagulant detection and interpretation. J. Thromb. Haemost. 2020, 18, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Vandevelde, A.; Chayoua, W.; de Laat, B.; Gris, J.C.; Moore, G.W.; Musial, J.; Zuily, S.; Wahl, D.; Devreese, K.M.J. Semiquantitative interpretation of anticardiolipin and antibeta2glycoprotein I antibodies measured with various analytical platforms: Communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost. 2022, 20, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Montaruli, B.; De Luna, E.; Erroi, L.; Marchese, C.; Mengozzi, G.; Napoli, P.; Nicolo, C.; Romito, A.; Bertero, M.T.; Sivera, P.; et al. Analytical and clinical comparison of different immunoassay systems for the detection of antiphospholipid antibodies. Int. J. Lab. Hematol. 2016, 38, 172–182. [Google Scholar] [CrossRef]
- Grossi, V.; Infantino, M.; Benucci, M.; Li Gobbi, F.; Bandinelli, F.; Damiani, A.; Bodio, C.; Borghi, M.O.; Mahler, M.; Aure, M.A.; et al. Two Novel Technologies for the Detection of Anti-cardiolipin and Anti beta2-Glycoprotein Antibodies in the Real Life: Chemiluminescent in Comparison to the Addressable Laser Bead Immunoassays. Immunol. Investig. 2020, 49, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.J. Solid Phase Assays for Antiphospholipid Antibodies. Semin. Thromb. Hemost. 2022, 48, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Vandevelde, A.; Devreese, K.M.J. Laboratory Diagnosis of Antiphospholipid Syndrome: Insights and Hindrances. J. Clin. Med. 2022, 11, 2164. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J.; Pasalic, L.; Lippi, G. Classification Criteria for the Antiphospholipid Syndrome: Not the Same as Diagnostic Criteria for Antiphospholipid Syndrome. Semin. Thromb. Hemost. 2024, 50, 605–608. [Google Scholar] [CrossRef]
- Chayoua, W.; Kelchtermans, H.; Moore, G.W.; Gris, J.C.; Musial, J.; Wahl, D.; Zuily, S.; Gianniello, F.; Fontana, P.; Remijn, J.; et al. Detection of Anti-Cardiolipin and Anti-beta2glycoprotein I Antibodies Differs between Platforms without Influence on Association with Clinical Symptoms. Thromb. Haemost. 2019, 119, 797–806. [Google Scholar] [CrossRef]
- Erkan, D.; Barbhaiya, M.; Zuily, S.; Bertolaccini, M.L.; Willis, R.; Devreese, K. Response to: Correspondence on ‘2023 ACR/EULAR antiphospholipid syndrome classification criteria’ by Miro-Mur et al. Ann. Rheum. Dis. 2024, 83, e3. [Google Scholar] [CrossRef] [PubMed]
- Miro-Mur, F.A.; Alijotas-Reig, J.; Anunciacion-Llunell, A.; Marques-Soares, J.; Esteve-Valverde, E.; Pardos-Gea, J. Correspondence on ‘2023 ACR/EULAR antiphospholipid syndrome classification criteria’. Ann. Rheum. Dis. 2024, 83, e2. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.N.; Gharavi, A.E.; Patel, S.P.; Hughes, G.R. Evaluation of the anti-cardiolipin antibody test: Report of an international workshop held 4 April 1986. Clin. Exp. Immunol. 1987, 68, 215–222. [Google Scholar] [PubMed]
- Ichikawa, K.; Tsutsumi, A.; Atsumi, T.; Matsuura, E.; Kobayashi, S.; Hughes, G.R.; Khamashta, M.A.; Koike, T. A chimeric antibody with the human gamma1 constant region as a putative standard for assays to detect IgG beta2-glycoprotein I-dependent anticardiolipin and anti-beta2-glycoprotein I antibodies. Arthritis. Rheum. 1999, 42, 2461–2470. [Google Scholar] [CrossRef]
- Vandevelde, A.; Gris, J.C.; Moore, G.W.; Musial, J.; Zuily, S.; Wahl, D.; Devreese, K.M.J. Toward harmonized interpretation of anticardiolipin and anti-beta2-glycoprotein I antibody detection for diagnosis of antiphospholipid syndrome using defined level intervals and likelihood ratios: Communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost. 2024, 22, 2345–2362. [Google Scholar] [CrossRef]
| Antiphospholipid Antibody | ELISA | CLIA |
|---|---|---|
| aCL IgG (GPL-U/mL) | >14.4 | >20 |
| aCL IgM (MPL-U/mL) | >7.2 | >10 |
| aß2GPI IgG (U/mL) | >14.4 | >20 |
| aß2GPI IgM (U/mL) | >14.4 | >10 |
| A | B | C | D | E | |
|---|---|---|---|---|---|
| Entire cohort | All aPL tests negative | Any aPL test positive | Any aPL test positive using ELISA | Any aPL test positive using CLIA | |
| N | 946 | 810 | 136 | 92 | 94 |
| Women | 466 (49%) | 386 (48%) | 80 (59%) | 56 (61%) | 56 (60%) |
| Age | 51 (35–61) | 50 (33–61) | 54 (38–65) | 51 (36–65) | 54 (39–65) |
| Clinical findings | |||||
| Arterial or venous thrombosis | 143 (15%) | 89 (11%) | 54 (40%) | 25 (27%) | 30 (32%) |
| Ischemic stroke | 482 (51%) | 436 (54%) | 46 (34%) | 29 (32%) | 34 (36%) |
| Transient ischemic attack | 40 (4%) | 35 (4%) | 5 (4%) | 3 (3%) | 2 (2%) |
| Obstetric complications | 36 (4%) | 27 (3%) | 9 (7%) | 8 (9%) | 8 (9%) |
| Autoimmune diseases | |||||
| Connective tissue diseases | 36 (4%) | 19 (2%) | 17 (13%) | 16 (17%) | 13 (14%) |
| Vasculitis | 21 (2%) | 18 (2%) | 3 (2%) | 2 (2%) | 2 (2%) |
| Others | 13 (1%) | 7 (1%) | 6 (4%) | 2 (2%) | 6 (6%) |
| APS diagnosis | 55 (6%) | 4 (<1%) | 51 (38%) | 47 (51%) | 41 (44%) |
| Primary APS | 38 (4%) | 3 (<1%) | 35 (26%) | 33 (36%) | 29 (31%) |
| Secondary APS | 17 (2%) | 1 (<1%) | 16 (12%) | 14 (15%) | 12 (13%) |
| Laboratory findings | |||||
| LA (positive) | 46 (5%) | 22 (3%) | 24 (18%) | 23 (25%) | 20 (21%) |
| dRVVT (screen) (s) | 31 (29–34) | 31 (29–34) | 33 (29–45) | 37 (31–52) | 32 (29–47) |
| aPTT (Actin FS) (s) | 24 (23–27) | 24 (23–27) | 25 (23–27) | 26 (23–29) | 25 (23–28) |
| aPTT (Actin FSL) (s) | 27 (25–28) | 26 (25–28) | 27 (25–31) | 29 (26–35) | 25 (27–31) |
| Manufacturer thresholds | |||||
| aCL IgG (positive) | 57 (6%) | - | 57 (42%) | 49 (53%) | 30 (32%) |
| aCL IgM (positive) | 96 (10%) | - | 96 (71%) | 49 (53%) | 63 (67%) |
| aß2GPI IgG (positive) | 54 (6%) | - | 54 (40%) | 31 (34%) | 30 (32%) |
| aß2GPI IgM (positive) | 88 (9%) | - | 88 (65%) | 24 (26%) | 65 (69%) |
| Moderate threshold (according to 2023 ACR/EULAR criteria) | |||||
| aCL IgG (>40 GPL-U/mL) | 41 (4%) | - | 41 (30%) | 20 (22%) | 28 (30%) |
| aCL IgM (>40 MPL-U/mL) | 24 (3%) | - | 24 (18%) | 6 (7%) | 20 (21%) |
| aß2GPI IgG (>40 U/mL) | 29 (3%) | - | 29 (21%) | 10 (11%) | 28 (30%) |
| aß2GPI IgM (>40 U/mL) | 25 (3%) | - | 25 (18%) | 12 (13%) | 21 (22%) |
| CLIA | ||
|---|---|---|
| ELISA | ||
| aCL IgG | negative | positive |
| negative | 891 | 25 |
| positive | 6 | 24 |
| ELISA | ||
| aCL IgM | negative | positive |
| negative | 861 | 22 |
| positive | 36 | 27 |
| ELISA | ||
| aß2GPI I IgG | negative | positive |
| negative | 908 | 8 |
| positive | 7 | 23 |
| ELISA | ||
| aß2GPI I IgM | negative | positive |
| negative | 877 | 4 |
| positive | 45 | 20 |
| CLIA | ||
|---|---|---|
| ELISA | ||
| aCL IgG | negative | positive |
| negative | 20 | 3 |
| positive | 8 | 24 |
| ELISA | ||
| aCL IgM | negative | positive |
| negative | 23 | 8 |
| positive | 7 | 17 |
| ELISA | ||
| aß2GPI I IgG | negative | positive |
| negative | 25 | 6 |
| positive | 3 | 21 |
| ELISA | ||
| aß2GPI I IgM | negative | positive |
| negative | 33 | 5 |
| positive | 1 | 16 |
| ELISA | CLIA | p-Value | |
|---|---|---|---|
| aCL IgG (GPL-U/mL) | 30 (10–47) | 38 (0–304) | 0.0071 |
| aCL IgM (MPL-U/mL) | 6 (3–23) | 10 (0–36) | 0.0258 |
| aß2GPI IgG (U/mL) | 10 (2–34) | 56 (0–407) | 0.0003 |
| aß2GPI IgM (U/mL) | 5 (2–22) | 7 (2–29) | 0.0022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocijancic, M.; Goj, T.; Peter, A.; Klein, R.; Hörber, S. Antiphospholipid Antibody Testing in a Maximum Care Hospital: Method-Dependent Differences. J. Clin. Med. 2024, 13, 4528. https://doi.org/10.3390/jcm13154528
Kocijancic M, Goj T, Peter A, Klein R, Hörber S. Antiphospholipid Antibody Testing in a Maximum Care Hospital: Method-Dependent Differences. Journal of Clinical Medicine. 2024; 13(15):4528. https://doi.org/10.3390/jcm13154528
Chicago/Turabian StyleKocijancic, Marija, Thomas Goj, Andreas Peter, Reinhild Klein, and Sebastian Hörber. 2024. "Antiphospholipid Antibody Testing in a Maximum Care Hospital: Method-Dependent Differences" Journal of Clinical Medicine 13, no. 15: 4528. https://doi.org/10.3390/jcm13154528
APA StyleKocijancic, M., Goj, T., Peter, A., Klein, R., & Hörber, S. (2024). Antiphospholipid Antibody Testing in a Maximum Care Hospital: Method-Dependent Differences. Journal of Clinical Medicine, 13(15), 4528. https://doi.org/10.3390/jcm13154528






